An iridium oxide reference electrode for use in microfabricated biosensors and biochips
Haesik
Yang
*,
Sun Kil
Kang
*,
Chang Auck
Choi
,
Hyokyum
Kim
,
Dong-Ho
Shin
,
Yong Shin
Kim
and
Youn Tae
Kim
BioMEMS Group, Electronics and Telecommunications Research Institute (ETRI), Daejeon 305-350, Korea. E-mail: hsyang@etri.re.kr; strider2@etri.re.kr; Fax: +82 42 860 6836; Tel: +82 42 860 6876
First published on 3rd December 2003
Abstract
In this paper we argue for the use of iridium oxide (IrOx) electrodes as quasi-reference electrodes in microfabricated biosensors and biochips that operate in buffered solutions. The simple microfabrication of these electrodes consists of a one-step electrodeposition of IrOx onto a microfabricated platinum (Pt) electrode. The IrOx electrode potential was found to vary less than 20 mV over 9 days after stabilization for 1 day in a phosphate-buffered saline (PBS) solution; this behavior of the electrode potential was found to be easily reproduced. Moreover, the electrode potential was found to vary by less than 15 mV in the initial hour of its use; this behavior of the electrode potential was also found to be reproducible. The performance of a microfabricated glucose sensor employing an IrOx reference electrode is characterized in this paper in order to evaluate the usefulness of this new IrOx electrode as a quasi-reference electrode. The glucose sensor consists of a recessed microfabricated Pt electrode array, an electrodeposited IrOx film, an inner layer composed of an electropolymerized poly(m-phenylenediamine)/glucose oxidase (PMPD/GOx) film, and an outer or protective layer composed of Teflon and polyurethane (PU) films. The response of this sensor was found to be equivalent to the response of the same sensor employing a commercial Ag/AgCl reference electrode. These results show that a microfabricated IrOx electrode can be used as a quasi-reference electrode in microfabricated biosensors and biochips operating in buffered solutions.
Introduction
Many research groups have been developing microfabrication methods for use in the cheap, reproducible and mass production of biosensors and biochips.1–4 The microfabrication of biosensors and biochips utilizing electrochemical detection schemes, such as electrochemical DNA sensors5 and enzyme-catalyzing glucose sensors,6 are particular targets of this research, because semiconductor microfabrication techniques can be applied directly to sensor fabrication,7 and the easy miniaturization of whole systems would be beneficial for the creation of implantable devices8 and point-of-care testing devices.9,10 Reference electrodes11 and working electrodes are crucial components of these electrochemical biosensors and biochips, in terms of both their microfabrication and performance.A reference electrode that can easily and reproducibly be microfabricated has long been required for use in microfabricated electrochemical biosensors and biochips. To date, miniature Ag/AgCl reference electrodes12–21 have been used for this purpose because their exchange current is large and their potential is stable. These electrodes can be microfabricated via a two-step process: firstly, Ag film formation by electrodeposition or vacuum deposition, and secondly, electrochemical or chemical formation of the AgCl film.22,23 The long-term stability of the potential of these microfabricated reference electrodes is highly dependent on the stability of the AgCl film. AgCl gradually dissolves in solutions with a high Cl− concentration and is susceptible to reactions with electroactive species; these susceptibilities eventually lead to the degradation of the AgCl layer on the Ag electrode.17 Moreover, because the quantity of AgCl is very small in microfabricated Ag/AgCl electrodes, these electrodes have a short lifetime. After complete AgCl dissolution or consumption, the reference electrode potential changes radically. This change in the potential results in a large shift in the working electrode potential and causes erroneous sensor responses. Modified AgCl formation methods15–17 and protective layers such as Nafion24,25 have been employed in the attempt to minimize these problems. Though protective layers have been found to be efficient in suppressing dissolution, this approach is not a reliable solution for long-term measurements.
