Determination of phosphorus and metals in human brain proteins after isolation by gel electrophoresis by laser ablation inductively coupled plasma source mass spectrometry
J. Sabine
Becker
*a,
Myroslav
Zoriy
a,
J. Susanne
Becker
b,
Carola
Pickhardt
a and
Michael
Przybylski
b
aCentral Division of Analytical Chemistry, Research Center Juelich, D-52425 Juelich, Germany. E-mail: s.becker@fz-juelich.de
bLaboratory of Analytical Chemistry, Department of Chemistry, University of Konstanz, D-78457 Konstanz, Germany
First published on 8th December 2003
Abstract
Phosphorus, sulfur, silicon and metal concentrations (Al, Cu and Zn) were determined in human brain proteins by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) after separation of protein mixtures by two dimensional (2-D) gel electrophoresis. The analysis of phosphorus, silicon and metals in single protein spots in the gel was performed with an optimized microanalytical method using a double-focusing sector field inductively coupled plasma mass spectrometer coupled to a commercial laser ablation system (LA-ICP-MS). Relative ion intensities for P, Si and metals with respect to sulfur in protein spots were determined by LA-ICP-MS. The detection limits for phosphorus and sulfur in protein spots with a silver staining procedure on the 2-D gels were compared with the Coomassie staining technique described previously.
1. Introduction
Laser ablation ICP-MS (LA-ICP-MS) is a powerful mass spectrometric technique for fast trace element determination and isotope analysis and it has been used to an increasing extent for microlocal analysis of solid materials.1,2 The development of microanalytical techniques involving LA-ICP-MS for element determination in biological and medical samples is a challenging task. Special interest has recently focused on the determination of phosphorus in proteins.3–5,16 In these experiments LA-ICP-MS was applied for microlocal analysis of phosphorus in selected protein spots in two dimensional gels after 2-D gel electrophoresis. A major problem is the quantification of analytical data, therefore new quantification strategies for direct microlocal analysis of phosphorus in protein spots are required. Marshall et al.5 have developed a simple quantification strategy for phosphorus determination in protein gel spots which showed good detection sensitivity for β-casein. We have recently described a solution based calibration strategy for quantitative phosphorus determination in proteins in 2-D gel by LA-ICP-MS.3A serious disadvantage and limiting factor of LA-ICP-MS (and also of ICP-MS) for the determination of monoisotopic phosphorus at m/z 31 are isobaric interferences of high intensity such as 15N16O+, 14N17O+ and 14N16O1H+. For the separation of isobaric interferences, a double-focusing sector field ICP-MS (ICP-SFMS) with sufficient mass resolution (m/Δm ≥ 1500) has been applied to phosphorus determination in protein samples. An alternative approach is the application of ICP-MS with a dynamic reaction cell by measurement of 31P16O+ molecular ions, proposed by Baranov et al.6 This technique is useful if no sector field ICP-MS is available. However, at optimized experimental conditions we found significantly lower detection limits by ICP-SFMS (20 pg g−1) for phosphorus determination in aqueous solution in comparison to quadrupole based ICP-MS with a collision cell (ICP-CC-QMS) (1.3 ng g−1).7
In our previous work7 the Coomassie staining technique for visualization of protein spot in 2-D gel was applied, which provided a relatively high background of P and S in gel and protein spots and therefore high detection limits for both elements. To improve the detection limits for selected elements of interest, silver staining of separated protein spots in 2-D gels has been studied. Via the determination of phosphorus concentration by LA-ICP-MS, the occurrence of phosphorylations in proteins can be identified, a most important post-translational modification which is of crucial relevance for many physiological as well as pathophysiological processes such as in carcinogenesis and neurodegenerative diseases.8,9 Numerous recent studies have established soft ionisation biological mass spectrometry using electrospray (ESI) and matrix assisted laser desorption ionisation (MALDI) as powerful techniques for the identification and structure determination of proteins from biological material, including the determination of post-translational modifications such as glycosylation, fatty acylation and phosphorylation.10–12 While MALDI and ESI mass spectrometry are efficiently used for the identification of phosphorylation structures in proteins,3,13 these techniques cannot provide direct quantitative determinations of phosphorus and metals in biological samples.
In the present study, we have developed a direct microlocal technique for protein gel spots using LA-ICP-MS for the simultaneous multielement determination of phosphorus, sulfur and other elements (silicon, copper, zinc and aluminium) which were detected in human brain sample with Alzheimer’s disease.
