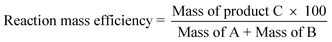The development of an environmentally benign synthesis of sildenafil citrate (Viagra™) and its assessment by Green Chemistry metrics†
Peter J.
Dunn
*a,
Stephen
Galvin
b and
Kevin
Hettenbach
c
aDepartment of Chemical Research and Development, Pfizer Global Research and Development, Sandwich, Kent, UK CT13 9NJ
bPfizer Ireland Pharmaceuticals, Ringaskiddy API Plant, P.O. Box 140, Ringaskiddy, County Cork, Ireland
cDepartment of Chemical Research and Development, Pfizer Global Research and Development, Groton, CT-06340, United States
First published on 16th December 2003
Abstract
The process development and scale-up of the sildenafil citrate process is reviewed. Key environmental metrics are reported at several time points during the development process. Significant achievements during the development of sildenafil citrate were (i) discovering a convergent, efficient synthetic route (ii) designing 7 process steps so that there was no extractive work-up in any step (iii) implementing efficient solvent recovery early in the product's commercial lifetime. The result of this work is that the E-factor for the process is very low with just 6 Kg of waste per kilogram of product compared with an industry average of 25–100 Kg. Pfizer received the 2003 UK Award for Green Chemical Technology (Best Process category) for the sildenafil citrate process.
Introduction
Sildenafil citrate is a selective inhibitor of phosphodiesterase 5 (PDE5) and was the first agent with this mode of action for the treatment of male erectile dysfunction (a disease that was more commonly known as male impotence). This new drug was approved for prescription use within the United States, the European Union and Japan during 1998 and immediately became a major seller, achieving sales of more than $1 billion during its first year on the market. With such a rapid sales take off it was critical that the environmental performance of the synthesis was good from the outset.A crude assessment of the environmental performance of a synthesis is provided by the E-factor1 and Sheldon1 has given broad levels of environmental performance for different categories of the Chemical Industry as shown in Table 1.
| Industry segment | Annual product tonnage | Kg waste/Kg product |
|---|---|---|
| Oil refining | 106–108 | ca. 0.1 |
| Bulk chemicals | 104–106 | <1–5 |
| Fine chemicals | 102–104 | 5–>50 |
| Pharmaceuticals | 10–103 | 25–>100 |
There are many reasons why waste levels are generally higher for pharmaceutical compounds. For example, typical syntheses involve complex molecules with demanding quality standards, often requiring batch processing; there are greater regulatory constraints and in general high value, low volume products are being made. Some earlier publications by GlaxoSmithKline have looked at assessing general reaction types by Green Chemistry metrics.2,3 This paper assesses a specific process, the sildenafil citrate process, using various Green Chemistry metrics, including the E-factor proposed by Sheldon, and examines how the process compares with industry norms. Data at various points in the chemical development process are also reported.
Medicinal chemistry route and “quick fixes” for early scale-up
The medicinal chemistry route is shown in Scheme 1.4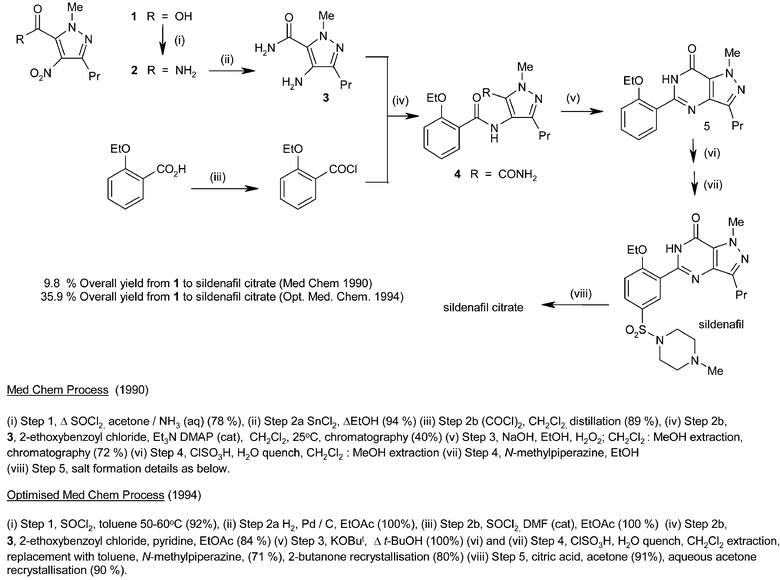 | ||
| Scheme 1 The medicinal chemistry route to sildenafil. | ||
The first task of the process chemist was to prepare a few kilograms of material for early toxicology studies. The following four changes were made for early batches to improve the environmental performance of the synthesis.
