An undergraduate teaching exercise that explores contemporary issues in the manufacture of titanium dioxide on the industrial scale
Steven
Grant
a,
Andrew A.
Freer
a,
John M.
Winfield
a,
Craig
Gray
b,
Tina L.
Overton
c and
David
Lennon
*a
aDepartment of Chemistry, Joseph Black Building, The University of Glasgow, Glasgow, UK G12 8QQ
bTeaching and Learning Service, Florentine House, 53 Hillhead Street, University of Glasgow, Glasgow, UK G12 8QQ
cDepartment of Chemistry, University of Hull, Cottingham Road, Kingston upon Hull, UK HU16 7RX
First published on 10th December 2003
Abstract
A teaching exercise is described that aims to expose undergraduate chemistry students to the type and range of complex and interacting issues that a chemist active in an industrial organisation might typically encounter. The business area selected is the titanium dioxide pigment manufacturing sector, that is meant to be representative of large-scale chemical manufacturing. Although the students are exposed to a range of chemical topics, they are also expected to understand some of the associated economic, environmental and legislative drivers that, inevitably, impinge on the business operations. Indirectly, the exercise aims to make the students aware of the concepts of sustainable development, as applied to the chemicals industry.
The students are asked to engage in a mature discussion as to whether a new titanium dioxide manufacturing facility should be constructed within their area. The background information supplied to the students is based around a real situation that existed in 2001. Evaluation of the exercise by the students indicates a definite positive response and suggests that such teaching packages can play a positive role in demonstrating the real issues related to enhanced environmental awareness within the chemicals industry.
1 Introduction
The Department of Chemistry at the University of Glasgow has recently applied the concepts of problem based learning1–3 to produce new teaching materials for its 2nd year (≡ 1st year in England, Wales and Northern Ireland) undergraduate programme. Two new packages have been produced, that are designed to increase student awareness of the modern chemicals industry. These packages have been termed interactive teaching units (ITUs)4 and address issues relevant to the industrial scale manufacture of (i) refrigerants (ITU 1 ‘The Age of Refrigeration’) and (ii) chlorine (ITU 2 ‘Mercury, Membrane or Diaphragm’). The background, approach adopted and an evaluation of these units are described elsewhere.4 In October 2001 it was decided to produce a new ITU in order to broaden the range of chemistry covered within the ITU format and the purpose of this communication is to describe the background, methodology, content and effectiveness of the new material. It is hoped that, through a wider appreciation of modern industrial chemistry, our students will have a better understanding of the range of topics they are likely to encounter as chemists operating in the industrial sector.The topic selected for the new unit (ITU 3) was the titanium dioxide industry. The main reason for this selection was because there already existed a problem based learning exercise on this sector that we felt could be extended and adapted to fit our current ITU format. In 1998 researchers at the University of Hull produced a comprehensive package that required undergraduate students, firstly, to understand the background of the modern titanium dioxide industry, and then to consider issues relevant to the successful and continued operation of an existing titanium dioxide manufacturing facility. That work, ‘The Titanium Dioxide Project’ was produced as a result of the Project Improve initiative and was circulated widely amongst the UK chemistry higher education sector.5 Unfortunately, the structure of the Hull documentation was not readily transferable to the Glasgow ITU format, which is based on students working through specific tasks in small groups and giving several oral presentations within a designated three hour period. Consequently, we decided to retain the basic theme of ‘The Titanium Dioxide Project’5 but to adapt it and extend it to fit more closely our educational requirements. Specifically, we wished to broaden and increase the number of issues that the students should be aware of when evaluating the large-scale production of commodity chemicals.
A major aim of our new educational package was to expose the students to the general concepts of sustainable development6–9 and to create a scenario where the importance of chemistry, economics, the distribution of the world market, environmental issues, legal requirements and politics could be discussed amongst the student body. These themes commonly come under the banner of Green Chemistry.10,11 Recent literature had applied the tools of Life Cycle Analysis12 to the titanium dioxide industry and so we included this important means of evaluating sustainability in the course content. Additionally, it was deemed important to ensure that the new unit had a direct connection to material presented within the traditional lecture-based courses of the 2nd year chemistry programme. This ensures that the students do not see ITU 3 as being something separate from their core studies but rather as a supplement to material they encounter in lectures. Structure/property relationships of metal oxides feature prominently in the solid state chemistry course undertaken by our 2nd year undergraduates and so an exploration of the different structural phases of TiO2 were included within the exercise.