2. Experimental
2.1. LA-ICP-MS instrumentation
A double-focusing sector field ICP-MS (ICP-SFMS, ELEMENT, Finnigan MAT, Bremen, Germany) coupled with a commercial laser ablation system LSX 200 (Cetac LSX 200, Cetac Technologies, Omaha, NE, USA) was used for the microlocal analysis of phosphorus and sulfur, silicon and selected metals in protein spots. The ablated material was transported by argon as a carrier gas into the inductively coupled plasma (ICP). The ions formed in the ICP were extracted in the sector field mass spectrometer and separated according to their mass-to-charge ratios. To separate interfering molecular ions from atomic ions 31P+, 32S+, 28Si+, 27Al+, 63Cu+ and 64Zn+, all LA-ICP-SFMS measurements were performed at a mass resolution of m/Δm 4000. The 31P+ ions are clearly separated from 15N16O+, 14N17O+ and 14N16O1H+, hence providing accurate phosphorus determinations. For the sulfur determination the most abundant interference of 16O2+ ions at m/z = 32 is separated using ICP-SFMS at medium mass resolution. The ICP torch was shielded with a grounded platinum electrode (GuardElectrode™, Finnigan MAT). For calibration a single gas flow solution-based procedure was applied using an ultrasonic nebulizer (USN, Cetac Technologies) described elsewhere.3 Using this arrangement, simultaneous optimization of the nebulizer gas flow rate for the USN and the carrier gas flow rate for the transport of laser-ablated material in ICP is possible. The experimental parameters of LA-ICP-MS were optimized with respect to the maximal ion intensity of 31P+ using a 1 µg L−1 phosphorus solution introduced by the USN, which is coupled on-line to the laser ablation chamber. Maximal ion intensity was observed at a carrier gas flow rate of 1 L min−1 for the transport of ablated material to the ICP-MS and an optimal mixing of nebulized standard solutions and laser-ablated solid sample directly in the ablation chamber is possible. The background intensity of 31P+, 32S+, 28Si+, 27Al+, 63Cu+ and 64Zn+ were determined after digestion of a small cut of the blank gel with HNO3 and measurement using ICP-SFMS.14 The optimized experimental parameters of LA-ICP-SFMS and ICP-SFMS measurements are summarized in Table 1.| Technique | LA-ICP-SFMS | ICP-SFMS |
|---|---|---|
| a Element (Finnigan MAT) for determination of P, S, Al, Si, Cu and Zn in proteins. | ||
| Laser ablation system | LSX 200 (CETAC) | |
| Nebulizer type | USN (for calibration) | Microconcentric |
| Spray chamber | With desolvator | Minicyclonic |
| Rf power/W | 1250 | 1200 |
| Cooling gas flow rate/l min−1 | 18 | 14 |
| Auxiliary gas flow rate/l min−1 | 1.1 | 1.4 |
| Nebulizer (carrier) gas flow rate/l min−1 | 1.32 | 0.7 |
| Solution uptake rate/ml min−1 | 2 | 0.05 |
| Extraction lens potential/V | 2000 | 2000 |
| Mass resolution (m/Δm) | 4000 | 4000 |
| Analysis time/min | 5 | 5 |
| Number of runs | 6 | 20 |
| Number of blocks of runs | 5 | 6 |
2.2. Standards and reagents
Concentrated nitric acid of Supragrade purity from Merck (Darmstadt, Germany) was used for sample digestion. Phosphorus, sulfur, silicon, aluminium, copper and zinc standard stock solutions for the calibration procedures were obtained from Merck (Darmstadt, Germany) and from the National Institute of Standards and Technology (NIST). For all dilutions, deionized Milli-Q water (18 MΩ) was used from a Millipore Milli-Q-Plus water purifier.A certified standard (CRM) BCR-273 (single-cell proteins with a P concentration of 26.8 ± 0.4 mg g−1) was obtained from IRMM (Geel, Belgium). The results of analysis of BCR-273 were mainly reported in a previous paper. We found that BCR-273 is not suited to microlocal analysis by LA-ICP-MS owing to serious inhomogeneity of the CRM.3
2.3. Samples and sample preparation
Human brain samples obtained post mortem from patients with Alzheimer’s disease were analysed for phosphorus, sulfur, copper, aluminium, silicon and zinc content by LA-ICP-MS after 2-D gel electrophoresis directly. For preparation of the brain sample for 2-D electrophoresis cell lysis and afterwards ultracentrifugation were used. For the lysis a buffer system with different proteases (e.g., aprotinin, antipain, leupeptin, pepstatin, from Sigma, Deisenhofen, Germany) was applied.2.4. Protein separation by two-dimensional gel electrophoresis
