Ene reaction of allylbenzene and N-methylmaleimide in subcritical water and ethanol
Antero Laitinen*ab, Yoshihiro Takebayashib, Irene Kylänlahtic, Jari Yli-Kauhaluomac, Tsutomu Sugetab and Katsuto Otakeb
aIndustrial Chemistry Research Group, VTT Processes, Biologinkuja 7, P.O. Box 1602, FIN-02044 VTT, Espoo, Finland. E-mail: antero.laitinen@vtt.fi
bResearch Institute for Green Technology, National Institute of Advanced Industrial Science and Technology (AIST), Higashi 1-1, Tsukuba, Ibaraki 305-8565, Japan
cDepartment of Pharmacy, Viikki Drug Discovery Technology Center, P.O. Box 56, FIN-00014 University of Helsinki, Finland
First published on 14th November 2003
Abstract
Ene reaction of allylbenzene and N-methylmaleimide was studied in water and ethanol solvents at subcritical temperatures (220–310 °C). Subcritical water was inappropriate for this reaction, because it rapidly hydrolyzed N-methylmaleimide. Subcritical ethanol was found to be a very promising solvent. The highest ene product yield in ethanol reached 40% in 480 min, and the highest trans-selectivity was 92%. The yields in pure ethanol were comparable to those in 1,2,4-trichlorobenzene with 10% hydroquinone added as a polymerization inhibitor. Addition of hydroquinone had a negligible effect on the yield in ethanol, suggesting that the solvent ethanol itself acts as an inhibitor of the side reactions. It is also expected that the polar environment and the high vapor pressure of ethanol favored pericyclic association between the apolar starting compounds.
Green ContextThe replacement of volatile organic solvents in organic reaction processes is an important green chemistry goal. The use of water and ethanol being biodegradable and readily available is attractive. Here ethanol is shown to be effective in the synthetically useful ene reactions of allylbenzene and N-methylmaleimide.JHC |
1 Introduction
Ene reaction is a general category of pericyclic organic reactions, and is of great potential use in chemical industries associated with polymer cross-linking and paper chemical synthesis.1–3 However, the application of ene reactions in practical synthesis has been hindered by their necessity for high temperatures. High temperature brings about dirty side reactions, e.g. polymerization and decomposition of starting materials. To avoid this problem, the conventional method requires the use of polymerization inhibitors, dangerous and expensive catalysts, as well as harmful chlorinated solvents with high boiling temperatures. In the present work, we investigate the applicability of water and ethanol as efficient and environmentally benign solvents for the ene reaction shown in Fig. 1. | ||
| Fig. 1 Ene reaction between allylbenzene and N-methylmaleimide. | ||
Pericyclic reactions, including ene reactions and Diels–Alder ones,4 are the most powerful tools in carbon–carbon bond-forming synthesis. In the case of ene reaction, an alkene containing an ene double bond reacts with a compound containing an enophilic double or triple bond (e.g. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C
O, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) to form a new σ-bond with a migration of the double bond and a concomitant [1,5]-hydrogen shift via a cyclic transition state.1–3 Unfortunately, ene reactions typically need higher reaction temperatures (140–300 °C) than those of the related Diels–Alder reactions, limiting the synthetic utility of ene reactions. A major limiting factor is the polymerization of the reactants at the high temperatures. Thus additives such as hydroquinone are commonly used to slow down the rate of polymerization. It is also well-known that the rate of ene reaction can be enhanced by using Lewis acid catalysts, e.g. AlCl3, SnCl4 and TiCl4.5,6 However, the catalysts are often expensive and hazardous to use.
C) to form a new σ-bond with a migration of the double bond and a concomitant [1,5]-hydrogen shift via a cyclic transition state.1–3 Unfortunately, ene reactions typically need higher reaction temperatures (140–300 °C) than those of the related Diels–Alder reactions, limiting the synthetic utility of ene reactions. A major limiting factor is the polymerization of the reactants at the high temperatures. Thus additives such as hydroquinone are commonly used to slow down the rate of polymerization. It is also well-known that the rate of ene reaction can be enhanced by using Lewis acid catalysts, e.g. AlCl3, SnCl4 and TiCl4.5,6 However, the catalysts are often expensive and hazardous to use.
