Electron transfer mediator systems for bleaching of paper pulp
D.
Rochefort
a,
D.
Leech
*b and
R.
Bourbonnais
c
aDépartment de chimie, Université de Montréal, C. P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7, Canada
bDepartment of Chemistry, National University of Ireland, Galway University Road, Galway, Ireland. E-mail: donal.leech@nuigalway.ie
cPulp and Paper Research Institute of Canada, 570 Boul. St-Jean, Pointe-Claire, Québec, Canada H9R 3J9
First published on 12th December 2003
Abstract
The participation of biological agents in pulp bleaching systems has received a lot of attention from research teams around the world, driven by the environmental benefits that biobleaching could bring. Nature showed us the ability of some of its agents, such as wood-decaying fungi, to delignify and bleach wood and wood pulp. What we need to do is to enhance the efficiency of such agents to make them cope with the fast pace of our modern pulp mills. To do so, a profound understanding of the biobleaching system is required. Our efforts to discover new efficient mediators for the laccase-mediator system (LMS) brought us to use several techniques to analyse the reactions involved in mediated enzymatic delignification. Mostly based on electrochemistry, these techniques are reviewed in this paper, along with key results. Cyclic voltammetry was used to characterize electron transfer rates between each element of the LMS. We found, along with other authors, that the mediator redox potential has a great influence on its efficiency. We used bulk electrolysis to simulate the oxidative action of laccase on mediators and model compounds of lignin. Such electrolysis techniques allowed us to study mediated lignin oxidation outside of normal laccase working conditions. Finally, an electrolysis-based method for mediated pulp delignification that we developed, based upon our research on biobleaching, is presented.
Green ContextTraditional bleaching methods in the pulp and paper industry rely heavily on chlorine and its derivatives, and thus place a heavy burden on the environment. Newer methods are available, but often lack the power of the chlorine-based systems. This paper discusses the use of enzymatic systems in bleaching, in particular the use of mediated systems, which promise excellent levels of bleaching with a good environmental performance.DJM |
1.0 Introduction
Wood is the main source of fibre for pulp and paper production. It is composed of cellulose, lignin, hemicelluloses, and extractives compounds in proportions that vary with the type and essence of the wood. The structure of wood can be represented as an agglomeration of long and slim fibres that are maintained together by the lignin, acting as a natural glue to give wood its physical properties. The wood fibres are mainly composed of cellulose and hemicelluloses, but also contain lignin within the fibre wall. To produce chemical pulp, the lignin is removed in order to free the fibres, which will eventually be used to make paper. During mechanical pulping, however, the fibres are separated from one another by a mechanical action. Lignin remains in mechanical pulps and the use of such pulps (newspaper) does not require complete bleaching. The present text will only be concerned with chemical pulp. Lignin is a very complex polymer, which is composed of phenyl-propane subunits linked together by various types of bond. The aromatic groups, found in great proportion in the lignin, can be classified as either phenolic or non-phenolic and may contain different substituents. The lignin has therefore no defined structure and its composition may also vary with the type of wood. However, a detailed scheme showing the known lignin structural elements has been published by Adler in 1977 (Fig. 1).1 Chemical pulping processes use strong chemicals, mostly sodium hydroxide, to hydrolyse the covalent links of the lignin and to depolymerise it. The kraft pulping process uses a combination of sodium hydroxide and sodium sulfide, the latter being able to extend the delignification to lower lignin content than NaOH alone. Pulp obtained by this process is also stronger than without Na2S, hence the name kraft, which is the German word for strength. Along with these advantages, the kraft process can be operated in a closed circuit, where the cooking chemical solutions (known as cooking liquors) are burnt, allowing production of heat and chemical recovery. Kraft pulping is the most widely used method of pulp production, leading the market with almost 70% of all the pulp produced worldwide.2 The specific chemical reactions involved in kraft pulping will not be reviewed here as we will focus on bleaching reactions. The interested reader will find more information on chemical pulping in the literature.3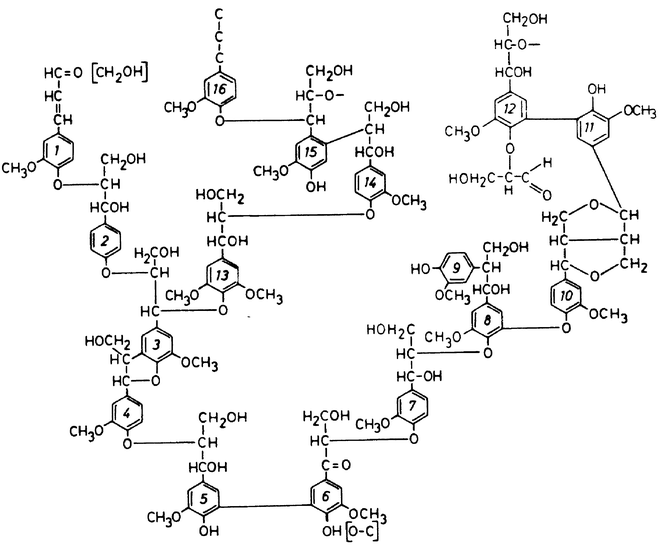 | ||
| Fig. 1 Softwood lignin structure as proposed by E. Adler, “Lignin Chemistry – Past, Present and Future”, Wood Science and Technology, 1977, 11, 169–218 (ref. 1); Figure 30, p. 203. © Springer-Verlag. | ||
Although very efficient, the kraft process does not remove all of the lignin contained in wood. The lignin surrounding fibres is removed but there is a residual of lignin within the fibre wall. Unbleached kraft pulp contains about 5% of lignin per unit of weight, lignin that must be removed since unbleached kraft pulp is very dark. This remaining lignin is tightly bound and strong oxidants must be used to remove it, which is a part of the objective of pulp bleaching. These oxidants, such as chlorine, chlorine dioxide, and oxygen, are necessary to oxidise and depolymerise the remaining lignin, to delignify pulp. The specific delignification reactions of these agents will be discussed in the text. Furthermore, side reactions during chemical cooking of wood produce light absorbing compounds such as quinones, which darken the pulp. To improve optical properties of the pulp, it must be bleached.
1.1 Traditional bleaching
Molecular chlorine appeared in the pulping industry in the early 1900s. It is easily produced at low cost, easy to use, and has a high reaction rate. Chlorine-bleached pulp is also of very good quality since Cl2 is very selective towards lignin, preventing cellulose-degrading reactions. Chlorine reacts with lignin via oxidation, substitution, and addition reactions. While the oxidation reactions take place on the substituents of the phenyl groups of lignin, substitution and addition occur mostly on the phenyl. All these reactions result in the depolymerisation of the lignin polymer, giving smaller, soluble fragments that can be removed from pulp with an alkaline extraction. A detailed overview of the reaction of chlorine with lignin can be found in other papers4,5 and in references cited therein. Overall, chlorine bleaching offers the best efficiency at low cost. However, as stated before, addition reactions of chlorine on lignin phenyl units can occur. The formation of toxic compounds such as dioxins and furans can result from addition, being the major drawback of the use of chlorine to bleach pulp. To avoid accumulation of these stable products in the environment, usage of chlorine decreased significantly since the late 1980s, being replaced by chlorine dioxide.Chlorine dioxide appeared in the pulping industry in the middle of the 20th century as an additive to chlorine bleaching agent.6 ClO2 gradually replaced chlorine as the so-called ECF (elemental chlorine free) bleaching method, and is now the principal kraft pulp bleaching agent. Chlorine dioxide, being an electrophile, will react preferentially with electron-rich structural elements, such as phenyl units. It degrades lignin by oxidation and aromatic substitution reactions. The exact reaction mechanisms leading to bond breaking and subsequent delignification depend upon which position the phenyl group will be oxidised. The radical cation formed on the phenyl unit will then degrade to depolymerise lignin.4 ClO2 bleaching is also very efficient and yields a very high quality pulp. However, one of the major environmental drawbacks of its use is the formation of chloride as a reaction product. The presence of chloride in the bleaching wastewater prevents system closure to avoid corrosion of the mill water circuit. The large amounts of water required to wash pulp between and during bleaching sequences must therefore be treated then released in the environment instead of being reused in the mill.