The 2-D gel electrophoresis separation of serum samples was performed as described by Tissot et al.15 Isoelectric focusing (IEF) in the first step was carried out on Immobiline DryStrip gels (immobilized pH gradient strip) with pH 4–7 and 3–10. All protein separations were performed and the selected separated proteins on the gel were analysed in respect of P, S, Si, Al, Cu and Zn directly by LA-ICP-MS. For future identification of separated protein spots by MALDI-FTICR-MS a duplicate gel was prepared under the same experimental conditions. After two-dimensional electrophoretic separation the gel was dried for several days before LA-ICP-MS measurements were carried out.3. Results and discussion
3.1. Separation and isolation of proteins by two-dimensional gel electrophoresis
Fig. 1 shows a graph of a 2-D gel separation of human proteins (Alzheimer’s disease) with different well-separated protein spots. At the standard conditions of isoelectric focusing and separation (see Experimental), the major and medium-abundant proteins are well resolved within the Immobiline pH gradients 4–7.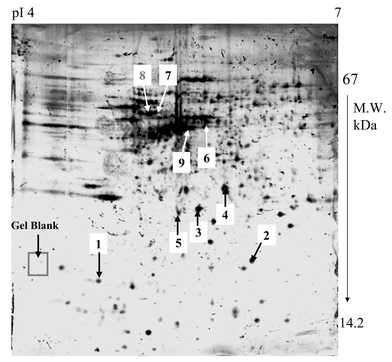 | ||
| Fig. 1 2-D gel electrophoresis separation of Alzheimer’s disease brain proteins within pH 4–7 using Immobiline gel strip; staining was performed with silver staining. | ||
In this work selected protein spots (marked by 1–9) are analyzed by LA-ICP-MS. Several areas from these 2-D gels were employed as references and gel blanks for elemental determinations by LA-ICP-MS, as described below.
3.2. Determination of element ratios in protein spots after separation of 2-D gel electrophoresis by LA-ICP-SFMS
LA-ICP-MS is a promising surface analytical technique for the direct µ-local analysis of protein spots in a two-dimensional gel, whereby rapidly and simultaneously the existence of P, S, Al, Cu, Zn and other elements in several proteins can be detected. Owing to high background of elements studied in the gel blank, it was sometimes difficult to analyze these elements in protein spots. Table 2 summarizes the qualitative results of LA-ICP-MS on investigated protein spots in the gels and the detection limits of elements measured in the gel blank.In protein spots 1, 2 and 4, no element studied was found in comparison with the background intensity in the gel. In protein spot 3, only sulfur (about 3–4 times higher than the background signal) was measured. Whereas for P and Cu, continuous background signals in the LA-ICP mass spectra for different µ-local analysis in the blank and investigated spots were observed, transient signals were measured for sulfur in the investigated gel spots and blank. The detection limit of phosphorus in protein spots was determined to be 0.6 µg g−1.
In protein spot 5, mainly P and S were detected. The relative ion intensities of 31P+ and 32S+ measured by LA-ICP-MS for the protein spot 5 are shown in Fig. 2. The ion intensity of phosphorus in the protein spot was determined to be about a factor of 6–7 higher than the background intensity. For the other elements studied, continuous background signals were observed.
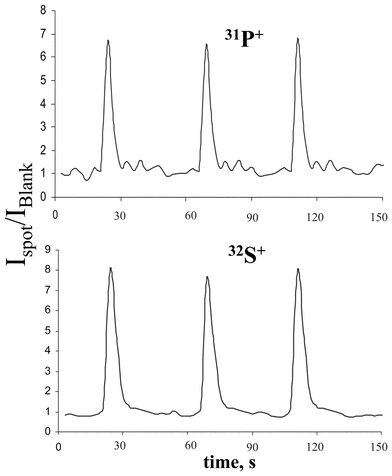 | ||
| Fig. 2 Transient signals of 31P+ and 32S+ in protein spot 5. | ||
Of special interest are protein spots 6–9, where a multielement analysis of several analytes via transient signals was possible. Fig. 3 compares the ion intensities of analytes in these proteins in comparison to background signal in gel blank. Transient ion signals of 31P+, 63Cu+, 27Al+, 64Zn+, 28Si+ and 32S+ in different protein spots measured by LA-ICP-SFMS with 500 laser shots using single point µ-local analysis are shown. The highest ion intensity of Al+ in the protein of spot 7 correlates with relative high P+, Cu+ and Zn+ ion intensities. In contrast, the protein in spot 8, which also contains all elements studied by LA-ICP-MS, shows similar signal intensity for Zn+ but lesser intensities for Al+, Cu+ and P+.