• The removal of the tin chloride reduction. Tin is a heavy metal and a major environmental polluter.
• The use of stoichiometric quantities of thionyl chloride in a solvent, rather than thionyl chloride as solvent in the preparation of compound (2). This is much safer and has a reduced environmental impact.
• The elimination of hydrogen peroxide from the synthesis. Hydrogen peroxide causes burns on contact with skin and is a fire and transportation hazard especially in contact with organic materials.
• The use of thionyl chloride rather than oxalyl chloride for the preparation of 2-ethoxybenzoyl chloride. This change gave an improvement in worker safety by avoiding carbon monoxide emissions.
Medicinal chemists employed a tin chloride reduction because standard reduction by hydrogenation was not effective. Investigation showed that the failure of the hydrogenation was due to trace levels of sulfur impurities, from the previous thionyl chloride reaction, poisoning the hydrogenation reaction. Careful control of these sulfur impurities (for example, switching to stoichiometric thionyl chloride use) allowed tin to be removed from the process and replaced with a simple catalytic hydrogenation reaction. Catalytic hydrogenations are among the cleanest environmental chemical steps. For a reduction of a nitro group to an amine, the only by-product is water and there are recovery options for solvent and catalyst.
The medicinal chemistry method to cyclise compound (4) to the pyrazolopyrimidinone (5) used an aqueous alcoholic solution of sodium hydroxide and hydrogen peroxide in 72% yield. A literature review of alternative cyclisation methods revealed that such reactions had only previously been performed in moderate yield (30–70%).5 It was found that by employing KOBut in tBuOH in the cyclisation reaction, a 100% isolated yield was obtained with no impurities detected. Finding this very clean and high yielding reaction impacted on the way the commercial route was designed.
Selection and development of the commercial route
A summary of the two routes is outlined in Scheme 2. The flow diagrams in Scheme 2 clearly show that the strategic advantages of the commercial route were as follows:• Greater convergency
• The clean cyclisation reaction is now at the end of the synthesis in the final bond-forming step. The potentially toxic materials now occur near the start of the synthesis.
• The environmental and scale-up issues associated with the chlorosulfonation step (i.e. large volumes of aqueous acidic waste that require neutralisation and increased levels of hydrolysis during increased quench time on a larger scale) are reduced by placing the chlorosulfonation reaction earlier in the synthesis. A simple, low molecular weight starting material (2-ethoxybenzoic acid) is used, hence less chlorosulfonic acid is needed and the problem is much reduced.
