HPLC-ICP-MS and ESI-Q-TOF analysis of biomolecules induced in Brassica juncea during arsenic accumulation
María Montes-Bayóna, Juris Meijab, Danika L. LeDucc, Norman Terryc, Joseph A. Carusob and Alfredo Sanz-Medel*a
aDepartment of Physical and Analytical Chemistry, University of Oviedo, C/Julián Clavería 8, 33006 Oviedo, Spain. E-mail: asm@correo.uniovi.es
bDepartment of Chemistry, University of Cincinnati, Mail Location 0172, Cincinnati, Ohio 45221-0172, USA
cDepartment of Plant and Microbial Biology, University of California Berkeley, Berkeley, California 94720-3102, USA
First published on 17th December 2003
Abstract
Arsenic (As) bioaccumulation by plants can be used as a strategy to detoxify arsenic polluted sites. Genetic engineering may provide a means of optimizing this natural process to increase its efficiency. However, this approach requires a thorough understanding of As metabolism and detoxification in plants. Identifying As-containing metabolites in plants is an important first step in elucidating As metabolism. Brassica juncea (Indian mustard) is studied here as a model for As accumulation in terms of total metalloid accumulation and its elemental speciation. A study on extraction conditions using 25 mM ammonium acetate buffer at increasing pH of 4.4, 5.6 and 7.8 has been performed. Those extracting solutions were also employed as mobile phases for the separation of the As species formed by size exclusion chromatography with inductively coupled plasma mass spectrometry (ICP-MS) as a selective As detector. Two main As containing species have been found in Brassica tissues (one of them at about 2 kDa and the other below 1.2 kDa). The first As species was found to be associated to thiol groups (monitoring 32S with double focusing ICP-MS). This can be ascribed to the presence of As-phytochelatin complexes. Electrospray-quadrupole-time of flight (ESI-Q-TOF) results indicated the presence of phytochelatins (apo-forms), the main metal bioligands in plants, which have also been shown to be induced by As. Oligomers of two, three and four sub-units, respectively (PC2, PC3 and PC4), with internal oxidation of the SH groups, have been extracted from Brassica leaves as well as a potential As–PC4 complex. These species have been further identified by collisional induced dissociation (CID).
Introduction
Phytoremediation is a developing technology that can potentially minimize the problems of contaminated agricultural land or intensely polluted areas affected by urban or industrial activities.1 In this approach, plants are used to uptake metal and metalloid ions from polluted sites (phytoextraction), followed by translocation and sequestration of the elements in plant specialized cells or vacuoles (accumulation).2 Sequestration may involve both physical compartmentalization and chemical complexation with ligands. Because multiple processes are involved, metal and metalloid accumulation in plants is likely to be controlled by multiple genes.3,4 For example, the induction of phytochelatins (PCs), binding peptides derived from glutathione (GSH) with the general structure (γ-Glu-Cys)n-Gly (n = 2–11), is thought to be the main detoxification and accumulation mechanism of heavy metals.5 These ions are subsequently complexed by PCs via thiolate coordination.In the case of metalloids such as As, there is a more limited knowledge of the species produced when As is accumulated by plants. Arsenic is a very toxic pollutant that adversely affects the health of millions of people worldwide.6,7 Plants are exposed to arsenical compounds mainly in the form of the inorganic anions arsenate (As(V)) and arsenite (As(III)).8 Arsenate seems to be a phosphate analogue9 and is readily taken up by plants. Reduction of arsenate