Here we study utilization of water and ethanol as solvents for ene reactions, instead of conventionally used solvents such as 1,2,4-trichlorobenzene. The beneficial effect of water solvent for pericyclic reaction was first suggested in 1939.7 An obvious example was reported by Breslow and Rideout that a cycloaddition between cyclopentadiene and methyl vinyl ketone is accelerated by a factor of 700 by using water as a solvent instead of isooctane.8 The acceleration was explained by the hydrophobic association of the apolar reactants. It was also pointed out by Albisetti et al. that in their ene experiments between alkenes and acrylonitriles at 200–300 °C the use of water as a solvent prevents the formation of tars of the polymerizable nitriles.9 In recent years, water above the critical temperature (374 °C) has received much attention as a novel medium for Diels–Alder reactions due to the high solubility of organic reactants in it.10 The observations above show that water can be an efficient solvent in both the acceleration of ene reactions and the inhibition of side reactions. In addition to water, ethanol is also tested here as a promising solvent for ene reaction due to its high polarity.
Ene reaction of allylbenzene and N-methylmaleimide (Fig. 1) was chosen here as a model system. This system is of great interest due to its potential use for the cross-linking of polymers, in particular between a cyanate ester resin and a bismaleimide one.11 The system is also closely related to the synthesis of paper sizing chemicals.12 We investigate the reaction in water and ethanol solvents at several temperatures below the critical point (<374 °C for water, and <241 °C for ethanol). The effects of temperature, reaction time, presence of hydroquinone, and amounts of starting compounds on the reaction are discussed in comparison with those in 1,2,4-trichlorobenzene solvent.
2 Experimental
Reagents
Allylbenzene (98%, FW 118.18), N-methylmaleimide (97%, FW 111.10), and trans-anethole (99%) were purchased from Aldrich Chemical Company, and hydroquinone (99%) from Nacalai Tesque. Trans-anethole was used as an internal standard in the product analysis. Ethanol (99.5%) and 1,2,4-trichlorobenzene (>95%, bp 214 °C) were purchased from Wako Pure Chemicals. Water was purified by Milli-Q Gradient (Millipore Co. Ltd.). The solvents were deoxygenated before use. Other reagents were used as received.Reaction and analysis
Experiments were carried out with a batch-type high-pressure reactor. The reactor tube (10.6 mL) was made of SUS-316 stainless steel. In typical experiments, allylbenzene (1.7–8.5 mmol), N-methylmaleimide (4.5–18 mmol), and hydroquinone (0.45 mmol, if added) were weighed into the reactor with solvent water (5 g, 0.28 mol), ethanol (4 g, 87 mmol), or 1,2,4-trichlorobenzene (4 g, 28 mmol). The free volume in the reactor was purged with argon to remove the air entrapped before the reactor was sealed. The reactor was immersed into a salt bath equipped with a mechanical shaking system (Perker Netsushori Kogyo Co. Ltd.). The shaker moved the ends of the reactor tube up and down, thereby stirring the reaction mixture, in the molten salt heated at the reaction temperature (220–310 °C). The mixture in the vessel was self-compressed up to the vapor pressure of the solvent at the reaction temperature: in the case of water, 2.4 MPa at 221 °C, 4.0 MPa at 251 °C, and 9.9 MPa at 310 °C,13 while in the case of ethanol, 4.2 MPa at 220 °C.14 After the required reaction time (30–780 min), the reactor was quickly cooled down by immersing it in water. In the case of water solvent, the crude reaction mixture was evaporated in vacuo, and subsequently extracted with ethyl acetate. In the case of ethanol and 1,2,4-trichlorobenzene solvents, the samples were directly injected into a gas chromatograph without evaporation of the solvent. This reaction proceeds at high temperature and pressure conditions and precautionary measures are necessary.The yields and conversions were determined by GC-MS: HP-6890 gas chromatograph (GC) equipped with HP-5973 mass selective detector (MS). The capillary column used was HP-1 (25 m × 0.2 mm, 0.33 μm). The carrier gas was He. The oven temperature program was 80 °C–10 °C min−1–290 °C. Injection and detection temperatures were 250 and 290 °C, respectively. The amounts of ene product and remaining starting compounds were calculated using trans-anethole as an internal standard. The yield of ene product is reported as a percentage of the theoretical maximum. The reaction produces tars as side products. It was very difficult to collect and analyse this polymerised material. For this reason we were not able to calculate the mass balances. The reproducibility of the method was tested by performing multiple runs using water and ethanol solvents. The RSD values were within 7%.