There have been some reports that show the possibility that iriduium oxide (IrOx)26 and Prussian blue27,28 can be used as quasi-reference electrodes. Recently, IrOx electrodes have received considerable attention, especially for their use in pH measurement19,29–35 and neural stimulation.36,37 Further, microfabricated IrOx electrodes have been studied as an alternative to glass pH electrodes, because it is difficult to microfabricate conventional glass pH electrodes and to use them in in vivo experiments. The pH dependence of the IrOx electrode potential is well-established, and the electrodes show good stability over a wide pH range and exhibit minimal long-term drift of electrode potential,38,39 so they are very stable in fixed pH solutions such as buffered solutions. For instance, the electrode potential drift of IrOx in blood and interstitial fluids is likely to be quite small, considering that the pH of human blood and interstitial fluids is in the range 7.31 to 7.45, and that the pH dependence of the IrOx potential is usually in the range of −59 to −80 mV/pH.31,39 Note also that the stability of the reference electrode potential is not as crucial to amperometric biosensors such as the H2O2-measuring glucose sensor as it is to potentiometric sensors. Thus, the IrOx electrode could be used as a quasi-reference electrode in continuous monitoring biosensors and in disposable biosensors and biochips that operate in buffered solutions.
In this paper, we argue for the use of IrOx electrodes as quasi-reference electrodes, and investigate their long-term and initial short-term performances in terms of their stability and reproducibility, which are two of the most important requirements of reference electrodes. The IrOx electrodes used in this study were fabricated using electrodeposition onto microfabricated platinum (Pt) electrodes,29,31,33,36,40–42 and the time dependence of their potentials was monitored in both a phosphate-buffered saline (PBS) solution and serum after their pH dependence and cyclic voltammetric performances were characterized. To evaluate the performance of the IrOx electrode in an actual biosensor, a glucose sensor employing an IrOx reference electrode was microfabricated and its performance is compared in this study with that of a sensor using a commercial Ag/AgCl electrode.
Experimental
Chemicals and electrochemistry
1,3-Phenylenediamine (MPD), hydrogen peroxide (30% solution in water), glutaraldehyde (25% solution in water), Teflon (60 wt. % dispersion in water), iridium tetrachloride, oxalic acid dihydrate, and anhydrous potassium carbonate were purchased from Aldrich. PBS (pH 7.4), glucose oxidase (GOx) (EC 1.1.3.4), glucose, and poly-L-lysine hydrobromide (MW = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were obtained from Sigma. Polyurethane (PU)
(SG85A) was purchased from Thermedics Inc. (Woburn, MA). All chemicals were used as received. Double-distilled water was used for the preparation of all solutions.
000) were obtained from Sigma. Polyurethane (PU)
(SG85A) was purchased from Thermedics Inc. (Woburn, MA). All chemicals were used as received. Double-distilled water was used for the preparation of all solutions.
All electrochemical experiments were performed with a CHI model 660A potentiostat/galvanostat (CH instruments, Austin, Tx) in a three-electrode cell. All potentials are reported relative to that of the Ag/AgCl (3 M NaCl) reference electrode. To monitor the IrOx electrode potential, the open circuit potential (OCP) was measured at room temperature. The pH was measured with a glass pH electrode.