produces arsenite, the more toxic and less bioavailable of the two. However, arsenite's strong affinity for thiol groups makes it a candidate for detoxification by complexation with peptide–thiols, such as those formed by γ-glutamylcysteine or glutathione.10 However, direct evidence for As–PC complexes is still lacking. This could be ascribed to some limiting steps either during the translocation of the metalloid ions from the soil to the vacuoles, where the chelating process takes place, or to the synthesis of PCs from GSH. Very recent genetic engineering studies have demonstrated that the co-expression of two bacterial genes, one responsible for As reduction from As(V) to As(III) and the other involved in the synthesis of γ-glutamylcysteine, enhanced arsenic tolerance when As(V) was the As source.11 In the case of As(III), the key to enhancing phytoremediation, therefore, appears to be increasing the formation of As–S conjugates (from GSH or PCs) that are expected to be stored in plant vacuoles.12 For this reason, in the present work, Brassica juncea plants have been genetically modified, over-expressing the enzymes that catalyze the formation of GSH from the individual amino acids: (i) formation of γ-Glu-Cys from Glu + Cys; and (ii) γ-Glu-Cys-Gly (GSH) from γ-Glu-Cys + Gly. Glutathione polymerization, catalyzed by a third enzyme (PC synthase) produces the formation of PCn where n is the number of γ-Glu-Cys units per molecule ((γ-Glu-Cys)nGly). Different analytical approaches have been proposed to study elemental speciation in plants, the coupling of size exclusion chromatography (SEC) to ICP-MS being the most widely used for detection of metallo-complexes in plant extracts.13,14 Reversed phase chromatography (RP-HPLC) using an acetonitrile gradient has been used to separate the different apo-PCs from plant extracts.15 However, the metal/metalloid complexes of PCs could not be successfully isolated by this mechanism due to the lability of the species.16 Since the key for understanding As accumulation in plants is the identification and characterization of the corresponding induced bio-ligands and/or complexes, the present work was aimed at developing a methodology for this purpose. SEC-ICP-MS has been evaluated as a hybrid technique to isolate and detect the different As compounds produced during the phytoremediation of this toxic metalloid. In order to study the possible binding of As to PCs, the monitoring of sulfur was carried out using a double focussing ICP-MS (DF-ICP-MS) following the 32S signal at resolving power m/Δm = 3000 after SEC separation. The nature of the thiol groups (SH) will be further confirmed by using a classical method which involves the post-column derivatization of the SH groups with Ellman's reagent17 (5,5-dithiobis(2-nitrobenzoic acid), DTNB).
Because of the lack of available standards for identification of the bio-induced molecules, the use of complementary techniques such as ESI-MS/MS18 is required. ESI-Q-TOF has been successfully used for the characterization of Se species (Se-amino acids and Se-peptides) in plant extracts.19 Therefore, this technique has been also employed here in order to identify the As bio-ligands in the plant extract.
Experimental
Procedures
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 g and 4 °C and the supernatant filtered through a 0.45 µm filter. The As concentration was determined by diluting the extracts 1 ∶ 100 in Milli-Q water and quantifying them by ICP-MS using external calibration with Ge as internal standard. In order to evaluate the extraction efficiency at different pH values, a nitric acid (50%) digestion of the leaf samples was performed using a microwave oven and the total As concentration determined by external calibration.