Synthesis of reference compounds
The trans and cis isomers of the ene product, 1-methyl-3-(3-phenylallyl)pyrrolidine-2,5-dione, were prepared by the ene reaction of allylbenzene and N-methylmaleimide with minor modifications of the general procedure reported by the Cunningham group.11 A stirred mixture of allylbenzene (6.64 mL, 50.0 mmol), N-methylmaleimide (5.56 g, 50.0 mmol), and hydroquinone (0.551 g, 5.00 mmol) in 1,2,4-trichlorobenzene (6.25 mL) was flushed with argon and heated under argon at 200 °C for 24 h. The cooled reaction mixture was distilled under a reduced pressure to give a distillation residue containing a mixture of the crude product and polymeric residue. The residue was dissolved in acetonitrile (ca. 1 g residue in 4–5 mL of acetonitrile). The oligomers and polymers were precipitated by a slow addition of diethyl ether to the magnetically stirred acetonitrile solution which was previously cooled down to the ice-water bath temperature. After filtration, the precipitated polymers were washed with small quantities of diethyl ether. The combined diethyl ether filtrates were evaporated in vacuo to give the crude ene product (1.53 g). The crude product was chromatographed on silica gel (100% n-hexanes gradient to n-hexanes ∶ CHCl3 3 ∶ 1 followed by the gradient to 100% CHCl3) to give the cis isomer (42 mg, 0.37%), the trans isomer (460 mg, 4.0%), and a mixture of these isomers (253 mg, 2.2%).Trans isomer
Rf (n-hexane ∶ acetone ∶ CHCl3 4 ∶ 1 ∶ 5) 0.60; LRGC-MS [HP-5MS, 12 m × 0.25 mm, 0.25 μm, 100 °C–20 °C min−1–310 °C, flow rate 1.1 mL min−1] tret 6.44 min m/z 229 (43%) [M]+, 157 (3%), 143 (6%), 129 (20%), 117 (100%), 104 (6%), 91 (25%), 77 (4%); 1H NMR (CDCl3, 300 MHz) δ 2.38 (m, 1 H), 2.43–2.57 (m, 1 H), 2.59–2.63 (m, 1 H), 2.74–2.83 (m, 2 H), 2.97 (s, 3 H), 6.03–6.13 (td, J 15.7, 7.2 Hz, 1 H), 6.63 (td, J 15.6, 1.3 Hz, 1 H), 7.17–7.37 (m, 5 H).Cis isomer
Rf (n-hexane ∶ EtOAc ∶ CHCl3 4 ∶ 1 ∶ 5) 0.67; LRGC-MS [HP-5MS, 12 m × 0.25 mm, 0.25 μm, 100 °C–20 °C min−1–310 °C, flow rate 1.1 mL min−1] tret 6.20 min, m/z 229 (43%) [M]+, 222 (25%), 143 (20%), 129 (20%), 117 (100%), 104 (4%), 91 (21%); 1H NMR (CDCl3, 300 MHz) δ 2.37 (m, 1 H), 2.57–2.66 (m, 1 H), 2.78–2.82 (m, 1 H), 2.88–2.97 (m, 2 H), 2.98 (s, 3 H), 5.50–5.59 (td, J 11.7, 7.1 Hz, 1 H), 6.63 (d, J 11.1 Hz, 1 H), 7.16–7.39 (m, 5 H); 13C NMR (CDCl3, 75.4 MHz) δ 24.5, 29.6, 33.7, 39.9, 126.7, 127.2, 128.4, 128.7, 132.7, 136.4.3 Results and discussion
Water solvent