 | ||
| Scheme 2 Strategic commercial route selection. | ||
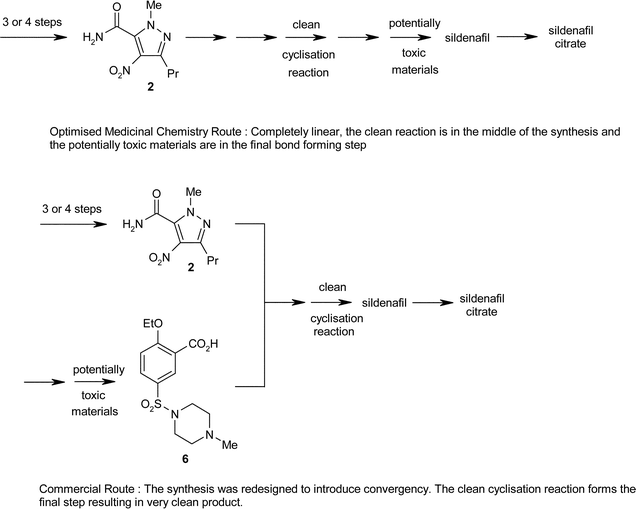
Once selected, the commercial route was subjected to further, detailed development and the optimised synthesis is outlined in Scheme 3.6
 | ||
| Scheme 3 The commercial route to sildenafil citrate. | ||
2-Ethoxybenzoic acid is chlorosulfonated using chlorosulfonic acid along with 1 mol of thionyl chloride, to ensure that the intermediate sulfonic acid is converted to the sulfonyl chloride. 2-Ethoxybenzoic acid is a low melting solid (mp 19–20 °C) and hence could be used as a melt, allowing the use of relatively low volumes of chlorosulfonic acid and thionyl chloride as there were no solubilisation issues. Following aqueous quench, the product is isolated as a water wet sulfonyl chloride. For simplicity and efficiency, the sulfonyl chloride is then resuspended in water and reacted with N-methylpiperazine. At the end of the reaction, the pH is adjusted and the sulfonamide (6) is collected by filtration. Hence, no organic solvent is used for the preparation of the sulfonamide.
As mentioned earlier, the original procedure to convert pyrazole (1) into the amide (2) used a neat reaction in thionyl chloride. The introduction of toluene as a heat sink/solvent both increased the safety of the process and allowed reduction of the levels of thionyl chloride to 1.2–1.6 equivalents. The toluene in this reaction is recovered and reused.
Heterogeneous hydrogenation in ethyl acetate was used to convert the nitropyrazole (2) to the amine (3). Activation of the acid (6) was examined with a number of reagents including thionyl chloride, oxalyl chloride and N,N′-carbonyldiimidazole (CDI). CDI was selected, despite its higher cost because this reagent:
• Provided clean, robust chemistry and high quality product.
• Allowed all three reactions (hydrogenation, activation and acylation) to be combined.
• Enabled a single solvent to be employed (ethyl acetate), allowing simple solvent recovery with low energy use.
• Avoided VOC emissions (EtCl) from the interaction of either thionyl chloride or oxalyl chloride with ethyl acetate.
• Provided a simple process – mix streams, concentrate and filter. This simplicity led to low energy use.
• High chemical yield, (90%) over three chemical steps (subsequently optimised to 96%).
The resulting coupled product (7) is then cyclised with t-BuOH and t-BuOK. The process is run at high concentration (2.5–3.75 L Kg−1) in order to minimise environmental waste. At the end of the reaction, water is added and the pH adjusted to the isoelectric point to precipitate pure sildenafil, which is collected by filtration. For Viagra™, the citrate salt is required. The process to covert sildenafil to sildenafil citrate has been carefully designed to give both a high yield and high quality. A 98–100% yield is routinely obtained for this step. By 1997 the overall yield from the pyrazole (1) to sildenafil citrate had risen to 75%, and the high yields late in the synthesis minimised the environmental impact of the earlier steps.
Once the steps were developed, diligent solvent recovery was required to fully optimise the environmental performance. Recovery of toluene and ethyl acetate was introduced in 1998, the year Viagra™ was launched, and production scale trials for the recovery of 2-butanone are now complete. A summary of the solvent usage at various stages of development is given in Fig. 1. Of particular note is the complete elimination of chlorinated solvents and the elimination of highly volatile solvents such as diethyl ether, methylene chloride, methanol and acetone. The elimination of these highly volatile solvents reduces the atmospheric emissions as will be seen later.