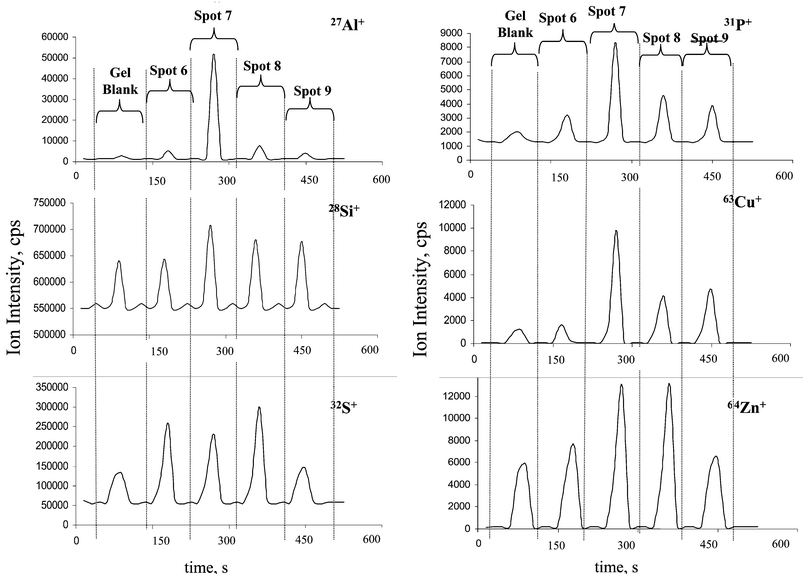 | ||
| Fig. 3 Transient signals of 27Al+, 28Si+, 31P+ ,32S+ , 63Cu+ and 64Zn+ in protein spots 6–9 in comparison with the blank gel. | ||
Concentrations of P and S in the blank gel were 0.010 and 0.052 mg g−1, respectively, measured by ICP-MS after digestion. These values are, in comparison with former measurements on Coomassie stained gels (background of P and S measured by LA-ICP-MS: 0.11 and 0.39 mg g−1, respectively3,14), an improvement for P background of about one order of magnitude, whereas the background of sulfur in silver staining gel is better by a factor of 7.5. Phosphorus concentrations in protein spots (assuming one phosphorylation site per protein molecule) were estimated to be detectable at ≥0.598 × 10−6 g g−1 by LA-ICP-MS. Owing to the high background of some elements in the gel (e.g., Si), the detection limit of LA-ICP-MS is relatively high.
An investigation via depth profiling by µ-local LA-ICP-MS on protein spots showed that, in most cases, the maximum P and S concentrations were found on one of the surfaces of the gel. Also in these measurements, concentrations of P and S changed with changing gel depth, but the ratio of P/S remained constant within measurement error. A lateral analysis of element distribution in the protein spots yielded maximum intensities for measured analyte ions in the middle of spot where the highest protein amount is concentrated. In the future, by scanning the laser beam over the gel surface the distribution of the elements will be studied.
Whereas, by LA-ICP-MS, a direct µ-local analysis of separated protein spots is possible, ICP-MS analysis requires a small trypsin (or HNO3) digested solution of protein spots from a gel. A major problem in element determination by ICP-MS in digested protein spots from gel is the possible contamination of the sample during the sample preparation.14 In LA-ICP-MS, the contamination problems can be minimized and therefore this technique provides an accurate analyte concentration if the sulfur content in the protein is known: therefore S was chosen in recent work3 as a suitable internal standard element. On the other hand, element ratios (e.g., P/S, Cu/S, Zn/S) of proteins can be determined. For example, in Table 3 the element ratios in the proteins 5–8 are summarized. The concentration of analyte X is mostly lower than the sulfur content, the element ratio X/S varied in different protein spots down to 0.003.