000 g and 4 °C and the supernatant filtered through a 0.45 µm filter. The As concentration was determined by diluting the extracts 1 ∶ 100 in Milli-Q water and quantifying them by ICP-MS using external calibration with Ge as internal standard. In order to evaluate the extraction efficiency at different pH values, a nitric acid (50%) digestion of the leaf samples was performed using a microwave oven and the total As concentration determined by external calibration.Results and discussion
Arsenic accumulation and extraction efficiency
Different extraction procedures have been studied in order to improve As extraction efficiency in plant material. The most recent results showed extraction recoveries for As of about 90% using tetramethylammonium hydroxide (TMAH) as the extracting solution.23 However, As has been found to trigger the formation of phytochelatins (PCs), cysteine rich peptides, in plants. Thus, it could be expected that some As could be present in the sample complexed with the thiol groups of the PCs. Since As–S association is labile and strongly pH dependent, an aggressive extracting solution such as TMAH might reduce or even destroy the As speciation information required in this study. Therefore, to preserve species integrity, mild extraction conditions (using an acetate buffer) at different pH values have been assayed as extracting solutions. Table 1 shows the results obtained for total As determination in the extractions performed with 25 mM ammonium acetate at pH 4.4, 5.6 and 7.8, respectively. As can be observed, no significant differences have been found in terms of the total elemental concentration extracted at the different pH values and only slightly higher results have been obtained at pH = 5.6. Even more importantly, the genetically modified plants over-expressing the ECS and GS enzymes, involved in GSH synthesis, do not seem to have increased the As uptake per gram of plant. Recoveries, calculated based on the results obtained for the sample digested using HNO3 and microwaves, showed extraction efficiencies ranging from 47–60% in all cases, in agreement with previous results obtained by other authors.23| 25 mM ammonium acetate buffered at— | As concentration/µg g−1 w.w.ab | ||
|---|---|---|---|
| WT | GS | ECS | |
| a In brackets are the extraction recoveries (%) based on the results obtained by acid digestion (using microwaves) of the tissues (n = 3).b w.w.: wet weight. | |||
| pH 4.4 | 30.6 ± 2.0 (60 %) | 25.1 ± 1.1 (47 %) | 29.0 ± 1.3 (55 %) |
| pH 5.6 | 38.5 ± 5.1 (75 %) | 30.0 ± 1.3 (56 %) | 25.5 ± 3.0 (48 %) |
| pH 7.8 | 31.3 ± 2.2 (61 %) | 34.6 ± 2.0 (64 %) | 38.1 ± 4.0 (69 %) |
Arsenic distribution among species
Size exclusion chromatography was selected as the separation mechanism to perform As speciation on Brassica leaves extracts. The extracting solutions, detailed in the previous section for As extraction, were also used as mobile phases to perform the chromatographic separation with ICP-MS detection. The pH of the mobile phase was varied because it may affect the retention times of the compounds, as documented by other authors.13Fig. 1 (A and B) shows the chromatograms corresponding to the extraction/separation of As species using 25 mM ammonium acetate buffer at pH 5.6 and 7.8, respectively, of the wild type Brassica juncea. As can be observed, three As containing species can be observed at pH 7.8 (Fig. 1 (B)), corresponding to a molecular weight distribution of about 12 kDa (tr = 10–12 min), 2 kDa (tr = 13 min) and <1kDa (non-specific retention) (tr = 20 min) in a ratio 1 ∶ 25 ∶ 74 (percentage of As per peak). This ratio was estimated after performing an As mass balance through the SEC column that provided quantitative results (101% of the As was recovered). At pH 5.6 (Fig. 1 (A)), the highest molecular weight As containing species (about 12 kDa) shows negligible peak area (see the insets in Figs. 1 (A and B)) while the retention time of the 2 kDa species appears shifted to higher retention times (as compared to pH = 7.8). This shift in retention time was even more apparent when the pH 4.4 buffer was used. This phenomenon can be ascribed to unspecific retention mechanisms apart from size exclusion (e.g., ion exchange) also operating in the column.13 According to previous studies,10 this molecular mass range could be ascribed to As–PC complexes, the species sought in the present study.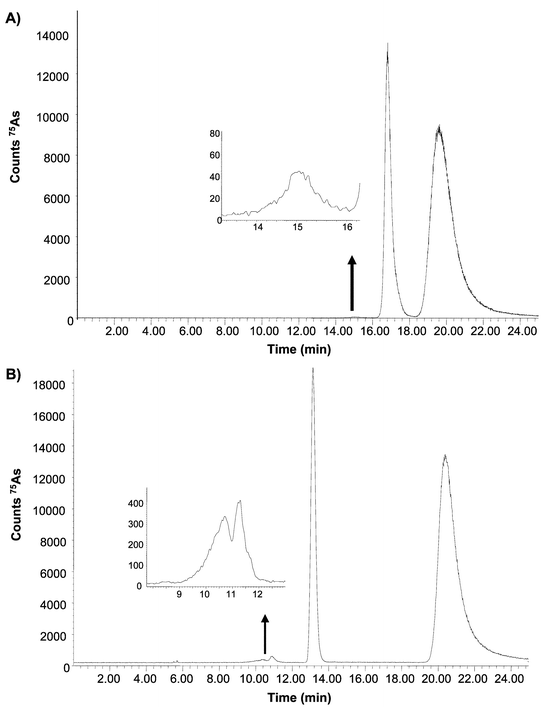 | ||