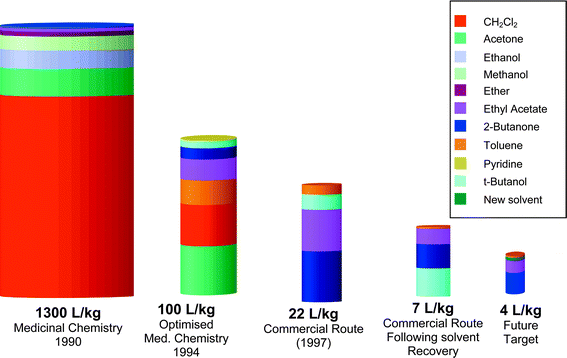 | ||
| Fig. 1 The amount of organic waste produced by the sildenafil citrate processes at various time points. | ||
A further improvement in the synthesis (described in Scheme 3) was the replacement of t-butanol. This solvent is completely water soluble in all proportions and is difficult to recover for reuse. There is a continued drive for improved environmental performance and t-butanol will be switched to another reaction solvent that can be recovered. This new process was developed and optimised in Ringaskiddy and has been demonstrated in the production plant in Ireland. When fully implemented this will give the final optimised solvent usage of 4 L Kg−1.
A more detailed environmental analysis of the optimised medicinal chemistry synthesis (1994), the 1997 commercial route and the future target follows, using such measures as atom economy, reaction mass efficiency, chemical yield, organic waste, aqueous waste, atmospheric emissions, energy usage and the E-factor. The original medicinal chemistry process (1990) is not analysed here, since it was only ever intended for laboratory scale synthesis.
Atom economy, reaction mass efficiency and chemical yield
The concept of atom economy was first introduced by Barry Trost7 as a prompt to synthetic chemists to pursue “greener chemistry”. The method of calculating atom economy was kept deliberately simple and is a percentage of how much of the reactants remain in the final product. Hence, atom economy ignores reaction yield and reagent excess. It also does not account for solvent usage. Further information on how to calculate atom economy can be found in Green Chemistry.3Reaction Mass Efficiency (RME)2,3 is a more sophisticated measure of “greenness” which allows for the effect of yield and the excesses of reagent used. RME does not account for solvent usage.
For a generic reaction A + B = C
The atom economy, reaction mass efficiency and chemical yields for the sildenafil citrate processes in 1994 (optimised medicinal chemistry route), 1997 (commercial route) and the future target (commercial route) are shown in Tables 2 to 4.
| Reaction type | Step No | RME | Atom econ. | Yield |
|---|---|---|---|---|
| Amide formation | 1 | 25% | 61% | 92% |
| Reduction (nitro to amine) | 2a | 83% | 83% | 100% |
| Activation/acylation | 2b | 48% | 71% | 84% from (2) |
| Cyclisation | 3 | 61% | 65% | 100% |
| Chlorosulfonation/sulfonamide formation | 4 Reaction | 73% | 90% | 71% |
| 4 Purification | 80% | 100% | 80% | |
| Salt formation | 5 Reaction | 91% | 100% | 91% |
| 5 Purification | 90% | 100% | 90% | |
| Overall process | 10%8 | 56% | 36% from (1) |
| Reaction type | Step number | RME | Atom econ. | Yield |
|---|---|---|---|---|
| Amide formation | 1 | 40% | 61% | 92% |
| Chlorosulfonation/sulfonamide formation | 2 | 30% | 74% | 68% |
| Reduction (nitro to amine) | 3a | 83% | 83% | 100% |
| Activation/acylation | 3b | 61% | 73% | 90% from (2) |
| Cyclisation | 4 | 65% | 83% | 92% |
| Salt formation | 5 | 98% | 100% | 99% |
| Overall process | 26%8 | 54% | 75% from (1) |
| Reaction type | Step number | RME | Atom econ. | Yield |
|---|---|---|---|---|
| Amide formation | 1 | 40% | 61% | 92% |
| Chlorosulfonation/sulfonamide formation | 2 | 30% | 74% | 68% |
| Reduction (nitro to amine) | 3a | 83% | 83% | 100% |
| Activation/acylation | 3b | 67% | 73% | 96% from (2) |
| Cyclisation | 4 | 73% | 83% | 95% |
| Salt formation | 5 | 99% | 100% | 99% |
| Overall process | 29%8 | 54% | 82% from (1) |
As can be seen from the data, the atom economy of the process has remained essentially constant over time. Improvements have been made in yield and through a greater degree of convergency in the synthesis, but these are not measured by atom economy. In contrast, there was a substantial improvement in both RME and chemical yield between 1994 and 1997 when the new route was introduced. There has been a further significant incremental improvement since 1997. A summary of the data in Tables 2–4 is given in Fig. 2.