2 Aims
The following specific aims were identified for ITU 3:(1) To develop a broad understanding of the range of complex issues arising in large-scale chemical manufacturing, using the TiO2 industry as a model.
(2) To develop an understanding of relevant sections of the undergraduate chemistry curriculum, using an applications-led approach.
(3) To promote the need for sustainable development6–9 and, in addition, demonstrate the negative environmental, social and economic consequences of non-compliance with this ideal.
3 Methodology and material selection
Having established the existence of a wealth of diverse information pertaining to the TiO2 industry, the next task was to select material conducive to the aims outlined above. As a consequence, the unit was developed with three main areas of focus in mind.Firstly, ITU 3 considers technical issues relating to the current TiO2 manufacturing alternatives. It was felt that the level of chemical complexity embodied within these processes offered a significant challenge in understanding for the students. Furthermore, the manufacturing options produce different crystal forms of TiO2 pigment with distinct physical-chemical properties. Accordingly, the new unit seeks to emphasise the ‘structure/properties/applications’ relationship fundamental to the comprehension of solid state chemistry.
Secondly, the volatile economics of TiO2 production over the last two decades mirrors the situation, past and present, affecting many other sectors of the bulk chemicals industry. The norm is that most students progressing to a career in industry will have had little or no exposure to such issues. In attempting to redress this imbalance, the unit focuses on two crucial elements disturbing the equilibrium of TiO2 economics: (1) capacity/demand ratios and, (2) growth levels in TiO2 markets across the major world regions.
Thirdly, consideration is given to environmental issues associated with TiO2 manufacture. With the push towards sustainable development continuing to shape the future of the modern chemicals industry, students need to understand this concept. Increasingly restrictive and, crucially, costly EU legislation13 covering effluent emissions from the TiO2 industry has made the move towards practising sustainable chemistry a top priority for most TiO2 manufacturers. ITU 3, therefore, looks at aspects of a contemporary industry's attempts to embrace sustainable development. However, past failures to do so are also highlighted in the unit, demonstrating the negative environmental, social and economic ramifications of non-compliance.
In the essential component of ITU 3, students are asked to consider the process by which a major global TiO2 producer decides where to locate its new manufacturing facility. The perspective of a major manufacturer, crucial to the debate, was gained from information available on the website of a global TiO2 company with operations in the UK. Titox Plc is a fictitious company, but some of the business scenarios used are related to those of Huntsman Tioxide.14 Also important to the discussion is a perspective on local economics, which was supplied by a major regional development agency, Scottish Development International.15
4 Course content
ITU 3 is composed of six separate sections, each of which is outlined in Fig. 1. The class size for each ITU session typically comprises 45 students, which during Sections B–E are sub-divided into 5 tutorial groups of 9 students, with each tutorial group operating independently in separate locations. An academic member of staff is assigned to each tutorial group, who are further required to form small sub-groups (sub-groups 1–3) to work together through a series of exercises. Sections B, C and D require each member of the sub-group to make a short oral presentation to the tutorial group and the tutor, who manages the whole exercise. Section E requires the group of 9 students to work together to consider the issues on whether or not a new TiO2 manufacturing facility should be located in Scotland. Section F reviews the conclusions arrived at by the 5 tutorial groups and finally the lead tutor summarises the current situation in the U.K. and abroad.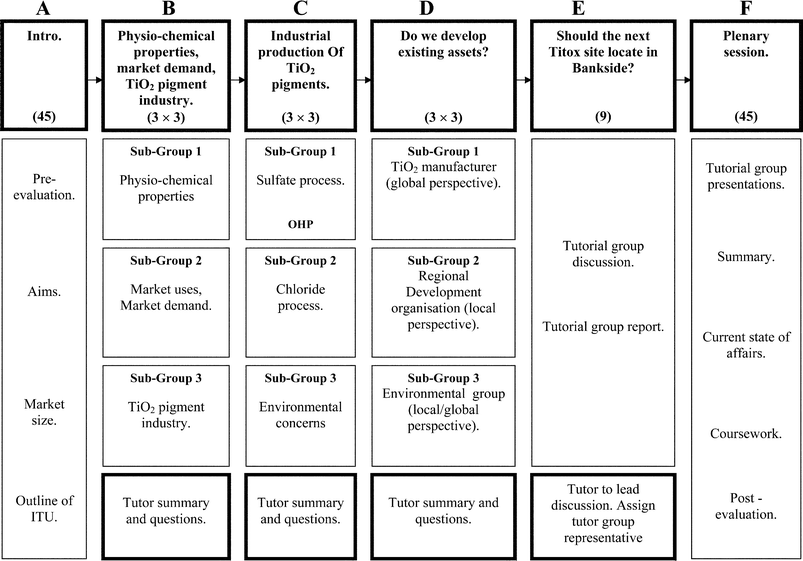 | ||