| Fig. 1 75As elution profile by SEC-ICP-MS of Brassica juncea extracts showing the separation of the As species using 25 mM ammonium acetate buffered at two pH values: (a) pH 5.6 and (b) pH 7.8. The same buffers were used for the extraction of the species. | ||
Regarding peak areas of those species, the 2 kDa As compound fluctuates among plant sets with peak areas ranging from 30 to 58% of the total As eluted. Within the same plant set, slight variations of the area of this As species, depending on the pH, were observed (lower pH values provided slightly higher peak areas). This finding agrees with the fact that slightly acid pH values are needed to maintain the integrity of As–S associations.10
In all cases, an As compound eluting at 20 min (non-specific retention from the column) was observed amounting to 50–70% of total As content. This compound was collected from the SEC column and injected in an anion-exchange chromatographic column (using 25 mM ammonium acetate buffer at pH 7.8). Results showed the presence of a single peak eluting in the void volume which could be ascribed to As(III). As was the case for total As concentration, the transgenic plants did not show any significant differences in terms of the peak area of the observed As species with respect to the wild type.
In order to determine whether the 2 kDa As species could be an As–PC complex, the specific monitoring of sulfur at its major isotope (32S) was performed by double focusing-ICP-MS using medium resolving power (m/Δm = 3000). Sulfur exhibited a single broad peak, for the ECS Brassica, as observed in Fig. 2, as well as the corresponding UV profile at 254 nm (inset). The maximum 32S signal was obtained at the retention time corresponding to As species (≅ 2 kDa) in the plant extracted/separated at pH 5.6 (Fig. 2). Plants grown on control media synthesized PCs to a much lesser extent than As exposed ones (see Fig. 2). Changes in the pH of the mobile phase affected the sulfur signals in a similar manner to that observed for As, shifting to shorter retention times at higher pH values. This fact further supports the assumption of a S–As association. Several attempts to identify such complexes by other authors have been unsuccessful so far (probably due to As lability, undergoing oxidation/reduction reactions during the chromatographic separation).
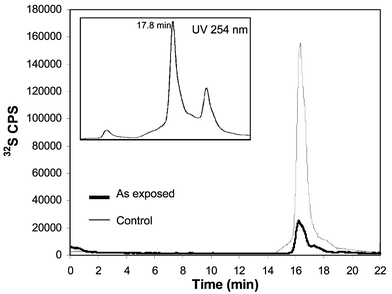 | ||
| Fig. 2 Sulfur specific monitoring of Brassica juncea extracts separated by SEC-ICP-MS at pH 5.6. Bold line for control plants and thin line for the As exposed plant. The UV absorption profile of the extract is showed in the inset at 254 nm. | ||
The UV absorption profile showed a broad peak (from 14 to 20 min) with its maximum slightly shifted (occurring at 18 minutes) with respect to the maximum of the observed sulfur signal. The presence of SH groups in the fraction corresponding to the maximum ICP-MS 32S signal was further confirmed by a conventional methodology developed for apo-phytochelatins described elsewhere.20 In this method, the PC-thiol groups are treated with DNTB post-column (with previous removal of any As by adding concentrated HCl) and the absorbance measured at 430 nm.20
Evidence for complexation of As by SH groups
In an attempt to study the complexation of As by SH groups as a function of the pH, in vitro experiments were performed by using GSH (as model) and As(III) (phytochelatin standards are not commercially available). GSH was prepared in de-oxygenated water and As solutions were prepared in the de-oxygenated working buffer (25 mM ammonium acetate at pH values of 4.4, 5.6 and 7.8). The peptide was incubated with arsenite ions in a 3 ∶ 1 molar ratio under a nitrogen stream (15 min) in order to prevent oxidation reactions from taking place.The formation of As(GSH)3 complexes was observed at all the three pH values by ESI-MS at m/z 994 (protonated molecular ion). For example, in the ESI-MS spectrum corresponding to the synthesized compound at pH 4.4 (Fig. 3), the protonated molecular ion at m/z 994 is observed as well as the non-complexed GSH (m/z 308) and GSH dimer (m/z 615). These results seem to agree with previous work in this field24 reporting that As–GSH species are stable between pH 1.5 and 7.5. Therefore, it is likely that As–PCs complexes would be stable over a similar range of pH values, as observed in the HPLC-ICP-MS studies shown in the previous section.