Water as a solvent was first studied at the subcritical temperatures of 221, 251, and 310 °C. The subcritical water proved to be inappropriate for our purpose due to the occurrence of hydrolytic side reaction. In all of the experimental runs shown in Fig. 2, N-methylmaleimide was totally hydrolyzed within a short reaction time (30 min) except for a small amount ene reacted, limiting the synthetic applicability of water as a solvent in this reaction. The major hydrolytic product was N-methylmaleic acid monoamide. The rapid hydrolysis can be explained by the catalytic effects of H+ and OH− produced from water; the value of the ionization constant of water reaches its maximum value (ca. 10−11) around 250 °C.15 The yield of ene product was only 2–6%, when equal amounts (0.5 g each, 4.2 mmol allylbenzene, 4.5 mmol N-methylmaleimide) of the starting compounds were used. The yield increased slightly with temperature from 2–3% at 220 °C to 5–6% at 310 °C. The temperature effect is partly due to the increase in the solubility of allylbenzene into water as well as the increase in the thermal energy.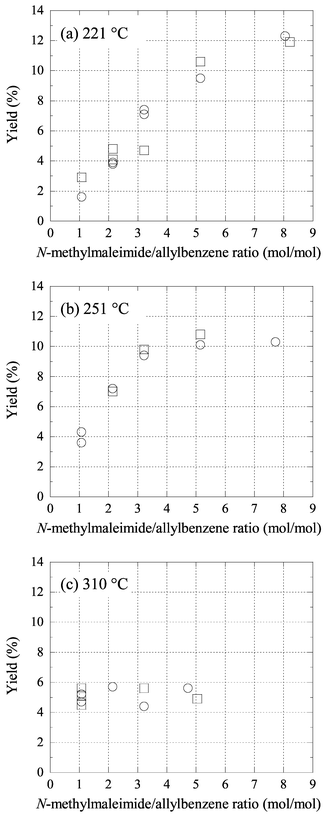 | ||
| Fig. 2 Yield of ene reaction between allylbenzene and N-methylmaleimide in water at (a) 221 °C, (b) 251 °C, and (c) 310 °C as a function of the excess mass ratio of N-methylmaleimide. □ without hydroquinone; ○ with hydroquinone. Reaction time is 30 min. | ||
By using a larger excess (2–8 times, mole based) amount of N-methylmaleimide at 221 and 251 °C, the ene yield increased up to 12%. However, the increase in the product yield cannot compensate the larger loss of N-methylmaleimide on an economical basis. Curiously, the yield decreased with increasing temperature from 251 to 310 °C. No higher yield than 6% was obtained at 310 °C, regardless of the excess ratio of N-methylmaleimide. At 221 and 251 °C, allylbenzene still remained after the reaction, whereas at 310 °C no starting compounds were found in the reaction mixture. It is thus suggested that the yield at 310 °C was limited by the rapid polymerization or other side reaction of allylbenzene accelerated with temperature. We should note, however, that the polymerization in water solvent was much less evident than that in apolar solvent, and that the addition of hydroquinone had a negligible effect on the ene yield. The selectivities for the trans isomer in water were 90–94%, which are slightly higher than those in 1,2,4-trichlorobenzene (87–91%). The trans-selectivity decreased slightly with temperature.