Electrode microfabrication
The electrodes were fabricated on a 5 in. diameter silicon wafer, and only two photomasks were used during the entire microfabrication process. After the use of a standard cleaning procedure, a low temperature silicon oxide film (1 µm) was deposited onto the wafer using low-pressure chemical vapor deposition. A titanium tungsten (TiW) adhesion layer (750 Å) and a Pt layer (2000 Å) were then deposited onto the wafer by dc magnetron sputtering. After TiW and Pt deposition, a photoresist layer was spin-coated onto the wafer and then exposed through the first mask. After removal of the sensitized photoresist, the exposed regions of the Pt layer were etched by wet etching in a 8:7:1 solution of H2O:HCl:HNO3. The exposed TiW regions were etched by reactive ion etching (RIE), and then the remaining photoresist was removed. The exposed platinum was covered with a plasma enhanced chemical vapor deposition (PECVD) silicon oxide (SiO2) layer (1 µm). An aluminium (Al) layer (8000 Å) was then deposited onto this layer by sputtering. Another photoresist layer was spin-coated on top of that and then exposed through the second mask. After removal of the sensitized photoresist, the exposed regions of the Al layer were etched by RIE. The exposed PECVD SiO2 regions were wet-etched, and then the remaining photoresist was removed. Another Pt layer (1000 Å) was deposited by e-beam evaporation. The sacrificial Al layer was then dissolved away, lifting off the unwanted Pt layer. The size of the resulting exposed recessed rectangular platinum electrode is 0.1 mm2 (0.1 × 1 mm) (Fig. 1). | ||
| Fig. 1 Photograph of a microfabricated bare Pt electrode and an IrOx electrode electrodeposited onto a Pt electrode. | ||
Film formation
IrOx films were electrodeposited onto the microfabricated Pt electrodes. The deposition solution was prepared as follows, according to the method described by Marzouk et al.33 A 75 mg portion of iridium tetrachloride was dissolved in 50 mL of water. The solution was stirred for 30 min. A 0.5 mL aliquot of aqueous 30% hydrogen peroxide solution was added and then stirred for 10 min. 250 mg of oxalic acid dihydrate was added and the solution was stirred again for 10 min. The pH of the solution was adjusted to 10.5 by adding small portions of anhydrous potassium carbonate. The resulting solution was allowed to sit quiescently for 48 h before electrodeposition. An anodic deposition of IrOx was created by cycling 100 times between the limits of 0.0 V and 0.6 V at 50 mV s−1.A poly(m-phenylenediamine)/GOx (PMPD/GOx) film was electropolymerized onto a microfabricated electrode at 0.7 V in a PBS solution containing 5 mM MPD, 20 units mL−1 GOx, 1 µL mL−1 of 0.25% glutaraldehyde, and 10 µL mL−1 of 1% poly-L-lysine hydrobromide. The electropolymerization time was 900 s, which is sufficient time for the film thickness to become self-limiting.43 This long electropolymerization time results in a reproducible film thickness and a reproducible glucose sensor response. The PBS solution containing MPD and GOx was prepared as described previously.44 The outer layer or protective layer of the glucose sensor consists of Teflon and PU films. The Teflon film was deposited by dipping the sensor in a 30% Teflon solution followed by drying at room temperature for 10 min. This step was then repeated. The PU was deposited by dipping the sensor in a 0.4% PU solution followed by drying. This step was also repeated. The 0.4% PU solution was prepared as described previously.44
Results and discussion
Stability and reproducibility
The uniform and reproducible formation of electrodeposited IrOx films on electrodes is dependent upon the electrode type and the electrodeposition conditions. In particular, film formation on Pt electrodes is potentially less reproducible because of the low adhesion of IrOx films to Pt surfaces, and is also affected by the state of the Pt surface.40 Thus, a clean Pt surface is required for reproducible deposition of IrOx films, and in this work this was achieved by delaying the formation of the Pt surface until the final fabrication process, at which stage there are no deposition or etching processes that adversely affect the Pt surface. Fig. 1 shows a photograph of a uniform IrOx film electrodeposited onto a microfabricated Pt electrode. Cyclic voltammograms of IrOx films vary with the conditions used during their electrodeposition. In this study, the cyclic voltammograms obtained in H2SO4 solution of the IrOx films exhibited similar behavior under similar conditions, indicating that the IrOx films are reproducibly formed on the microfabricated Pt electrodes. The thickness of the IrOx films was estimated by calculating the redox charge from the cyclic voltammogram obtained in H2SO4 solution, and using the reported surface density of the IrOx film (7.8 × 10−7 mol cm−2 µm−1).31 The calculated thickness is approximately 0.04 µm.IrOx electrodes also exhibit a pH response that varies with the electrodeposition conditions.39 The pH sensitivities of the electrodeposited IrOx electrodes were characterized. The pH dependence of the OCP consists of two lines with constant slopes (Fig. 2). Electrodeposited IrOx electrodes are known to have two different regions of linear pH response that meet near pH 6.42 The values we obtained for these slopes are −68 mV/pH below pH 6 and −77 mV/pH above pH 6. These values agree with those obtained in previous studies.42 This result confirms that IrOx films can be reproducibly fabricated on microfabricated Pt electrodes.