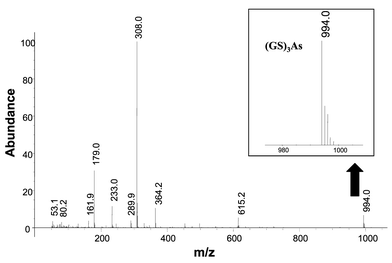 | ||
| Fig. 3 Electrospray mass (ESI-Q) spectra of the “in vitro” synthesized As(GSH)3 complex at pH 4.4. Molecular ion m/z 994. | ||
Induction of phytochelatins by As and possible As complexation
Recent findings have indicated that a mutant Arabidopsis lacking the ability to synthesize phytochelatins is much more sensitive to As than the wild type plant.25 Some authors have proposed a complexation model similar to As(GSH)3 but the only experimental data available has been obtained by incubating PC2 with As(III) in a 3 to 1 ratio (as in the case of GSH).10 No experimental evidence for the existence of As–PC complexes in plants has been provided, however. Therefore, the goal of this section was to identify possible As–PCn complexes produced by Brassica juncea exposed to 4 ppm As(III).Experiments for further purification of the 2 kDa As species were attempted by using complementary separation mechanisms (reversed phase and anion exchange) following SEC (and prior to ESI). Both chromatographic systems led to the presence of a single intense signal of As in the void volume of the columns. In other words, only SEC can be used to isolate the As species we are seeking. Thus, the As fraction occurring at 2 kDa was collected from the exit of the SEC column, freeze-dried, re-dissolved in 50% MeOH–0.1% acetic acid and analyzed by ESI-Q-TOF. Results revealed the presence of PC2, PC3 and PC4 (apo-forms) in the extract (Fig. 4), with PC2 being the most abundant. PC2, PC3 and PC4 protonated molecular ions are expected to appear at m/z 540, 772 and 1004, respectively. Instead, they appeared at m/z 538, 770 and 1000 as a result of internal disulfide bridge formation (internal oxidation). A similar fact has been recently reported by other authors in experiments carried out in vitro in Rauwolfia serpentina cells.10 It seems that the oxidation of PCs is catalyzed by As(III)/As(V) transitions and, indeed, we observed a much higher degree of oxidation in plant cells treated with As than those exposed to other elements, such as Cd. Although the addition of β-mercaptoethanol to the extraction buffer can decrease internal oxidation, this reagent also has the potential to remove As from the PC complexes. In any case, oxidized PC2 (C18H27N5O10S2), PC3 (C26H39N7O14S3) and PC4 (C34H48N9O18S4) showed an accuracy of −30, 14.9 and −25.7 ppm, respectively, with respect to the theoretical m/z of the molecular ions. Potassium conjugates of oxidized PC2 and PC4 at masses 576 and 1038 can also be observed in the mass spectra. The chemical identity of the observed oxidized PC2 and PC4 was confirmed by further fragmentation using the collisional induced dissociation (CID) and is shown in Figs. 5 (A) and 5 (B).