| Fig. 1 Structure plan for ITU 3. The bold letters indicate the six sections described in the text. The numbers in parentheses represent the number of students participating in each section. | ||
Thus, with reference to Fig. 1, the unit operates as an exercise in parallel learning, with each sub-group of 3 students educating their tutorial group of 9 students with distinctly different aspects of TiO2 production. This parallel format contrasts with that adopted in our first two ITUs,4 which essentially took a linear approach to raising and solving problems. Having 3 groups of students working on 3 different, but related, problems at the same time means that more material can be covered in a fixed time period and, ultimately, the topic can be explored to a greater degree than that achievable by an essentially linear approach.
A description of the individual Sections of the ITU now follows:
Section A. Introduction to ITU
The unit introduction begins by describing briefly essential properties of titanium metal. The ITU class learns of the metal's excellent strength to weight ratio and high resistance to corrosion, giving it applications in industries such as aerospace, sport and medicine, etc.16 The students are then made aware that the majority of titanium is not used in its elemental form but rather as the oxide, titanium dioxide (TiO2), which currently accounts for 96% of titanium consumption worldwide.17Fig. 2 presents the extent of TiO2 production since its humble beginnings in 1918.5 The significance of the scale of the TiO2 market is conveyed by informing the students that TiO2 is one of the leading inorganic compounds in production today.18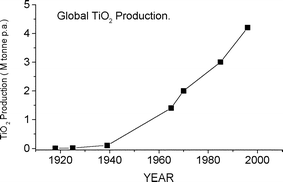 | ||
| Fig. 2 Global TiO2 production from the beginning of large-scale manufacture in 1918, through to the present day (1996).5 | ||
Section B. Physical-chemical properties and market demand
Logistics for Section B see the ITU class divided into tutorial groups containing a maximum of 9 students. Tutorial groups are then further divided into three sub-groups to perform exercises on (i) TiO2 physical-chemical properties, (ii) product demand and (iii) market share respectively. The sub-groups are required to digest a range of technical information relating to these topics then to give short oral presentations based around an overhead projector to the 9 students plus tutor.The first sub-group learns that lack of colour, chemical inertness and, most importantly, a high refractive index (conferring high opacity) are the three main desirable properties allotting TiO2 an increasingly dominant share of the white pigment market.18 They next examine the unit cells of rutile and anatase, the two commercially viable forms of TiO2, illustrated in Figs. 3 and 4 respectively.5,19 It is clear from comparison of the two unit cells that the rutile form is a more closely packed structure than that of anatase. An important consequence of the structural modifications is the extent to which UV light is absorbed. From the UV-visible absorption spectra presented in Fig. 5, the students can conclude that rutile TiO2 absorbs UV radiation over a greater wavelength range than the anatase form.20,21 This, coupled with a lower photocatalytic activity,21 makes rutile TiO2 the pigment of choice for applications susceptible to UV damage, for example automotive top-coatings. Brief mention is also made of other comparatively distinct properties arising between the rutile and anatase structures including density, refractive index, stability and abrasion.5