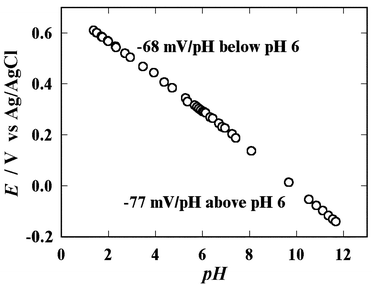 | ||
| Fig. 2 pH dependence of the OCP of an IrOx electrode to the addition of 0.1 M NaOH or 0.1 M HCl to a PBS solution. | ||
To investigate the stability of the potential of the IrOx electrode, its OCP was measured in a PBS solution at room temperature for 10 days, as shown in Fig. 3a. The initial slow negative shift of approximately 120 mV in Fig. 3a is known to result from structural changes of IrOx films by hydration after their formation.33,39 This stabilization time increases with the thickness of the film. After stabilization for approximately 1 day, the potential becomes stable and its drift was found to be less than 20 mV over the next 9 days. It is interesting to note that this potential drift is quite small for such a long period, even though no protective layers such as Nafion are present on the IrOx film.
 | ||
| Fig. 3 (a) Time dependence of the OCP of an IrOx electrode for 10 days in a PBS solution. For long-term reproducibility check, the time dependence of the OCPs of 25 electrodes was measured after 10 days as shown in the inset. (b) Time dependence of the OCP during the intital hour just after dipping of the electode in a solution. For short-term reproducibility check, time dependence of the OCPs of 25 electrodes was measured as shown in the inset. | ||
Twenty-five IrOx electrodes were electrodeposited in the same solution, and then their OCPs were compared after 10 days. As shown in the inset of Fig. 3a, the variation of the OCPs of these electrodes is negligible, with a mean value of 0.195 V, and a standard deviation of only 4 mV. This small deviation shows that electrodeposited IrOx electrodes can be produced reproducibly. The long-term stability and reproducibility of IrOx electrodes means that IrOx electrodes can be used as quasi-reference electrodes in continuous monitoring microfabricated biosensors that operate in buffered solutions for a long period.
The change in the OCP during the initial hour just after dipping of the electrodes in a PBS solution is shown in Fig. 3b. The total drift is less than 15 mV, which is a small change in potential as far as amperometric measurements are concerned. In disposable amperometric biosensors and biochips, the detection time is usually less than 1 h. Thus IrOx electrodes can be used as quasi-reference electrodes in constant pH solutions if the initial OCPs of IrOx are reproducible. To evaluate the reproducibility of the initial OCPs, OCPs for the 25 IrOx electrodes were obtained and are shown in the inset of Fig. 3b. Their mean value is 3.001 V, and their standard deviation is 10 mV. This indicates good reproducibility of the initial OCPs. Accordingly, the initial short-term stability and reproducibility of IrOx electrodes means that IrOx electrodes can also be used as quasi-reference electrodes in disposable microfabricated biosensors and biochips that operate in buffered solutions for a short period.
It is known that IrOx electrode potential has very low sensitivity to most ions and to oxygen, as well as to the electroactive species such as ascorbic acid and uric acid that are present in physiological samples.29,33 Moreover, H2O2 can be generated during the operation of many biosensors but has no effect on the OCPs of IrOx electrodes. The performance of IrOx reference electrodes in physiological buffer solutions was investigated indirectly by measuring the OCP of an IrOx electrode in serum after stabilizing it in a PBS solution (Fig. 4). The pH of serum increases in the open state because the concentration of CO2 in serum decreases (Fig. 4). The pH dependence of the IrOx electrode is −77 mV/pH in this pH range, as shown in Fig. 2. The pH-calibrated OCP was obtained taking this dependence into account. This pH-calibrated OCP was found to decrease by 10 mV over 1000 s, which occurs because of hydration of the IrOx film after drying, and then the OCP maintains its value. This small decrease indicates that the many ions and electroactive species that are present in serum have no significant effect on the OCP of the IrOx electrode.