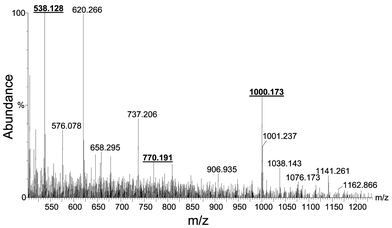 | ||
| Fig. 4 Electrospray-Q-TOF of the Brassica extract containing the internally oxidized forms of PC2, PC3 and PC4 at m/z 538, 770 and 1000, respectively. | ||
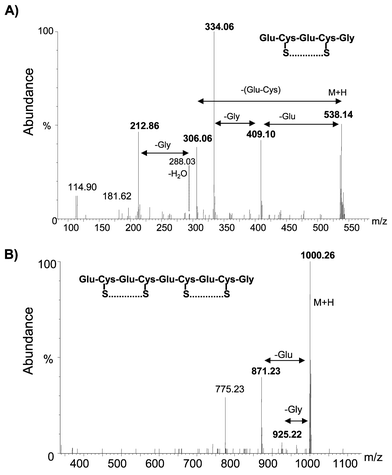 | ||
| Fig. 5 Collisional induced dissociation (CID) of the ions at m/z 538 and 1000 for sequencing of the peptides. | ||
A putative As–PC complex was observed at m/z 1076, a compound which could correspond to As(III) bound to three of the SH groups of PC4 and one free SH. Such a complex would have a theoretical mass of 1076.145 and would differ by only 25.9 ppm from the obtained value (1076.173). Fig. 6 shows the mass spectra of the plant extract magnified in the range m/z 1000–1100, showing the pattern of the compound. Also, the inset shows a molecular model of the proposed structure that shows good similarity with that obtained in the plant sample. Although no structure of the complex was documented, the recent finding of a complex containing three sulfur ligands associated to As(III) in Brassica shoots by As K-edge FT-EXAFS9 supports our results by ESI-Q-TOF. In this regard, it seems clear that molecules having vicinal sulfhydryl groups provide more avid complexation of As than monodentate sulfhydryl compounds such as GSH, which could explain the absence of As(GSH)3 species in Brassica extracts.
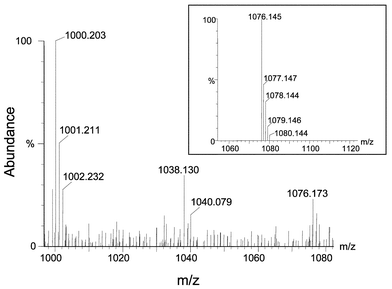 | ||
| Fig. 6 Magnification of the mass spectra shown in Fig. 4 to show the proposed As–PC3 complex at m/z 1076. The inset shows the molecular model calculated by the software and corresponding to the proposed structure. | ||
Conclusions
SEC-ICP-MS has proved to be a suitable technique for screening As compounds in biological tissue cytosols. Brassica juncea exposed to As(III) (as in phytoremediation experiments) shows the presence of As–thiolate complexes indicating possible induction of phytochelatins by this metalloid. Those complexes have proved to be more stable at slightly acid pH values, although no chromatographic separation mechanism except SEC could be applied for further purification.As expected, ESI-Q-TOF has proved to be very a valuable tool for characterizing the bio-ligands synthesized by the plant in response to As exposure. Mainly PC2, and also PC3 and PC4 in the oxidized forms, have been found to be the main phytochelatins produced during As exposure. A possible As–PC4 compound has been identified in Brassica juncea extracts although further confirmation of the corresponding As-phytochelatin species should be required.
Acknowledgements
The authors want to thank Dr Koka Jayashimhulu for his help on the ESI-Q-TOF analyses and the Ministry of Science and Technology of the Spanish Government for the financial support under the Ramon y Cajal contract of M. Montes-Bayón and for the project MCT-00-BQU-0468.References
- S. P. McGrath, F. J. Zhao and E. Lombi, Advan. Agron., 2002, 75, 1 Search PubMed.