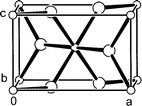 | ||
| Fig. 3 The contents of the rutile unit cell with a = b = 4.59, c = 2.96 Å viewed approximately along the b-axis. The smaller and larger spheres respectively represent Ti and O atoms. | ||
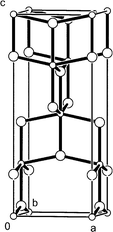 | ||
| Fig. 4 The contents of the anatase unit cell with a = b = 3.78, c = 9.52 Å viewed approximately along the b-axis. The smaller and larger spheres respectively represent Ti and O atoms. | ||
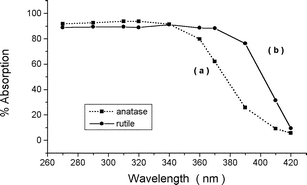 | ||
| Fig. 5 Absorption of UV-visible irradiation by (a) anatase and (b) rutile forms of titanium dioxide. The figure is re-drawn, with permission from the author and the Vincentz Network, from reference 21. | ||
The second sub-group examines the demand for TiO2 pigments. The information points to TiO2 accounting for almost two-thirds of the overall inorganic pigment market (1995) and, more specifically, a three-quarters share of the white pigment market (1996).18 Next, the major industrial consumers of TiO2 pigments in 1996 are shown to be paints and coatings, plastics and paper, accounting for 57%, 20% and 10% of world demand, respectively.22 The remaining demand is attributed to a number of other minor consumers. Sub-group 2 finally moves on to examine indicators for global TiO2 demand. They are made aware that the vast majority of products pigmented with TiO2 are quality of life items, examples being car paints, PVC windows/doors, glossy magazines, etc. As such, world gross domestic product (GDP), a broad measure of global living standards, correlates closely with world TiO2 demand, as illustrated by Fig. 6 for the period between 1980–1996.5 The sudden slump seen in global GDP between 1989–1991 serves to highlight the volatile nature of the TiO2 industry. The students learn of the excessively high capacity to demand ratio around this time, which subsequently left manufacturers with a product reduced in value. A further indicator of TiO2 demand alluded to, is the industrial operating rate,23i.e. the percentage of production capacity in use at any given time. Although affected by GDP, other factors such as customer de-stocking and competition between manufacturers, also contribute.
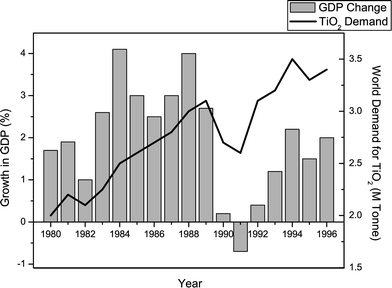 | ||
| Fig. 6 World TiO2 demand and global gross domestic product between 1980 and 1996. The figure is adapted from reference 5 with permission from the University of Hull. | ||
The third sub-group focuses on the distribution of market share within the TiO2 industry. Initially, they are informed of the world production capacities and geographical capacity distributions for the top four global TiO2 manufacturers (mid-1996). Next, growth rates in world regional markets are considered. The major finding is that the Asia/Pacific region has increased its world market share, a fact borne out by Fig. 7, which shows a near 9% rise for the period between 1981–1995.23 By contrast, the data also illustrate the decrease in world share for the remaining regions during the same period. The sub-group finds out that North America and Western Europe have suffered from maturing markets, whilst the decline in the rest of the world is tentatively attributed to political instability and civil unrest.
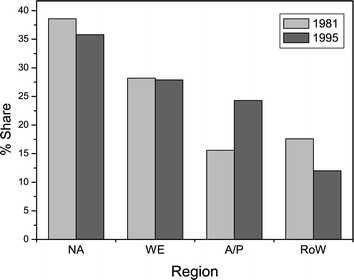 | ||
| Fig. 7 The geographical world share of the TiO2 market for 1981 and 1995 between the following major world regions: North America (NA), Western Europe (WE), Asia/Pacific (AP) and Rest of world (RoW). The figure is adapted from reference 23 with permission from the Oil and Colour Chemists Association. | ||
On completion of their set tasks, each sub-group elects a spokesperson to give an oral summary of the material they have covered. The aim is to provide the whole tutorial group with a collective knowledge of the topics discussed.