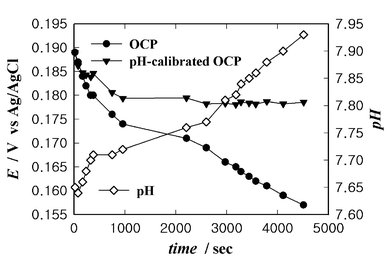 | ||
| Fig. 4 Time dependence of the OCP of an IrOx electrode in serum and the pH of serum. | ||
Microfabricated glucose sensor
Among the various biosensors and biochips that have now been developed, glucose sensors are the best established. However, there are many problems in their long-term use that remain to be overcome, including their need for a stable reference electrode. In this study, the performance of a microfabricated glucose sensor that uses an IrOx reference electrode is monitored in order to confirm the potential of IrOx electrodes for use as quasi-reference electrodes in buffered solutions.A microfabricated Pt electrode array fabricated on a silicon wafer was diced into many chips. Each diced chip contained four Pt electrodes and four pads. Three of these Pt electrodes were used as the working, reference, and counter electrodes. A schematic diagram of the microfabricated glucose sensor employing an IrOx reference electrode is shown in Fig. 5. This sensor utilizes an inner layer consisting of an electropolymerized PMPD/GOx film and an outer layer consisting of dip-coated Teflon and PU films. The inner layer and outer layer facilitate good permselectivity to electroactive interferents and stable sensor response respectively.44,45 The inner layer is formed only on the working electrode, while the outer layer covers the whole surface. This outer layer also functions as a protective layer for both the IrOx reference electrode and the Pt counter electrode. The IrOx reference electrode is created by one-step electrodeposition before the formation of the PMPD/GOx film. This one-step fabrication of the IrOx reference electrode is very simple compared to the complex microfabrication required for Ag/AgCl on the Pt electrode. This microfabrication advantage should facilitate cheaper and easier mass production of sensors.
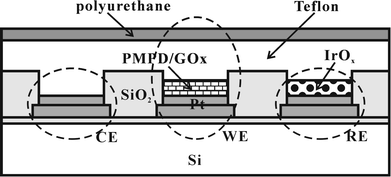 | ||
| Fig. 5 Schematic diagram of a microfabricated glucose sensor (CE = counter electrode; WE = working electrode; RE = reference electrode). | ||
Fig. 6 shows the hydrodynamic response of the glucose sensor to the addition of glucose to the stirred PBS solution. The potential of the working electrode was maintained at 0.45 V with respect to the IrOx reference electrode. The current shows a stable stepwise increase. The calculated dependence of the current on the glucose concentration is plotted in Fig. 7, as is that obtained with a commercial Ag/AgCl reference electrode (applied potential = 0.65 V). Though these data were measured with separate microfabricated sensors, the two curves coincide. The low drift of the IrOx OCP during the measurements contributes only negligibly to the sensor response. These results confirm that the IrOx electrode is a good quasi-reference electrode for use in microfabricated glucose sensors.
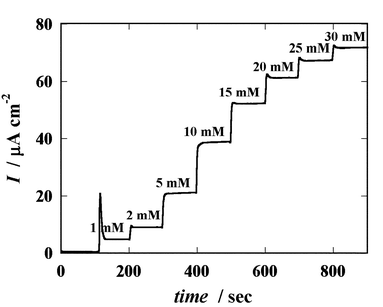 | ||
| Fig. 6 Hydrodynamic response of a microfabricated glucose sensor to the addition of glucose to a stirred PBS solution (applied potential = 0.45 V vs. IrOx). | ||
In H2O2-measuring glucose sensors, the current response depends on H2O2 oxidation on the Pt electrode, on the enzymatic reaction of glucose with GOx, and on glucose diffusion through the outer layer. If the applied potential of the Pt electrode is larger than a certain value, its current response depends mainly on the last two factors, and the H2O2 oxidation reaction has a negligible effect on the current response. It is known that H2O2 oxidation becomes significant above the potential corresponding to platinum oxide film formation, which occurs at approximately 550 mV vs. Ag/AgCl (sat. KCl) at pH 7.4.46 On the other hand, the operating potential of Pt must be limited to a value below 900 mV vs. Ag/AgCl (sat. KCl) because unwanted background current becomes significant above this potential.46 Thus, the window of applied potential is the range 550 to 900 mV. In a large part of this range, the sensor response depends mainly on the enzymatic reaction and on glucose diffusion, and any change in the applied potential results in a negligible effect on the sensor response. For instance, when the initial applied potential is in the middle of this range, a change in the potential of reference electrode during the measurements larger than ±100 mV would be possible without significant effect to sensor response. This means that the sensor response has a large tolerance to any drift in the potential of the reference electrode. The observed 20 mV drift of the IrOx electrode over 9 days is thus negligible compared to the large tolerance to change in the potential. Moreover, even the observed 120 mV drift over 10 days including the first day could have a minimal effect on the sensor response as long as the initial applied potential is properly chosen. Accordingly, the IrOx electrode seems to be a very promising quasi-reference electrode for use in H2O2-measuring continuous monitoring glucose sensors.