- C. S. Cobbett and P. B. Goldsbrough in Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment, eds. I. Raskin and B. D. Ensley, John Wiley and Sons. Inc., 2000 Search PubMed.
- N. Terry, A. M. Zayed, M. P. De Souza and A. S. Tarun, Annu. Rev. Plant Physiol. Plant Biol., 2000, 51, 401 Search PubMed.
- O. K. Vatamaniuk, S. Mari, Y.-P. Lu and P. A. Rea, J. Biol. Chem., 2000, 275, 31451 CrossRef CAS.
- M. H. Zenk, Gene, 1996, 179, 21 CrossRef CAS.
- National Research Council. Arsenic, National Academy Press, Washington, DC, 1977.
- S. C. Mukherjee, M. M. Rahman, U. K. Chowdhury, M. K. Sengupta, D. Lodh, C. R. Chanda, K. C. Saha and D. Chakraborti, J. Environ. Sci. Health, 2003, 38, 165 Search PubMed.
- J. Wang, F.-J. Zhao, A. A. Meharg, A. Raab, J. Feldmann and S. P. McGrath, Plant Physiol., 2002, 130, 1552 CrossRef CAS.
- I. J. Pickering, R. C. Prince, M. J. George, R. D. Smith, G. N. George and D. E. Salt, Plant Physiol., 2000, 122, 1171 CrossRef CAS.
- M. E. V. Schmöger, M. Oven and E. Grill, Plant Physiol., 2000, 122, 793 CrossRef CAS.
- O. P. Dhankher, Y. Li, B. P. Rosen, J. Shi, D. Salt, J. F. Senecoff, N. A. Sashti and R. B. Meagher, Nature Biotech., 2002, 20, 1140 CrossRef.
- E. Pilon-Smits and M. Pilon, Crit. Rev. Plant Sci., 2002, 21, 439 CAS.
- V. Vacchina, K. Polec and J. Szpunar, J. Anal. At. Spectrom., 1999, 14, 1557 RSC.
- I. Leopold and D. Günther, Fresenius' J. Anal. Chem., 1997, 359, 364 CrossRef CAS.
- W. E. Rauser, Annu. Rev. Biochem., 1990, 59, 61 CrossRef.
- R. Lobinski and J. Szpunar, Anal. Chim. Acta, 1999, 400, 321 CrossRef.
- R. Kneer and M. H. Zenk, Phytochemistry, 1997, 49, 71.
- V. Vacchina, H. Chassaigne, M. Oven, M. H. Zenk and R. Lobinski, Analyst, 1999, 124, 1425 RSC.
- M. Montes-Bayón, E. G. Yanes, C. Ponde de León, K. Jayasimhulu, A. Stalcup, J. Shann and J. A. Caruso, Anal. Chem., 2002, 74, 107 CrossRef CAS.
- W. Gekeler, E. Grill, E.-L. Winnacker and M. H. Zenk, J. Biosciences, 1989, 44, 361 Search PubMed.
- Y. L. Zhu, E. A. Pilon-Smits, L. Jouanin and N. Terry, Plant Physiol., 1999, 119, 73 CrossRef CAS.
- Y. L. Zhu, E. A. Pilon-Smits, A. S. Tarun, Ss. U. Weber, L. Jouanin and N. Terry, Plant Physiol., 1999, 121, 1169 CrossRef CAS.
- M. Quaghebeur, Z. Rengel and M. Smirk, J. Anal. At. Spectrom., 2003, 18, 128 RSC.
- J. Gailer and W. Lindner, J. Chromatogr. B, 1998, 716, 83 CrossRef CAS.
- S.-B. Ha, A. P. Smith, R. Howden, W. M. Dietrich, S. Bugg, J. O'Connell, P. B. Goldsbrough and C. S. Cobett, Plant Cell, 1999, 11, 1153 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