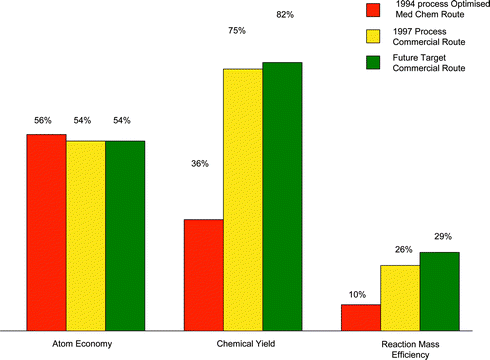 | ||
| Fig. 2 Atom economy, chemical yield and RME at various time points in the sildenafil citrate process. | ||
Organic waste, aqueous waste, atmospheric emissions and energy usage
The levels of organic waste, aqueous waste and atmospheric emissions for the sildenafil citrate process at three time points are shown in Fig. 3. The three time points are 1994, when the optimised medicinal chemistry was being used, 1997, following the switch to the commercial process and the future target, which includes solvent recovery, and switching from t-butanol to a solvent that can be recovered. Each time point is given as a percentage of the 1994 process.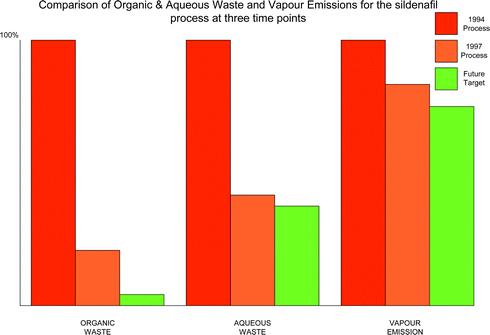 | ||
| Fig. 3 Comparison of organic and aqueous waste and vapour emissions for the sildenafil citrate process at three time points. | ||
The organic and aqueous wastes are actually measured; the atmospheric emissions are estimated from a modelling process using Batch Plus.9 It can be seen that for aqueous waste, there was a large reduction between 1994 and 1997 when the new route was introduced while subsequent improvements will be incremental. This shows the importance of route selection in controlling and eliminating waste. For the organic waste there was again a large reduction between 1994 and 1997 upon introduction of the commercial route. However, there was a further large reduction post 1997 due to the impact of introducing solvent recovery operations and reuse in the process. Interestingly, vapour emissions have also been reduced over time but by a much smaller amount. Energy use was also estimated by the “Batch Plus” software9 and it was found that there was a 35% reduction between 1994 and 1997. There were two possible reasons why reductions in vapour emissions and energy usage were smaller:
1. There is a significant level of solvent stripping performed in the commercial process to maximise the yield.
2. Parameters such as yield, aqueous waste and organic waste were actively managed and followed by the chemistry team as the process was developed, whereas volatile emissions were calculated retrospectively. It is interesting to speculate whether volatile emissions and energy use would have been lower if the chemistry team had used Batch Plus in real time to monitor volatile emissions and energy use as the process was being developed.