Total chlorine free (TCF) bleaching methods rely on non-chlorinated chemicals to bleach pulp. Oxygen, ozone, and hydrogen peroxide are examples of such TCF bleaching agents, the details of which can be found in other texts.7–9 Another alternative bleaching method is the use of biological agents that are able to degrade lignin, the biobleaching, which is part of the focus of this paper.
The goal of this paper is to review the electron transfer approaches to pulp bleaching, with a particular emphasis on biobleaching and the laccase-mediator system (LMS). We will present an overview of the mediators used, including recently proposed transition metal compounds, and the various approaches used to characterise their role in the system. Recently electron transfer approaches to pulp bleaching have expanded to include electrochemical methods for pulp bleaching, replacing the biological oxidant with an electrode: electrodelignification. An entire section will thus also be devoted to reviewing electrochemical methods for pulp bleaching and mediated oxidation of kraft pulp lignin.
1.2 Biobleaching
It has been known for over 40 years that some types of fungi, more particularly basidiomycetes (or white-rot fungi) are able to degrade lignin from wood.10 These fungi grow on dead wood to decay it. Experimental work later showed that basidiomycetes also degrade lignin remaining in unbleached kraft pulps,11–13 opening the way for biobleaching. Although capable of extensive delignification (up to 60%), the reaction rates of bleaching with fungi are very low and such delignification can only be obtained in a matter of days. Such reaction time is too long to cope with the high production rate of kraft pulp mills, even if the treatment represents non-negligible chemical savings. Basidiomycetes perform lignin degradation by secreting a variety of oxidative enzymes, including laccases,14 lignin peroxidases,15 and manganese peroxidases.16 Bearing in mind that the secreted enzymes are responsible for the delignifying action, isolating and concentrating these enzymes for direct use on pulp could improve the rate of biobleaching. This would make a more interesting system for a mill application. Among the several enzymes that have been isolated and used to bleach pulp,17 we focus on the laccases. Laccase is perhaps the most intensively studied enzyme for pulp delignification because of its excellent oxidative properties. However, since there is only one class of enzyme that currently is commonly used in pulp mills, the xylanases, their use is also described.Xylan is one type of hemicellulose found in wood that acts as a linking agent between lignin and cellulose.20 When wood is cooked using high alkaline charge, xylan is depolymerised and solubilised. As cooking goes on, the alkalinity of the solution decreases, causing some xylan to precipitate on the fibres. One mechanism that could explain the beneficial effect of xylanase on pulp bleaching is that the enzyme catalyses the depolymerisation of precipitated xylan. The bleaching chemical would therefore have easier access to lignin, increasing the efficiency of the bleaching sequence.19 Xylanase is therefore more a bleaching-aiding enzyme than a true delignification agent like oxidative enzymes such as the laccases.
Laccase is found in many plants and trees, but also in some bacteria and insects. It is however in fungi that laccase is most commonly found.27 Laccase, a blue copper enzyme, possesses an active site which consists of four copper atoms sub-classified into three different types, T1, T2, and T3, according to the different EPR spectrum of each copper atom.22 Laccase can oxidise a substrate via the four-electron reduction of dioxygen to water. Substrate oxidation occurs at the T1 monoatomic copper site, while the T2/T3 triatomic copper cluster is responsible for O2 reduction. This property of the laccase makes it very appealing for delignification since the only chemical necessary for substrate oxidation is oxygen. For several reasons, laccase alone has only a very limited effect on lignin degradation. Firstly, laccase can oxidise the non-phenolic groups (see Fig. 1) of lignin only at a very slow rate due to their high oxidation potential, which can reach values over 1 V vs. Ag/AgCl.28 Fungal laccases such as those from the basidiomycete Trametes versicolor),22 have a redox potential (E0) of 0.575 V vs. Ag/AgCl, which is considered to be high, compared to that of laccases from other sources. The large potential gap between laccase and these non-phenolic groups makes the oxidation reaction thermodynamically uphill, and thus results in slow reaction rates. The enzyme can however oxidise the phenolic groups (laccase's natural substrates) much more rapidly since they have low oxidation potentials. Since kraft pulp lignin contains an appreciable quantity of phenolic groups, this results in unwanted consequences, such as polymerisation. The second reason for the limited action of laccase alone on kraft pulp lies in its size. Due to its size, laccase is unable to rapidly diffuse into the fibre wall to reach the remaining fragments of lignin (see Fig. 2). This severely reduces the quantity of potentially oxidisable groups since only the outermost ones will be readily accessible to laccase. These size and thermodynamic related limitations can be partially overcome with the addition of another element to the system: a mediator.
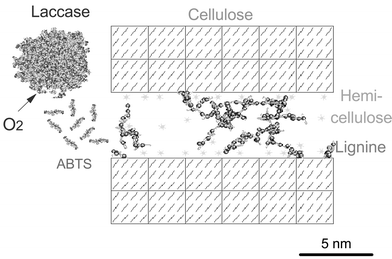 | ||
| Fig. 2 Representation of a fibre secondary wall from unbleached kraft pulp.74 The mediator (here ABTS) can diffuse back and forth between laccase and remaining lignin fragments. | ||
1.3 The laccase-mediator system (LMS)
A mediator is a small redox molecule that acts as an electron carrier between lignin and laccase. Once the mediator is oxidised, by giving one or several of its electrons to laccase, it will diffuse into the pulp fibre wall to oxidise lignin. The mediator then gains electrons from the lignin fragment that is oxidised to return to its reduced state, making it available once again for oxidation by laccase. Overall, the laccase-mediator system acts as a catalyst to oxidise lignin by transferring electrons from its oxidisable groups to oxygen. This simplifed mechanism is depicted in Scheme 1. This catalytic cycle will go on as long as there is oxygen present in the system and until oxidation of the lignin becomes too difficult. Contrary to laccase, the mediator is able to diffuse within the fibre wall due to its small size (see Fig. 2) and gain better access therefore to the oxidisable groups of lignin. Also a mediator with a high redox potential will likely react more efficiently with phenolic and non-phenolic groups in lignin than a low redox potential mediator, resulting in enhanced delignification. Finally, because of their smaller molecular weight in proportion to the enzyme, accumulation of a higher local concentration of mediator in the pulp fibre may explain the more effective oxidation of laccase-mediator systems. This more effective oxidation makes the laccase-mediator systems interesting for industrial kraft pulp delignification.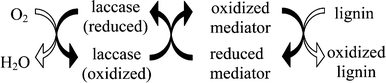 | ||
| Scheme 1 Representation of the successive oxidation and reduction reactions of the laccase mediator system to delignify pulp. | ||
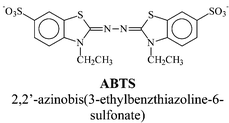
The first mediator that was used in the laccase-mediator system for pulp delignification is 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS), which was introduced by Bourbonnais and Paice in 1990.29 Their research results showed that laccase used in conjunction with a mediator (ABTS) was able to extend the oxidation of lignin to its non-phenolic groups. Further studies demonstrated that the laccase-mediator could be used to efficiently delignify unbleached kraft pulp to high levels (50% delignification).30,31 It was also shown that the mediated enzymatic oxidation with laccase and ABTS is selective towards lignin. Degradation of cellulose decreases bleached paper quality and so must be avoided during delignification. The laccase–ABTS system does not affect pulp viscosity, which measures the degree of cellulose polymerisation, and is thus indicative of pulp quality.31
| Mediator | Pulp delignification references | Lignin model compound oxidation references |
|---|---|---|
|
31,75,76 | 29,33,44,46,47,77–80 |
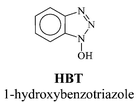
|
41,58,81–86 | 32,33,76,80,87–89 |
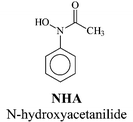
|
90–93 | 38 |
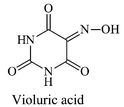
|
94 | 33,38 |
In an effort to improve the laccase-mediator system for pulp delignification, we recently proposed the use of transition metal coordination complexes as mediators.34,35 These compounds do not form radicals when oxidised by laccase, and the electron exchange is centred on the metallic atom of the complex. This type of electron exchange involving only transition metal redox reactions allows the use of mediators with high stability in both oxidation states. This is a great advantage over the other type of mediators. Comparison of their efficiency with that of radical-forming mediators will be discussed in the following sections.