Conclusions
We have argued here that IrOx electrodes can be used as quasi-reference electrodes in constant pH or buffered solutions. The long-term stability and reproducibility of the potential of IrOx electrodes after their initial stabilization means that IrOx electrodes can be used as quasi-reference electrodes in continuous monitoring microfabricated biosensors that operate for a long period, while their initial short-term stability and reproducibility means that they can also be used as quasi-reference electrodes in disposable microfabricated biosensors and biochips that operate for a short period. The IrOx electrode seems to be a very promising quasi-reference electrode for use in H2O2-measuring continuous monitoring glucose sensors because of both its long-term potential stability and the large tolerance of sensor response to change in the potential of the reference electrode.Acknowledgements
This work was supported financially by the Korea Ministry of Science and Technology through the NPL program.References
- M. J. Madou, Fundamentals of Microfabrication, CRC Press, Boca Raton, 2nd edn., 2002 Search PubMed.
- M. Madou and J. Florkey, Chem. Rev., 2000, 100, 2679–2692 CrossRef CAS.
- I. R. Lauks, Acc. Chem. Res., 1998, 31, 317–324 CrossRef CAS.
- M. R. Newman, R. P. Buck, V. V. Cosofret, E. Lindner and C. C. Liu, IEEE Eng. Med. Biol. Mag., 1994, 13, 409–419 CrossRef.
- J. J. Gooding, Electroanalysis, 2002, 14, 1149–1156 CrossRef CAS.
- J. Wang, Electroanalysis, 2001, 13, 983–988 CrossRef CAS.
- G. C. Fiaccabrino and M. Koudelka-Hep, Electroanalysis, 1998, 10, 217–222 CrossRef CAS.
- A. Heller, Annu. Rev. Biomed. Eng., 1999, 1, 153–175 Search PubMed.
- A. J. Tüdos, A. J. Besselink and R. B. Schasfoort, Lab Chip, 2001, 1, 83–95 RSC.
- J. Wang, Trends Anal. Chem., 2002, 21, 226–232 CrossRef CAS.
- A. W. Bott, Curr. Sep., 1995, 14, 64–68 Search PubMed.
- T. Matsumoto, A. Ohashi and N. Ito, Anal. Chim. Acta, 2002, 462, 253–259 CrossRef CAS.
- A. Hiratsuka, K. Kojima, H. Suzuki, H. Muguruma, K. Ikebukuro and I. Karube, Analyst, 2001, 126, 658–663 RSC.
- H. Suzuki, T. Hirakawa, S. Sasaki and I. Karube, Anal. Chim. Acta, 1999, 387, 103–112 CrossRef CAS.
- H. Suzuki, H. Ozawa, S. Sasaki and I. Karube, Sens. Actuators, B, 1998, 53, 140–146 CrossRef.
- H. Suzuki, T. Hirakawa, S. Sasaki and I. Kaurbe, Sens. Actuators, B, 1998, 46, 146–154 CrossRef.
- H. Suzuki, A. Hiratsuka, S. Sasaki and I. Karube, Sens. Actuators, B, 1998, 46, 104–113 CrossRef.
- D. Desmond, B. Lane, J. Alderman, J. D. Glennon, D. Diamo and D. W. M. Arrigan, Sens. Actuators, B, 1997, 44, 389–396 CrossRef.