Ethanol solvent
Ethanol at 220 °C was found to be a very promising solvent for the ene reaction, mainly because the side reaction in ethanol is slow enough. The results in ethanol are summarized in Table 1, in comparison with those in 1,2,4-trichlorobenzene. In ethanol solvent, the yield of ene product increased with reaction time. The highest yield obtained was 31%, when equal amounts (0.5 g each, 4.2 mmol allylbenzene, 4.5 mmol N-methylmaleimide) of the starting compounds were applied. When the amount of allylbenzene was doubled, the yield reached 40% at 480 min. No evident increase in the yield was observed at longer reaction times, where practically all the loaded N-methylmaleimide was already consumed due to side reactions, such as polymerization and ethanolysis. The selectivities for the trans isomer in ethanol (88–92%) were comparable to those in 1,2,4-trichlorobenzene (87–91%).| No. | Mole ratio of allylbenzene ∶ N-methylmaleimide/mmol | Reaction time/min | Allylbenzene conversion (%) | N-methylmaleimide conversion (%) | Ene product yield (%) |
|---|---|---|---|---|---|
| a With hydroquinone (0.45 mmol). | |||||
| Solvent: ethanol (87 mmol) | |||||
| 1 | 4.2 ∶ 4.5 | 90 | 43.8 | 46.0 | 13.4 |
| 2 | 4.2 ∶ 4.5 | 240 | 41.5 | 71.1 | 26.1 |
| 3 | 4.2 ∶ 4.5 | 480 | 41.0 | 87.1 | 27.2 |
| 4a | 4.2 ∶ 4.5 | 90 | 41.2 | 33.7 | 16.3 |
| 5a | 4.2 ∶ 4.5 | 240 | 47.9 | 60.7 | 27.0 |
| 6a | 4.2 ∶ 4.5 | 480 | 40.7 | 80.0 | 31.5 |
| 7 | 8.4 ∶ 4.5 | 90 | 47.4 | 61.2 | 16.5 |
| 8 | 8.4 ∶ 4.5 | 240 | 37.5 | 84.6 | 32.3 |
| 9 | 8.4 ∶ 4.5 | 480 | 33.0 | >99.0 | 33.9 |
| 10 | 8.4 ∶ 4.5 | 720 | 36.3 | >99.0 | 35.1 |
| 11 | 8.4 ∶ 4.5 | 720 | 38.7 | >99.0 | 38.3 |
| 12a | 8.4 ∶ 4.5 | 90 | 45.7 | 45.3 | 23.3 |
| 13a | 8.4 ∶ 4.5 | 240 | 42.6 | 85.3 | 33.4 |
| 14a | 8.4 ∶ 4.5 | 480 | 52.6 | 93.1 | 40.0 |
| Solvent: 1,2,4-trichlorobenzene (28 mmol) | |||||
| 15 | 4.2 ∶ 4.5 | 90 | 47.4 | 82.7 | 7.6 |
| 16 | 4.2 ∶ 4.5 | 240 | 55.7 | 97.0 | 10.7 |
| 17 | 4.2 ∶ 4.5 | 240 | 55.0 | 93.6 | 13.3 |
| 18a | 4.2 ∶ 4.5 | 90 | 27.6 | 50.6 | 17.3 |
| 19a | 4.2 ∶ 4.5 | 240 | 51.5 | 84.7 | 31.2 |
| 20 | 8.4 ∶ 4.5 | 90 | 34.1 | 89.9 | 9.4 |
| 21 | 8.4 ∶ 4.5 | 240 | 41.8 | 97.5 | 20.4 |
| 22a | 8.4 ∶ 4.5 | 90 | 34.5 | 70.2 | 23.0 |
| 23a | 8.4 ∶ 4.5 | 240 | 44.4 | 99.0 | 38.3 |
| 24a | 8.4 ∶ 4.5 | 780 | 52.7 | >99.0 | 34.2 |
In ethanol solvent, no hydroquinone was required as an additive into the reaction mixture. As shown in Table 1, the ene yield in ethanol was sufficiently high without hydroquinone, and the addition of hydroquinone had a small effect on the yield. This result is in good contrast to that in 1,2,4-trichlorobenzene. In the conventional solvent, the highest yields in the absence of hydroquinone (13–20%) were much lower than those in ethanol (27–38%). Additive hydroquinone was indispensable in 1,2,4-trichlorobenzene to obtain higher yields (31–38%) comparable to those in ethanol. This is mainly because the starting compounds, N-methylmaleimide in particular, were easily consumed in 1,2,4-trichlorobenzene by the polymeric side reactions. The polymerization was less evident in ethanol, suggesting that the solvent ethanol itself acts as an inhibitor of the side reactions. A possible explanation is that the ethanol molecule serves as a radical scavenger: the radicals thermally formed are immediately trapped by the surrounding ethanol molecules. Another possible reason is the high polarity of ethanol solvent: the polar environment is less favorable for the radical reactions than apolar conditions.