From the results of the numerous studies on the laccase mediator system, it is possible to define the most essential characteristics required for an efficient mediator.
• The mediator must be a substrate for laccase, which is generally not a problem since this enzyme can oxidise a large variety of compounds. This relatively low selectivity is due to the exposed T1 active site, which is located near the surface of the laccase proteinic skeleton.
• Results of studies on mediator formal potentials (E0′)36–39 point out that altering the mediator E0′ has a very important effect on the overall efficiency of the system. The most significant explanation for its effect lies in the thermodynamic point of view of the electron exchange reaction. As stated earlier in the text, laccases have an oxidation potential that is too low to efficiently oxidise all the groups in lignin, which makes the reaction thermodynamically uphill. To have a thermodynamically favourable reaction, a mediator with a formal potential higher than the oxidation potential of the non-phenolic groups should be used. Unfortunately, this high E0′ mediator would be only slowly oxidised by laccase, if at all, for the same thermodynamic explanation. These limits heavily restrain the number of compounds that could be used as a mediator.
• The mediator should be stable in both of its oxidation states to prevent losses and unwanted side-reactions during the treatment.
• The electron exchange rate between laccase and mediator, as well as between the mediator and lignin, should be fast.
• Considering an eventual mill application, the mediator should be available in large quantities at a low cost and be non-hazardous for the environment.
With a suitable mediator at hand, the laccase-mediator can be studied either for evaluating the efficiency of a new mediator or to elucidate mechanisms. Since the exact mechanisms of pulp delignification are still unclear, the use of different mediators can be useful in explaining the reactions occurring during delignification.
2.0 Characterisation of reactions involved in laccase-mediator system
There are numerous publications on different methods used to study the efficiency of the laccase-mediator system. This section will present the direct pulp delignification technique, the use of lignin model compounds and of electroanalytical methods, to gain knowledge of delignification chemistry as well as to evaluate the efficiency of the laccase-mediator system.Perhaps the most simple and representative method is the direct pulp treatment, which consists of a lab-scale version of a mill treatment to reproduce mill conditions. The pulp is afterward analysed to evaluate the efficiency of the treatment, most often by measuring three parameters; kappa number, brightness level and viscosity. These three parameters of pulp will be described, since the reader will find several references to these in the text.
The kappa number (K) is used to evaluate the residual lignin content of a pulp. This number corresponds to the volume of a 0.1 N KMnO4 solution that is consumed (reduced) by the lignin contained in one gram of dry pulp. Therefore, a treatment with high delignification efficiency will result in a large decrease of the kappa number. The brightness level of pulp is simply a measure of the light reflected by the sample at a fixed wavelength.40 Brightness is useful as an indication of the efficiency of a bleaching treatment for highly bleached pulps as kappa number becomes less accurate near very low values. Finally, the viscosity index gives an evaluation of the pulp quality. The viscosity loss due to a treatment should be minimal as a decrease in viscosity indicates a decrease in the average cellulose chain length, which weakens the eventual paper's physical properties.
Although useful to evaluate the overall efficiency of a pulp treatment, measuring these three properties provides neither details on the reactions mechanisms nor on the identity or type of lignin subunits that are being oxidised during the treatment. Furthermore, no information relative to the reaction rates can be extracted from these measurements so we must rely on different, more powerful methods. The most widely used method to obtain detailed insights into the reactions governing pulp delignification by the laccase-mediator system is the study of the effects of the system on lignin model compounds. A literature database search for lignin model compounds over the last ten years returned over 250 entries with nearly 50 concerning studies with the laccase-mediator system. The next section is therefore devoted to this subject.
2.1 Lignin model compounds
Lignin model compounds (LMCs) are soluble low molecular weight molecules that are used to represent some of the functional groups or linkages of lignin. As mentioned in the introduction and as depicted in Fig. 1, lignin is a very complex and irregular polymer that, in addition, varies in structure from wood species to species. Due to these characteristics and to its very low solubility, experimentation on lignin is difficult. To avoid the limitations inherent to the use of lignin, experiments can be conducted on lignin model compounds. Another noteworthy advantage of using LMCs over lignin is that it allows the evaluation of the reactivity of a specific type of linkage, which is represented by the model compound. Most commonly used LMCs are β-O-4 and β-1 dimers, which are used to represent these bonds in lignin. For an example of such types of bond, the reader is referred to the links between phenyls #7 and #8 for β-O-4 model and phenyls #14 and #15 for β-1 model (Fig. 1). Model compounds representing these particular bonds are shown in Fig. 3. The relevance of the use of such model compounds is often questioned on their ability to really represent the reactivity of bonds in the lignin. The LMCs are indeed solvated molecules while the real bonds in lignin are in a complex insoluble matrix. The LMC studies are however undoubtedly useful for mediator screening. It is clear that an oxidative system that is able to oxidise a particular chemical bond will not automatically be able to oxidise the corresponding functional group found in lignin. On the other hand, a mediator that is unable to oxidise the most reactive model compound will have a rather limited efficiency for lignin degradation.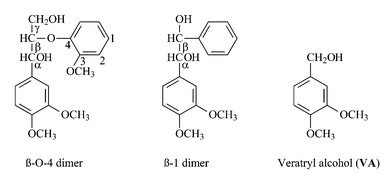 | ||
| Fig. 3 Non-phenolic lignin model compounds representing important oxidisable groups in kraft pulp residual lignin. | ||
The procedure used is almost constant throughout the literature on the subject. A solution containing an oxidising agent (such as laccase), a mediator, and the model compound is left to react and then analysed to measure the concentration of unreacted LMC and to identify and quantify oxidation products. This information can then be used to evaluate the efficiency of the treatment and to elucidate oxidation mechanisms from the nature of the oxidation products detected.