| Element ratios | Spot 5 | Spot 6 | Spot 7 | Spot 8 |
|---|---|---|---|---|
| Al/S | — | 0.003 | 0.49 | 0.033 |
| Si/S | — | 0.52 | 0.75 | 0.29 |
| P/S | 0.323 | 0.002 | 0.063 | 0.017 |
| Cu/S | — | 0.066 | 0.13 | 0.028 |
| Zn/S | — | 1.53 | 0.15 | 0.10 |
When ablating proteins from the wet gel the analyzed material is evaporated rapidly from the gel surface. In the case of single ion detector ICP-MS, the detector must switch from one isotope to another. In addition, the possible instability of the mass calibration at medium mass resolution in the ICP-SFMS applied required a relatively wide mass window to be scanned, which resulted in longer scanning time per isotope and was disadvantageous when monitoring fast processes, especially if multielemental analysis was being performed. Therefore, in this case, a multiple ion collector ICP-MS would be beneficial because it allows determination of several analytes, e.g., 31P+, 27Al+, 28Si+and 32S+, simultaneously. Future work will be focused on developing a new screening technique for two-dimensional gels to detect fast phosphorus, sulfur and metals in well-separated protein spots, development of a quantification procedure by LA-ICP-MS and identification of proteins by high resolution MALDI-FTICR-MS.
4. Conclusions
In the present study we demonstrated the multielement determination in separated protein spots of two-dimensional gels by LA-ICP-MS as the microlocal analytical techniques. By application of LA-ICP-MS using a sector field mass spectrometer, interfering molecular ions such as 15N16O+ and 14N16O1H+ can be separated from analyte ions 31P+, which is crucial for accurate phosphorus determinations in protein spots. The most important problem for the determination of phosphorus and other elements in the gel are possible contaminations during sample preparation. Future work will focus on further minimizing the gel blank with respect to other elements by improving the 2-D gel electrophoretic separation of proteins and avoiding possible contamination.Acknowledgements
The work at the University of Konstanz was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (Biopolymer-MS). The first author is also very grateful to H.-J. Dietze (Juelich) and A. Vonderheide (Cincinnati) for valuable discussions.References
- J. S. Becker, Spectrochim. Acta, 2002, 57, 1805 CrossRef.
- S. F. Durrant, J. Anal. At. Spectrom., 1999, 14, 1385 RSC.
- J. S. Becker, S. F. Boulyga, J. Su. Becker, C. Pickhardt, E. Damoc and M. Przybylski, Int. J. Mass Spectrom., 2003, 228, 985 CrossRef CAS.
- J. L. Neilsen, A. Abildtrup, J. Christensen, P. Watson, A. Cox and C. W. McLeod, Spectrochim. Acta, Part B, 1998, 53, 339 CrossRef.
- P. Marshall, O. Heudi, S. Bains, H. N. Freeman, F. Abou-Shakra and K. Reardon, Analyst, 2002, 127, 459 RSC.
- V. I. Baranov, Z. A. Quinn, D. R. Bandura and S. D. Tanner, J. Anal. At. Spectrom., 2002, 17, 1148 RSC.
- S. F. Boulyga, C. Pickhardt, J. Su. Becker, M. Przybylski and J. S. Becker, in Plasma Source Mass Spectrometry,, eds. G. Holland and S. D. Tanner, The Royal Society of Chemistry, Cambridge, 2003, p. 55 Search PubMed.
- Protein Phosphorylation, eds. B. M. Sefton and T. Hunter,Academic Press; San Diego, CA, 1st edn., 1998 Search PubMed.
- M. J. Davies, R. T. Dean and D. Davies, Radical-Mediated Protein Oxidation: From Chemistry to Medicine, Oxford University Press, Oxford, UK, 1998 Search PubMed.
- A. V. Loboda, A. N. Kruchinsky, M. Bromirski, W. Ens and K. G. Standing, Rapid Commun. Mass Spectrom., 2000, 14, 1047 CrossRef CAS.
- K. L. Bennett, A. Stensballe, A. V. Podtelejnikov, M. Moniatte and O. N. Jensen, J. Mass Spectrom., 2002, 37, 179 CrossRef CAS.
- T. A. Fligge, C. Reinhard, C. Harter, F. T. Wieland and M. Przybylski, Biochemistry, 2000, 39, 8491 CrossRef CAS.
- J. S. Rossier, N. Youhnovski, N. Lion, E. Damoc, J. Su. Becker, F. Reymond, H. H. Girault and M. Przybylski, Angew. Chem. Int. Edn. Engl., 2003, 42, 53 CrossRef.
- J. S. Becker, S. F. Boulyga, C. Pickhardt, J. Su. Becker, S. Buddurus and M. Przybylski, Anal. Bioanal. Chem., 2003, 375, 561 CAS.
- J.-D. Tissot, F. Invernizzi, J. A. Schifferi, F. Sperteni and P. Schneider, Electrophoresis, 1999, 20, 606 CrossRef CAS.
- M. Wind, I. Feldmann, N. Jakubowski and W. Lehmann, Electrophoresis, 2003, 24, 1276–1280 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