The E-factor
The future target process generates 4 L Kg−1 of organic waste along with 2 Kg Kg−1 of salts (mainly from neutralising chlorosulfonation waste). Hence the total kilos of waste per kilo active of sildenafil citrate is around 6 Kg Kg−1. The performance of the sildenafil citrate process against industry norms is shown in Table 5. The sildenafil citrate process is more similar to a bulk chemical or an efficient fine chemical synthesis in terms of its waste generation. However the annual volumes produced are in the lower end of the range for a Pharmaceutical given in Table 5.| Industry segment | Annual product tonnage | E-factor |
|---|---|---|
| Oil refining | 106–108 | ca. 0.1 |
| Bulk chemicals | 104–106 | <1–5 |
| Sildenafil citrate | 30–40 | 6 |
| Fine chemicals | 102–104 | 5–>50 |
| Pharmaceuticals | 10–103 | 25–>100 |
As total annual waste is the multiple of the E-factor and the annual product volume it can be seen that the sildenafil citrate process produces exceptionally low levels of waste per annum.
Conclusions
Green Chemistry metrics have been presented at different time points in the development of the sildenafil citrate process. With the exception of atom economy, there was an improvement in the environmental performance as the process was developed, and for the most important measures there was a major improvement. The future target for the E-factor is 6 (for 7 process steps) which is significantly less than the industry standard (25–100). When both E-factor and the relatively low volume (of this high value, relatively complicated chemical product) are taken into account, the amount of waste produced per year is extremely low. Pfizer received the UK Award for Green Chemical Technology for the sildenafil citrate process in 2003.10Experimental
Details of the commercial route to sildenafil citrate can be found in reference 6. Details of the 1990 medicinal chemistry synthesis can be found in reference 4. Aqueous waste, organic waste, atom economy and reaction mass efficiency were determined by the Batch Plus program then reality checked by human calculation. Energy usage and atmospheric emissions were estimated using the Batch Plus software.9 Batch Plus is a recipe based modelling program designed for simulating pharmaceutical/chemical batch operations. Batch Plus has the capability of performing process mass balance calculations based on a specified amount of product, and estimating the corresponding aqueous and organic waste stream amounts and atmospheric emissions. Batch Plus was shown to be a quick and efficient tool for assigning green chemistry metrics as the process was developed from laboratory to production scale.Acknowledgements
The authors would like to thank a number of colleagues who contributed to this project notably Jens Ahman, Brian Corby, David Dale, Jon Ericson, Michael Hughes, Rosemary Prior, Patrica Searle, Colette Shirran, Andrew Pearce and Albert Wood.References
- R. A. Sheldon, Chem. Ind., 1992, 903–906 Search PubMed; R. A. Sheldon, Chem. Ind., 1997, 12–15 CAS.
- A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- A. S. Bell, D. Brown and N. K. Terrett, EP-0463 756 (1991).
- H. W. Hamilton, D. F. Ortwine, D. F. Worth and J. A. Bristol, J. Med. Chem., 1987, 30, 91–96 CrossRef CAS.
- D. J. Dale, P. J. Dunn, C. Golightly, M. L. Hughes, P. C. Levett, A. K. Pearce, P. M. Searle, G. Ward and A. S. Wood, Org. Proc. Res. Dev., 2000, 4, 17–22 Search PubMed.
- B. M. Trost, Science, 1991, 254, 471.
- The RME of the overall process is not the multiple of the RMEs for the individual steps, intermediates are cancelled out as detailed in the literature3.
- Aspen Technology, Inc., Boston, MA, USA. Batch Plus software version 11.1.
- The UK Awards for Green Chemical Technology were sponsored by the Crystal Faraday Partnership and administered by the Green Chemistry Network.
Footnote |
| † This chemistry was presented at the Green Chemistry Symposium at the American Chemical Society in Florida, April, 2002 and at the IUCT 4th Green Chemistry conference in Barcelona 11th/12th November, 2002. |
| This journal is © The Royal Society of Chemistry 2004 |