2.2 Electrochemistry of laccase-mediator reactions
An approach that is often neglected is the use of electrochemical techniques to study the reactions of the laccase-mediator system on pulp and on model compounds. Since these reactions involve electron exchange, electrochemical analyses can provide useful information on the system that could otherwise be difficult to obtain. These electrochemical methods are either potentiostatic (electrolysis) or potentiodynamic (polarography and cyclic voltammetry).2.2.1.1. laccase-mediator reaction. The oxidation of a substrate (a mediator in our case) by laccase, with concomitant conversion of oxygen to water is composed of nine steps involving electron transfers between the substrate, the different copper atoms of the enzyme active site, and oxygen. For the purpose of the text, these nine steps will be regarded as one reaction as we will focus on the oxidation rate of mediator by the enzyme. Detailed information on electron transfer reactions of laccase can be found elsewhere.22 Thus the substrate (mediator) oxidation can be oversimplified by eqn. 1:
 | (1) |
Whilst sweep rate-independent pseudo-first-order rate constants (kf) for this process can be evaluated using CV, according to the method developed by Cass et al.56 based on the Nicholson and Shain57 approach (vide infra), the main difficulty encountered when attempting to evaluate the homogenous second-order rate constant (kmed) for a mediator reaction with laccase is the need to determine the active laccase concentration. While precise measurement of enzymatic activity is easily accomplished by spectrophotometric assay with a substrate, such as ABTS,58 the laccase concentration determination relies on a single absorbance reading at λ = 620 nm (ε = 4900 M−1 cm−159). This absorption band, which corresponds to a d–d transition of the T1-type copper atom of the active site, is also responsible for the blue colour of the laccase in solution. The absorbance measurement however only yields the total amount of T1 Cu atoms of laccase molecules, regardless of the activity or inactivity of the enzyme. For instance, the T2/T3 copper atoms cluster site responsible for O2 reduction could be inhibited without affecting the absorption band at 620 nm. Furthermore, we have noted that storage conditions affect this absorption and the possible contamination of the laccase sample results in an increased absorbance reading with time, whereas laccase activity decreases, as depicted in Fig. 4. For this reason we present, and recommend that others do likewise, only the pseudo-first order rate constants (kf) for the laccase-mediator reaction with the mediator present at a fixed, excess, concentration. In addition, in order to be able to compare the kf values for different mediators, all experiments have been conducted using the same laccase activity (based on ABTS as a substrate).
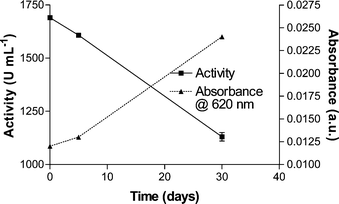 | ||
| Fig. 4 A comparison between Trametes versicolor laccase activity and absorbance measurements, supposedly reflecting laccase concentration changes, over the period of a month. | ||
To evaluate kf, cyclic voltammograms of 0.2 mM mediator in buffer solution were recorded at several scan rates (1 to 50 mV s−1). The cathodic peak current is measured at peak maximum intensity (labelled diffusion current (id)). An example of such a curve obtained for K4[Mo(CN)8] is presented in Fig. 5. Following this step sufficient laccase to yield activity of 5 U ml−1 is added to the solution. CVs are recorded at the same scan rates. At scan rates slow enough, a decrease of oxidation peak intensity is observed, along with an increase in reduction currents, and a change in shape of CV from tailed diffusion-controlled peaks to sigmoidal, catalytic, curves (see Fig. 5). These changes arise from the enzymatic oxidation of mediator (eqn. 1). In presence of laccase there is continuous regeneration of the oxidised state by the enzyme. Under cathodic polarisation, when the scan rate is low enough, the continuously oxidised mediator will be continuously reduced at the electrode (eqn. 2), generating more current than by the mediator alone in solution.
 | (2) |
![Diffusion controlled (id) and catalytic (ik) cyclic voltammograms for K4[Mo(CN)8] mediator (0.2 mM) in presence and absence of laccase (Lc, 5 U ml−1). CVs were recorded in pH 4.5 citrate buffer at a scan rate of 2 mV s−1.](/image/article/2004/GC/b311898n/b311898n-f5.gif) | ||
| Fig. 5 Diffusion controlled (id) and catalytic (ik) cyclic voltammograms for K4[Mo(CN)8] mediator (0.2 mM) in presence and absence of laccase (Lc, 5 U ml−1). CVs were recorded in pH 4.5 citrate buffer at a scan rate of 2 mV s−1. | ||
The faster the enzymatic oxidation rate is, the larger the current increase will be. This cathodic current, labelled catalytic current (ik) is also measured and the ratio of diffusion current over catalytic current is calculated for each scan rate. The kf values for the enzymatic oxidation of each mediator are then obtained according to the method developed by Cass et al.56 based on the Nicholson and Shain57 approach.
In order to investigate optimal conditions for laccase-mediator electron transfer, we have evaluated the effect of pH and mediator E0′ on kf. Fig. 6 illustrates the importance of the effect of mediator redox potential (E0′) on the enzymatic oxidation rate. The general trend for kf values is to decrease as the E0′ increases towards the laccase redox potential of 575 mV vs. Ag/AgCl, as the reaction becomes less thermodynamically favourable. While mediators with very low E0′ (with respect to laccase) are rapidly oxidised, they may not, however, be suitable mediators for delignification. To oxidise lignin in pulp, the mediator must have a redox potential as high as possible in order to perform efficient lignin oxidation, as discussed in the next section. The ideal mediator should therefore have at the same time a high kf and a high E0′. This is the case with the ABTS mediator for which kf is clearly higher than the general trend shown in Fig. 6. This high reactivity of ABTS with laccase is a result of several factors. Firstly, the organic aromatic structure of ABTS makes it closer to that of laccase natural substrates, the phenols. The interaction with the laccase active site is probably more favourable with ABTS than with transition metal-based mediators. The fact that ABTS undergoes disproportionation when oxidised must also be taken into account. This disproportionation mechanism, which is the reaction of two radical cation ABTS˙+ to form one reduced ABTS and one dicationic ABTS++ molecule,60 has already been invoked to explain the high efficiency of ABTS to lignin and its model compound oxidation.36 The ABTS++ produced by such a mechanism has a redox potential higher than that of the radical cation (0.91 compared to 0.47 vs. Ag/AgCl, respectively). Finally, pH has an effect on kf for mediators. All measurements have been performed in pH 4.5 citrate buffer, which is the optimal pH for ABTS oxidation by the Trametes versicolor laccase (Fig. 7). Other mediators may have optimal pHs other than 4.5, their kf will subsequently be lower compared to ABTS at this pH.