The high yields in ethanol can be explained partly by the following two factors, the high polarity and high vapor pressure of the ethanol solvent. The high solvent polarity is generally known to enhance the solvophobic association of the apolar reactants, and the high pressure to accelerate the pericyclic reactions with negative activation volume.16 The vapor pressure of ethanol amounts to 4.2 MPa at 220 °C, thus is in favor of the pressure effect, although the pressure obtained here is much lower than those commonly employed in high-pressure organic synthesis (ca. 50–1000 MPa).
Finally, we should note that methanol is unsuitable as a solvent for this reaction due to the occurrence of methanolytic side reactions. Methanol has a much higher reactivity than that of ethanol, and the too high reactivity destroyed N-methylmaleimide within 30 min, as in water solvent. This is the reason why ethanol was chosen here as a solvent instead of methanol or water.
4 Conclusion
The ene reaction of allylbenzene and N-methylmaleimide was studied in water and ethanol solvents at subcritical conditions. Subcritical water was unsuitable for this reaction, because the reactant N-methylmaleimide was easily hydrolyzed due to the high reactivity of water. Subcritical ethanol proved to be a very promising solvent. The yields of ene product in pure ethanol were comparable to those in 1,2,4-trichlorobenzene with hydroquinone. In ethanol solvent, the highest yield amounts to 40% in 480 min, and the yield was weakly influenced by the presence of hydroquinone, possibly because the solvent ethanol itself acts as a polymerization inhibitor. In addition, the high polarity and high vapor pressure of ethanol is considered to accelerate the pericyclic association of the apolar reactants. As a result, subcritical ethanol serves as an efficient and relatively environmentally benign solvent compared with the conventional solvents such as 1,2,4-trichlorobenzene, without any additives. In the subsequent work, we will explore other feasible reaction systems in order to elucidate the novel potential of the subcritical polar solvent in organic synthesis.Acknowledgements
We thank the Academy of Finland for financial support (J. Y.-K. grants 75527 & 79890). Also the intriguing discussions with Prof. Lajunen from Oulu University are gratefully acknowledged.References
- H. M. R. Hoffmann, Angew. Chem., Int. Ed. Engl., 1969, 8, 556 CrossRef CAS.
- E. C. Keung and H. J. Alper, J. Chem. Educ., 1972, 49, 97 CAS.
- B. B. Snider, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 5, p. 1 Search PubMed.
- W. Oppolzer, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 5, p. 315 Search PubMed.
- W. Oppolzer, Angew. Chem., Int. Ed. Engl., 1984, 23, 876 CrossRef.
- K. Mikami and M. Shimizu, Chem. Rev., 1992, 92, 1021 CrossRef CAS.
- H. Hopf and C. W. Rautenstrauch, US Pat. 2 262 002, 1939.
- D. Rideout and R. J. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS.
- C. J. Albisetti, N. G. Fisher, M. J. Hogsed and R. M. Joyce, J. Am. Chem. Soc., 1956, 78, 2637 CrossRef CAS.
- M. B. Korzenski and J. W. Kolis, Tetrahedron Lett., 1997, 38, 5611 CrossRef CAS.
- I. D. Cunningham, A. Brownhill, I. Hamerton and B. J. Howlin, Tetrahedron, 1997, 53, 13473 CrossRef CAS.
- P. Mäkipeura, M. Kapanen, J. Tulisalo and S. Koskimies, US Patent 5 939 562, 1999 and references therein.
- CRC Handbook of Chemistry and Physics, 80th Edn., ed. D. R. Lide, CRC Press, Boca Raton, FL, 1999, pp. 6––14 Search PubMed.
- JSME Data Book, Thermodynamic Properties of Fluids, ed. H. Uchida, The Japan Society of Mechanical Engineers, Tokyo, Japan, 1983, p. 323 Search PubMed.
- W. L. Marshall and E. U. Franck, J. Phys. Chem. Ref. Data, 1981, 10, 295 CAS.
- K. Matsumoto and A. Sera, Synthesis, 1985, 999 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