- P. J. Kinlen, J. E. Heider and D. E. Hubbard, Sens. Actuators, B, 1994, 22, 13–25 CrossRef.
- S. D. Collins, Sens. Actuators, B, 1993, 10, 169–178 CrossRef CAS.
- L. J. Bousse, P. Bergveld and H. J. M. Geeraedts, Sens. Actuators, 1986, 9, 179–197 CrossRef CAS.
- X. Cai, N. Klauke, A. Glidle, P. Cobbold, G. L. Smith and J. M. Cooper, Anal. Chem., 2002, 74, 908–914 CrossRef CAS.
- I.-Y. Huang and R.-S. Huang, Thin Solid Films, 2002, 406, 255–261 CrossRef CAS.
- M. A. Nolan, S. H. Tan and S. P. Kounaves, Anal. Chem., 1997, 69, 1244–1247 CrossRef CAS.
- F. Moussy and D. J. Harrison, Anal. Chem., 1994, 66, 674–679 CrossRef CAS.
- O. C. Keller and J. Buffle, Anal. Chem., 2000, 72, 936–942 CrossRef CAS.
- A. Noll, V. Rudolf and E. W. Grabner, Electrochim. Acta, 1998, 44, 415–419 CrossRef CAS.
- P. J. Kulesza and Z. Galus, Electrochim. Acta, 1997, 42, 867–872 CrossRef CAS.
- A. N. Bezbaruah and T. C. Zhang, Anal. Chem., 2002, 74, 5726–5733 CrossRef CAS.
- S. A. M. Marzouk, R. P. Buck, L. A. Dunlap, T. A. Johnson and W. E. Cascio, Anal. Biochem., 2002, 308, 52–60 CrossRef CAS.
- D. O. Wipf, F. Ge, T. W. Spaine and J. E. Baur, Anal. Chem., 2000, 72, 4921–4927 CrossRef CAS.
- H. Suzuk, H. Arakawa, S. Sasaki and I. Karube, Anal. Chem., 1999, 71, 1737–1743 CrossRef CAS.
- S. A. M. Marzouk, S. Ufer, R. P. Buck, T. A. Johnson, L. A. Dunlap and W. E. Cascio, Anal. Chem., 1998, 70, 5054–5061 CrossRef CAS.
- W. Olthuis, M. A. M. Robben, P. Bergveld, M. Bos and W. E. Van Der Linden, Sens. Actuators, B, 1990, 2, 247–256 CrossRef.
- K. Kinoshita and M. J. Madou, J. Electrochem. Soc., 1984, 131, 1089–1094 CAS.
- R. D. Meyer, S. F. Cogan, T. H. Nguyen and R. D. Rauh, IEEE Trans. Rehab. Eng., 2001, 9, 2–11 Search PubMed.
- J. D. Weiland and D. J. Anderson, IEEE Trans. Biomed. Eng., 2000, 47, 911–918 CrossRef CAS.
- M. Wang, S. Yao and M. Madou, Sens. Actuators, B, 2002, 81, 313–315 CrossRef.
- S. Yao, M. Wang and M. Madou, J. Electrochem. Soc., 2001, 148, H29–H36 CrossRef CAS.
- S. A. M. Marzouk, Anal. Chem., 2003, 75, 1258–1266 CrossRef CAS.
- M. A. Petit and V. Plichon, J. Electroanal. Chem., 1998, 444, 247–252 CrossRef CAS.
- J. E. Baur and T. W. Spaine, J. Electroanal. Chem., 1998, 443, 208–216 CrossRef CAS.
- Q. Yang, P. Atanasov and E. Wilkins, Sens. Actuators, B, 1998, 46, 249–256 CrossRef.
- H. Yang, T. D. Chung, Y. T. Kim, C. A. Choi, C. H. Jun and H. C. Kim, Biosens. Bioelectron., 2002, 17, 251–259 CrossRef CAS.
- S. A. Emr and A. M. Yacynych, Electroanalysis, 1995, 7, 913–923 CAS.
- Y. Zhang and G. S. Wilson, J. Electroanal. Chem., 1993, 345, 253–271 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |