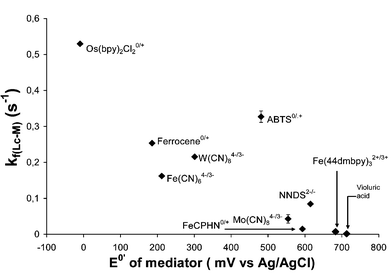 | ||
| Fig. 6 Effect of mediator E0′ on pseudo-first-order rate constants, kf, for the laccase-mediator oxidation (citrate buffer, pH 4.5, laccase = 5 U ml−1, mediator = 0.2 mM). The error bars represent standard deviation calculated from triplicate experiments. The abbreviation used for mediators are: ABTS = 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid), FeCPHN = iron(II) dicyano-bis-(1,10-phenantroline), NNDS = 1-nitroso-2-naphtol-3,6-disulfonic acid, Fe(44dmbpy)3 = iron(II) tris-(4,4′-dimethyl-2,2′-bipyridine). | ||
![Effect of pH on pseudo-first-order rate constants for the laccase (5 U ml−1)-mediator (0.2 mM) oxidation. Mediators are ABTS (circles) and K4[Mo(CN)8]
(squares).](/image/article/2004/GC/b311898n/b311898n-f7.gif) | ||
| Fig. 7 Effect of pH on pseudo-first-order rate constants for the laccase (5 U ml−1)-mediator (0.2 mM) oxidation. Mediators are ABTS (circles) and K4[Mo(CN)8] (squares). | ||
The pH can affect several parameters of the laccase-mediator reaction. It is known that the oxidation potential of the enzyme T1 active site is not significantly affected by pH.61,62 Hydroxyl anions however are known inhibitors of the T2/T3 site, where oxygen reduction takes place.23 Laccase activity is maximum over a pH range of 3 to 5, depending on the laccase source and on the type of substrate. For mediators that possess E0′s that are pH-independent, such as ABTS and K4[Mo(CN)8], the variation in rate constant observed with pH should decrease with an increase in OH− concentration. The plot in Fig. 7 shows that this is not the case. Whilst the redox potential of the active site does not change with pH, the structure surrounding the T1 copper atom is affected by it. These changes, protonation and deprotonation with pH variations, produce different electrostatic interactions between the mediator and the active site. ABTS for instance is neutral at pH lower than 3.5 and anionic at pH above that. Since histidines and methionines at the substrate-binding site of laccase are positively charged at acidic pH, ABTS would be more attracted to this site at pH above 3.5. The maximal rate for K4[Mo(CN)8] interaction with laccase is attained, in contrast, at pH 3. It seems appropriate, thus, to state that the optimal pH of laccase-mediator reactions should be determined for each mediator. Furthermore, since the laccase-mediator system for pulp delignification comprises another element, the lignin in the pulp, the overall system optimal pH must take the mediator–lignin reaction into account.
2.2.1.2. Mediator–lignin reaction. We have adopted a method similar to that presented in the last section to evaluate the oxidation rate of lignin in unbleached kraft pulp by mediators. Initially, the method was developed for rapid determination of kappa number and pulp bleachability.63 While most automated kappa number measurement techniques are based on optical reading, our proposed method correlates a measure of catalytic currents determined using cyclic voltammetry with kappa number. One the main advantages of using an electrochemical method over an optical one is its independence on pulp sample consistency, which varies through the different bleaching stages.
To evaluate lignin oxidation rates, a 10 mg pulp sample is impregnated with a 0.2 mM mediator solution. The pulp sample is then compressed at the surface of an electrode and held in place. The electrode is then immersed in the mediator solution for electrochemical measurements. As for the experiments described in the previous section, cyclic voltammograms are recorded at several scan rates. As shown in Fig. 8, a catalytic response is observed when the mediator is able to oxidise the lignin in pulp. Also presented in Fig. 8 is the CV obtained with a fully bleached pulp sample (negligible residual lignin). As expected, no catalytic current is noted and the CV shape exhibits the characteristic semi-infinite diffusion-controlled response of that obtained for solutions containing the mediator alone. In order to evaluate comparable rates of reaction between mediators and lignin in pulp, the mediator diffusion co-efficient in the pulp, rather than in solution, should be used. This is evaluated from CVs recorded with the mediator and a fully bleached pulp sample, using the Randles–Sevcik equation for reversible semi-infinite diffusion.64 Since the exact molar concentration of lignin cannot be determined due to its complex and irregular structure, the second order rate constant for the reaction between the mediator and lignin, kmed is again unavailable. The pseudo-first order rate constants, kf, can however be used with confidence to compare the oxidation rates amongst the different mediators, using the same bulk pulp sample.
![Cyclic voltammograms of 0.2 mM K4[Mo(CN)8] recorded at 250 mV s−1 with fully bleached (kappa number = 0) and unbleached (kappa number = 19.9) kraft pulp. The buffer solution is 100 mM phosphate at pH 8.](/image/article/2004/GC/b311898n/b311898n-f8.gif) | ||
| Fig. 8 Cyclic voltammograms of 0.2 mM K4[Mo(CN)8] recorded at 250 mV s−1 with fully bleached (kappa number = 0) and unbleached (kappa number = 19.9) kraft pulp. The buffer solution is 100 mM phosphate at pH 8. | ||
The plot in Fig. 9 shows the different kf values obtained for the mediator–lignin reaction as a function of the mediator redox potential. As expected, from a thermodynamic point of view, mediators with high E0′ tend to have faster oxidation rates. As for the laccase-mediator reaction, ABTS does not follow the general trend for the same reasons as outlined previously. Regarding the optimal mediator, a mediator with high E0′ that produces a free radical upon oxidation would be best to perform pulp delignification. However, since this mediator must be oxidised by laccase prior to pulp delignification, its oxidation potential should be near or under that of the T1-type copper of laccase. The best mediator for the LMS is therefore that with highest kf but lowest E0′. Among the mediators investigated by us, this criteria ranks ABTS, K4[Mo(CN)8], and Fe(II)(4,4′-dimethyl-2,2′-bipyridine)3 as the three best mediators for LMS delignification. Indeed, ABTS is the most efficient of these when tested for pulp delignification. However, its efficiency is very close to that of K4[Mo(CN)8], which reacts more slowly with both laccase and lignin.34,35 The high delignification efficiency of K4[Mo(CN)8] is a result of its excellent stability; even if this mediator reacts less rapidly than ABTS it can perform oxidation for a longer period of time without degradation of its oxidised form. Analysis of the solution after pulp treatment reveals that the K4[Mo(CN)8] concentration remains unchanged from the initial concentration.
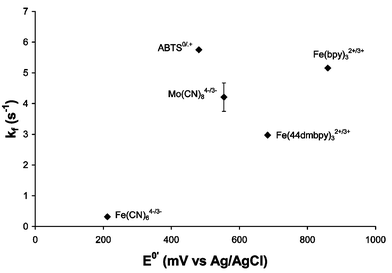 | ||
| Fig. 9 Pseudo-first-order rate constants for the mediator–lignin oxidation for several mediators (phosphate buffer, pH 8). The abbreviation used for mediators are: ABTS = 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid), Fe(44dmbpy)3 = iron(II) tris-(4,4′-dimethyl-2,2′-bipyridine). | ||
The rate constant for the mediator–lignin reaction was also evaluated for K4[Mo(CN)8] at varying solution pH values (Fig. 10). The optimal pH range for mediated pulp delignification is located between 8 and 10. Once again, the bell-shaped curve is a result of two factors: lignin reactivity and mediator behaviour. Oxidisable lignin groups such as phenolic moieties are ionised at high pH, making the lignin more solvated and thus more accessible. At high pH however, the K4[Mo(CN)8] mediator loses its stability, making the lignin oxidation less efficient. The results (not shown) of stability experiments on K4[Mo(CN)8] in alkaline solution showed a decrease of 25% of this mediator concentration within a day.
![Variation of pseudo-first-order rate constant of unbleached kraft pulp lignin (K
= 19.9) oxidation by K4[Mo(CN)8] mediator with different pH values.](/image/article/2004/GC/b311898n/b311898n-f10.gif) | ||
| Fig. 10 Variation of pseudo-first-order rate constant of unbleached kraft pulp lignin (K = 19.9) oxidation by K4[Mo(CN)8] mediator with different pH values. | ||
The conclusion from these oxidation rate experiments is that, unfortunately, the conditions for an efficient enzymatic mediator oxidation (low E0′ mediator, acidic solution) are unfavourable for the mediator–lignin reaction. A compromise must be achieved at lower than optimal efficiencies of both reactions.
In order to circumvent such a compromise, it has been proposed that the use of bulk electrolysis to replace the enzyme–O2 biocatalyst as the oxidising agent in the laccase mediator system can result in a system that is much less condition-sensitive than the LMS and allow efficient mediator oxidation under conditions that favour lignin oxidation.37,65,66
Other information on the role of the mediator can also be derived from electrolysis experiments. For instance, the role of transition metal based mediators as electron carriers has been clearly demonstrated using electrolysis.50 In such experiments, a solution of potassium hexacyanoruthenate(II), K4[Ru(CN)6], mediator was prepared and oxidised to completion in the electrolysis cell. Veratryl alcohol (VA, see Fig. 3) was then added to the solution and left to react, giving a 27% VA oxidation with a 50 to 1 mediator ∶ model compound ratio. No VA oxidation occurred if the mediator was not first oxidised, nor when the experiment was repeated with equal concentrations of K4[Ru(CN)6] and of VA. This suggests that the oxidised mediator is the oxidising agent of the model compound and that there must be a large excess of mediator to drive the oxidation of the LMC. In enzymatic oxidations, when low levels of mediator are used, the turnover of the cycle presented in Scheme 1 is necessary to drive the reaction. These results clearly indicate that the system must provide a continuous source of oxidised mediator in order to efficiently oxidise the lignin model compounds. Stemming from these results, we became interested in exploring the use of an electrochemical means to supply a continuous source of oxidised mediator for pulp delignification.
3.0 Electrochemical aspects of pulp delignification
Several electrochemical techniques have been used for numerous years in the pulp and paper industry. These techniques, mainly focussed on electrosynthesis of bleaching chemicals, such as chlorine, chlorine dioxide, and hypochlorite, were reviewed and presented by Oloman.68 The application of electrochemistry in the pulping industry is however not limited to the production of these bleaching agents and, quoting Oloman, “The possibilities for new applications are limited only by the imagination”.68 The goal of the last section of this paper is to present a possible role of electrochemistry in mediated kraft pulp delignification.Of the rich literature content for electrochemistry in pulp and paper production, only few publications directly address delignification.37,65,66,69–71 This is surprising, as delignification is a result of oxidation (an electrochemical reaction) of functional groups in lignin. Direct oxidation of dissolved lignin at Ni, PbO2, and dimensionally stable anodes (DSA)71 has been attempted, but the efficiency of this concept is limited when using pulp, since the lignin may not be accessible to the electrode. Another type of electrochemical delignification found in the literature concerns the in situ formation of a delignification70,72 or a bleaching73 agent. The latter consists of applying a voltage to an electrolysis cell containing the pulp in an alkaline chloride solution to produce hypochlorite, which bleaches the pulp. Such techniques result in a bleaching efficiency comparable to normal multi-stage bleaching methods but with less AOX produced and a shorter bleaching time. In situ electrochemical formation of activated forms of oxygen for use as delignification agents has also been reported.70,72 For example, when bleaching liquor containing ferrocyanide and dissolved oxygen is passed over an electrode to which a potential is applied, the ferricyanide produced at the electrode appears to ionise some phenolic moieties in lignin, allowing them to react with oxygen to achieve bleaching.72 Dissolved oxygen could also react with the ferricyanide to form bleaching-active species.
In mediated electrochemical delignification, the laccase and oxygen is substituted by an electrode. The mediator acts as an electron carrier and is the sole agent responsible for the lignin oxidation. Such systems have been proposed using ferrocyanide,69 violuric acid,65,66 and K4[Mo(CN)8].37 We focus on transition metal based mediators as we believe that these mediators are well suited for this system because of their stability and of the wide redox potential range offered by this class of mediators. Indeed, mediators such as the iron(II) tris-dipyridyl complexes have high redox potentials, allowing them to oxidise some functional groups in lignin that could not be attacked by other mediators.37
Three major advantages arise from the replacement of an enzymatic system with an electrolytic system. Firstly, the problems of enzyme sensitivity to experimental conditions (pH, temperature, presence of inhibitors) are avoided. Secondly, the range of mediators is no longer limited to those which act as substrates of laccase. Finally, mediators of high redox potential can be selected to favour the oxidation of lignin; such mediators are only slowly oxidised by laccase (vide supra). The major disadvantage arises from the fact that the use of an electrode creates a heterogeneous system. Since the pulp itself is a heterogeneous suspension, it is more difficult, using electrolysis, to obtain uniform distribution of the oxidised mediator. In an electrochemical system, one oxidation cycle could be viewed as the following sequence: (I) the mediator is oxidised at the electrode, (II) it diffuses in the solution and into the fibre cell walls where the residual lignin is located then (III) oxidises lignin, being itself reduced. The mediator must then (IV) diffuse back to the electrode to begin a new cycle. The solubility of laccase confers a huge advantage since the oxidation agent is uniformly distributed, resulting in a low mediator diffusion time. In an attempt to improve mediator access to lignin in the pulp our electrochemical system comprises an isolated electrolysis cell, in which the mediator is continuously oxidised and then pumped (continuously) through the pulp, which is compressed in a dense pad in a separated cell (see Fig. 11).
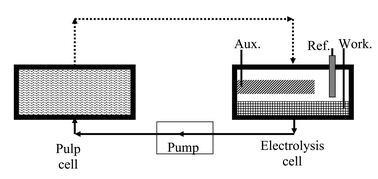 | ||
| Fig. 11 Electrodelignification experimental setup scheme, showing the flow of mediator solution through the electrolysis cell (right) and the pulp sample (left). | ||
Significant delignification can be achieved using this system: ∼30% for a two hour treatment using 1% potassium octacyanomolybdate(IV) as the mediator at pH 5.37 This result is statistically equivalent to the delignification efficiency obtained with laccase and the same mediator under the same conditions. When conducting the experiment at a pH of 8, delignification (30%) is obtained with the electrochemical system, whilst laccase is completely inactive at this pH and no delignification occurs with the LMS. It is worth noting that no delignification is observed when the electrochemical system is used without mediator or without applying any potential at the electrode. Finally, a 60% delignification can be attained after an extensive 45 hour electrolysis treatment. The importance of this long term experiment is that the electrolysis treatment showed no damage to the cellulose fibre. The viscosity loss of the electrochemical delignification with a transition metal based mediator is minimal, demonstrating that pulp physical properties are preserved during such a treatment.
4.0 Future of mediator bleaching systems
The laccase-mediator system appears to represent a very promising method to bleach kraft pulp. Of course, when dealing with a potential application in the industry, other parameters must be considered. Chlorine dioxide is a cheap, efficient bleaching agent that could not be totally substituted by a laccase-mediator stage without affecting the final bleaching of pulp. Benefits can be accrued, however, by substitution of a fraction of ClO2 through the addition of a LMS stage in the bleaching sequence of an ECF pulp mill. So far, the cost of mediator has been the limiting step to this substitution. The same cost-related problem is observed for an application in a TCF mill. On the other hand, the laccase-mediator system has the great advantage of being capable of implementation in a mill without any major change in the infrastructure of the bleaching sequence. A solution containing laccase and mediator would just have to be pumped in via a currently existing oxygen tower for example. Since the water used in a bleaching sequence is not reused but sent to the activated sludge waste system, one also must ensure that the mediator is not harmful to this biosystem. Since mediators that may be used to bleach pulp may be very stable, particularly the transition metal based complexes, sending them to the water treatment represents a waste of a chemical (and of money) that could be reused. The application of the laccase-mediator system could therefore greatly benefit from the development of a means to separate and recycle the mediator from bleaching waters. To our knowledge, no such attempts have been made to recycle the mediator.The mediated electrochemical delignification process that we describe holds great promise for application to bleaching. Further research on additional mediators, particularly those of high redox potential, high stability and that are inexpensive and non-toxic, for efficient bleaching of pulps using this approach is warranted. The major drawback to industrial acceptance of this delignification technology, however, is the requirement for capital infrastructure investment by the industry for installation of electrolysis cells, and the alteration of present bleaching processes.
Acknowledgments
Support for this research was provided by the National Science and Engineering Research Council of Canada, Enterprise Ireland, the Higher Education Authority of Ireland, the European Union and Pulp and Paper Research Institute of Canada member companies.References
- E. Adler, Wood Sci. Technol., 1977, 11, 169 Search PubMed.
- CPPA, Statistics tables from CPPA, Canadian Pulp and Paper Association, 1999.
- Alkaline Pulping, ed. M. J. KocurekT. M. Grace and E. W. Malcolm, Pulp & Paper Technical Association of Canada, Montreal, 1989 Search PubMed.
- C. W. Dence, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 125 Search PubMed.
- R. Hise, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 241 Search PubMed.
- D. W. Reeve, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 379 Search PubMed.
- T. J. McDonough, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 213 Search PubMed.
- B. vanLierop, A. Skothos and N. Liebergott, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 321 Search PubMed.
- J. R. Anderson and B. Amini, in Pulp Bleaching – Principles and Practice, ed. C. W. Dence and D. W. Reeve, TAPPI, Atlanta, 1996, p. 411 Search PubMed.
- T. K. Kirk and A. Kelman, Phytopathology, 1965, 55, 739 Search PubMed.
- I. D. Reid, M. G. Paice, C. Ho and L. Jurasek, TAPPI J., 1990, 73, 149 Search PubMed.
- T. K. Kirk and H. H. Yang, Biotechnology Lett., 1979, 1, 347 Search PubMed.
- K. Fujita, R. Kondo, K. Sakai, Y. Kashino, T. Nishida and Y. Takahara, TAPPI J., 1991, 74, 123 Search PubMed.
- T. Higuchi, in Plant Cell Wall Polymers, Vol. 399, ed. N. G. Lewis and M. G. Paice, American Chemical Society, Washington, DC, 1989, 482 Search PubMed.
- M. Tien and T. K. Kirk, Methods Enzymol., 1988, 161, 238 CAS.
- M. H. Gold and J. K. Glenn, Methods Enzymol., 1988, 161, 258 CAS.
- M. G. Paice, R. Bourbonnais, I. D. Reid, F. Archibald and L. Jurasek, J. Pulp Pap. Sci., 1995, 21, J280 Search PubMed.
- L. Viikari, M. Ranua, A. Kantelinen, J. Sundquist and M. Linko, Third International Conference on Biotechnology in the Pulp and Paper Industry, STFI, Stockholm, 1986, p. 67 Search PubMed.
- R. L. Farrell, L. Viikari and D. Senior, in Pulp Bleaching – Principles and Practice, Vol. C, ed. W. Dence and D. W. Reeve, Atlanta, 1996, p. 363 Search PubMed.
- D. J. Senior and J. Hamilton, Pulp Paper, 1992, 66, 111 Search PubMed.
- H. Yoshida, J. Chem. Soc., 1883, 43, 472 RSC.
- E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Chem. Rev., 1996, 96, 2577 CrossRef.
- B. Reinhammar, in Copper Proteins and Copper Enzymes, Vol. 3, ed. R. Lontie, Franklin Book Company, Elkins Park, PA, 1984, p. 2 Search PubMed.
- C. F. Thurston, Microbiology, 1994, 140, 19 Search PubMed.
- A. I. Yaropolov, O. V. Skorobogat′ko, S. S. Vartanov and S. V. Varfolomeyev, Appl. Biochem. Biotechnol., 1994, 49, 257–280 Search PubMed.
- K. Piontek, M. Antorini and T. Choinowski, J. Biol. Chem., 2002, 227, 37663 CrossRef.
- L. Gianfreda, F. Xu and J.-M. Bollag, Biorem. J., 1999, 3, 1 Search PubMed.
- P. J. Kersten, B. Kalyanaraman, K. E. Hammel, B. Reinhammar and T. K. Kirk, Biochem. J., 1990, 268, 474.
- R. Bourbonnais and M. G. Paice, FEBS Lett., 1990, 267, 99 CrossRef CAS.
- R. Bourbonnais and M. G. Paice, Appl. Microbiol. Biotechnol., 1992, 36, 823 CrossRef CAS.
- R. Bourbonnais and M. G. Paice, TAPPI J., 1996, 76, 199 Search PubMed.
- K. C. Li, R. F. Helm and K. E. L. Eriksson, Biotechnol. Appl. Biochem., 1998, 27, 239 Search PubMed.
- K. C. Li, F. Xu and K. E. L. Eriksson, Appl. Environ. Microbiol., 1999, 65, 2654 CAS.
- R. Bourbonnais, D. Rochefort, M. Paice, S. Renaud and D. Leech, TAPPI J., 2000, 83, 68 Search PubMed.
- R. Bourbonnais, D. Rochefort, M. G. Paice, S. Renaud and D. Leech, in Oxidative Delignification Chemistry. Fundamentals and Catalysis, Vol. 785, American Chemical Society, Washington, DC, 2001, 391 Search PubMed.
- R. Bourbonnais, D. Leech and M. G. Paice, Biochim. Biophys. Acta, 1998, 1379, 381 CrossRef CAS.
- D. Rochefort, R. Bourbonnais, D. Leech, S. Renaud and M. Paice, J. Electrochem. Soc., 2002, 149, D15 CrossRef CAS.
- F. Xu, J. J. Kulys, K. Duke, K. C. Li, K. Krikstopaitis, H. J. W. Deussen, E. Abbate, V. Galinyte and P. Schneider, Appl. Environ. Microbiol., 2000, 66, 2052 CrossRef CAS.
- F. Xu, H. J. W. Deussen, B. Lopez, L. Lam and K. C. Li, Eur. J. Biochem., 2001, 268, 4169 CrossRef CAS.
- J.-E. Levlin and L. Söderhjelm, in Pulp and Paper Testing, ed. J. Gullichsen and H. Paulapuro, Vol. 17, Fapet Oy, Helsinki, Finland, 1999 Search PubMed.
- H. P. Call and I. Mucke, J. Biotechnol., 1997, 53, 163 CrossRef CAS.
- M. Fabbrini, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2002, 16, 231 CrossRef CAS.
- M. Fabbrini, C. Galli, P. Gentili and D. Macchitella, Tetrahedron Lett., 2001, 42, 7551 CrossRef CAS.
- A. Muheim, A. Fiechter, P. J. Harvey and H. E. Shoemaker, Holzforschung, 1992, 46, 121 Search PubMed.
- A. Potthast, T. Rosenau, C.-L. Chen and J. S. Gratzl, J. Org. Chem., 1995, 60, 4320 CrossRef CAS.
- M. Y. Balakshin, C. L. Chen, J. S. Gratzl, A. G. Kirkman and H. Jakob, Holzforschung, 2000, 54, 165 Search PubMed.
- M. Y. Balakshin, C. L. Chen, J. S. Gratzl, A. G. Kirkman and H. Jakob, Holzforschung, 2000, 54, 171 Search PubMed.
- J. Gierer, Holzforschung, 1997, 51, 34 Search PubMed.
- M. Fabbrini, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2002, 18, 169 CrossRef CAS.
- D. Rochefort, R. Bourbonnais, D. Leech and M. G. Paice, Chem. Commun., 2002, 11, 1182 RSC.
- F. Peter, J. Polcin and W. H. Rapson, Pulp Pap. Mag. Can., 1973, 74, 89 Search PubMed.
- J. J. Lindberg, K. Penttinen and F. Sundholm, Pap. Puu, 1969, 11, 823 Search PubMed.
- D. Limosin, G. Pierre and G. Cauquis, Holzforshung, 1985, 39, 91 Search PubMed.
- E. Evstigneyev, H. Maiyorova and A. Platonov, J. Wood Chem. Technol., 1999, 19, 379 Search PubMed.
- P. Zuman, Substituent Effects in Organic Polarography, Kluwer Academic, New York, 1967, 384 Search PubMed.
- A. E. G. Cass, G. Davis, G. D. Francis, H. A. O. Hill, W. J. Aston, I. J. Higgins, E. V. Plotkin, L. D. L. Scott and A. P. F. Turner, Anal. Chem., 1984, 56, 667 CrossRef CAS.
- R. S. Nicholson and I. Shain, Anal. Chem., 1964, 36, 706 CrossRef CAS.
- R. Bourbonnais, M. G. Paice, B. Freiermuth, E. Bodie and S. Borneman, Appl. Environ. Microbiol., 1997, 63, 4627 CAS.
- D. M. Dooley, J. Rawlings, J. H. Dawson, P. J. Stephens, L.-E. Andréasson, B. G. Malmström and H. B. Gray, J. Am. Chem. Soc., 1979, 101, 5038 CrossRef CAS.
- R. E. Childs and W. G. Bardsley, Biochem. J., 1975, 145, 93 CAS.
- F. Xu, J. Biol. Chem., 1997, 272, 924 CAS.
- F. Xu, R. M. Berka, J. A. Wahleithner, B. A. Nelson, J. R. Shuster, S. H. Brown, A. E. Palmer and E. I. Solomon, Biochem. J., 1998, 334, 63 CAS.
- R. Bourbonnais and M. G. Paice, 11th International Symposium on Wood and Pulping Chemistry, Nice, France, 2001, p. 371 Search PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical methods, John Wiley & Sons, Inc., Hoboken, NJ, 1980 Search PubMed.
- C. Padtberg, H. C. Kim, M. Mickel, S. Bartling and N. Hampp, TAPPI J., 2001, 84 Search PubMed.
- H. C. Kim, M. Mickel, S. Bartling and N. Hampp, Electrochim. Acta, 2001, 47, 799 CrossRef CAS.
- H. E. Schoemaker, Rec. Trav. Chim. Pays-Bas, 1990, 109, 255 Search PubMed.
- C. Oloman, Electrochemical Processing for the Pulp & Paper Industry, The Electrochemical Consultancy, Electrosynthesis Company, Inc., Lancaster, NY, 1996 Search PubMed.
- Q. Y. Hu, M. M. Sain and C. Daneault, Pap. Puu, 1999, 81, 63 Search PubMed.
- Y. S. Perng and C. W. Oloman, TAPPI J., 1994, 77, 115 Search PubMed.
- E. M. Belgsir, A. P. Bettencourt, A. M. Carvalho and P. Parpot, Port. Electrochim. Acta, 1997, 15, 413 Search PubMed.
- M. P. Godsay, M. N. Hull and V. M. Yasnovsky, TAPPI Pulping Conference, Atlanta, 1998, p. 731 Search PubMed.
- S. Varennes, C. Daneault and M. Levesque, Appita, 1994, 47, 45 Search PubMed.
- F. S. Archibald, R. Bourbonnais, L. Jurasek, M. G. Paice and I. D. Reid, J. Biotechnol., 1997, 53, 215 CrossRef CAS.
- R. Bourbonnais, M. G. Paice and I. D. Reid, in Biotechnology in Pulp and Paper Industry, Vol. 1, ed. M. Kuwahara and M. Shimada, UNI Publishers, Tokyo, 1992, 181 Search PubMed.
- R. Bourbonnais, M. G. Paice, D. Leech and B. Freiermuth, TAPPI Proc. TAPPI Biol. Sci. Symp., San Francisco, 1997, p. 335 Search PubMed.
- C. L. Chen, A. Potthast, T. Rosenau, J. S. Gratzl, A. G. Kirkman, D. Nagai and T. Miyakoshi, J. Mol. Catal. B: Enzym., 2000, 8, 213 CrossRef CAS.
- A. A. Leontievsky, N. M. Myasoedova, B. P. Baskunov, N. N. Pozdnyakova, T. Vares, N. Kalkkinen, A. I. Hatakka and L. A. Golovleva, Biochemistry (Moscow), 1999, 64, 1150 CAS.
- A. Majcherczyk, C. Johannes and A. Hüttermann, Appl. Microbiol. Biotechnol., 1999, 51, 267 CrossRef CAS.
- A. Potthast, T. Rosenau, H. Koch and K. Fischer, Holzforschung, 1999, 53, 175 Search PubMed.
- F. S. Chakar and A. J. Ragauskas, TAPPI Pulping Conference, Atlanta, 1998, p. 109 Search PubMed.
- R. P. Chandra, R. P. Beatson, E. de Jong and J. N. Saddler, J. Wood Chem. Technol., 1999, 19, 61 Search PubMed.
- C. Crestini, Abstr. Pap. Am. Chem. Soc., 2000, 219, U280 Search PubMed.
- K. Poppius-Levlin, M. Wang, T. Tamminen, B. Hortling, L. Viikari and M. L. Niku-Paavola, J. Pulp Pap. Sci., 1999, 25, 90 Search PubMed.
- J. Sealey and A. J. Ragauskas, J. Wood Chem. Technol., 1998, 18, 403 Search PubMed.
- J. Sealey, A. J. Ragauskas and T. J. Elder, Holzforschung, 1999, 53, 498 Search PubMed.
- S. Kawai, M. Asukai, N. Ohya, K. Okita, T. Ito and H. Ohashi, FEMS Microbiol. Lett., 1999, 170, 51 CrossRef CAS.
- S. Kawai, M. Nakagawa and H. Ohashi, FEBS Lett., 1999, 446, 355 CrossRef CAS.
- E. Srebotnik and K. E. Hammel, J. Biotechnol., 2000, 81, 179 CrossRef CAS.
- H. P. Call, PCT World patent application No. WO 94/29510, 1994.
- F. S. Chakar and A. J. Ragauskas, J. Wood Chem. Technol., 2000, 20, 169 Search PubMed.
- F. S. Chakar and A. J. Ragauskas, Holzforschung, 2000, 54, 647 Search PubMed.
- K. J. Poppius-Levlin, T. L. Tamminen and A. Kalliola, Abstr. Pap. Am. Chem. Soc., 2000, 219, U280.
- H. P. Call, PCT World patent application No. WO 97/36041, 1997.
| This journal is © The Royal Society of Chemistry 2004 |