Section C. Industrial production
Remaining in their sub-groups, students move on to consider the industrial production of TiO2 pigments. Two sub-groups focus on the two TiO2 manufacturing alternatives, the Sulfate Process or the Chloride Process. The remaining sub-group is concerned with environmental issues associated with TiO2 manufacture.Before examining the various stages of their respective processes, the first two sub-groups discuss the titanium-containing raw starting materials used in each.18,23 The sulfate process utilises ilmenite (FeTiO3) or its derivative Ti slag, with maximum TiO2 contents of around 60% and 85% respectively. Naturally occurring rutile or synthetic rutile, the latter again derived from ilmenite, is used in the chloride processing option. Although both these satisfy a desirable TiO2 content, many manufacturers are reluctantly using Ti slag, as deposits of natural rutile become increasingly scarce. For both manufacturing processes, students are made aware of the inverse relationship existing between TiO2 content and the contribution to the waste stream. The main steps of each process are then described in some detail.5,18,24,25 Using this information, both sub-groups are required to complete an industrial flow diagram summarising their particular process. Completed flow diagrams, outlining the various stages pertinent to the sulfate and chloride manufacturing options, are shown in Figs. 8 and 9 respectively. Linkage between the chemical equations and specific stages of the chemical flow sheet are emphasised by the tutor when questioning the groups' oral presentations. Finally, both sub-groups look at a number of comparative advantages and disadvantages existing between the TiO2 manufacturing alternatives.5,23,26 For example, the newer chloride process is limited to the production of rutile TiO2 pigment. The older sulfate process can, however, produce both the anatase and rutile TiO2 forms, depending on the nature of seed crystals introduced during hydrolysis (see Fig. 8). Consequently, the latter process has exclusivity in supplying markets demanding anatase TiO2, such as fine quality paper and ceramics. On the other hand, rutile TiO2 produced by the chloride process is free from anatase contamination and is used in a wide range of applications including normal quality paper, paper laminates, plastics, paints, etc., in fact anything other than fibres, food, cosmetics and most inks. Also brought to the attention of the students are the favourable production economics of the continuous chloride technology as opposed to the batch methodology necessary in the sulfate process.
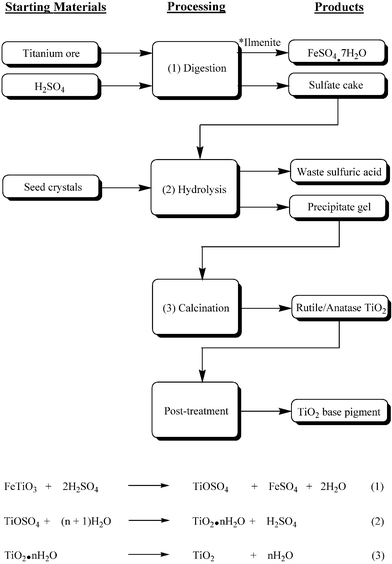 | ||
| Fig. 8 Industrial flow diagram representing the various stages of the Sulfate Process used for the manufacture of rutile and anatase TiO2 base pigments. Reaction equations, where relevant, are shown for the processing steps. * The use of ilmenite as feedstock produces a significant quantity of iron sulfate heptahydrate by-product. The figure is adapted from reference 18 with permission from Wiley-VCH. | ||
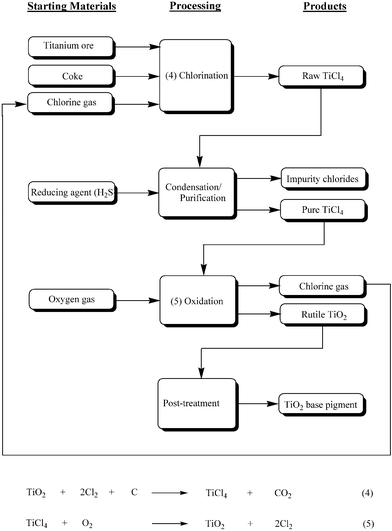 | ||
| Fig. 9 Industrial flow diagram representing the various stages of the Chloride Process used for the manufacture of rutile TiO2 base pigments. Reaction equations, where relevant, are shown for the processing steps. The figure is adapted from reference 18 with permission from Wiley-VCH. | ||
The remaining sub-group is first made aware of the 1978 EU environmental legislation (and later amendments) imposed on the TiO2 industry.13 This was in response to the high volume of wastes, (solid, liquid and gaseous) arising from production, especially through the sulfate processing option. Sulfate technology is becoming increasingly unsustainable, owing to the spiralling cost of compliance with the EU directives in place.26 The sulfate route is shown to generate up to 8 tonnes of dilute sulfuric acid and 0.6–2.7 tonnes of iron sulfate, whilst the chloride process gives rise to 0.4–0.9 tonnes of mainly impurity metal chlorides, for every tonne of TiO2 produced.23 The nature and quantity of these wastes obviously depends on the titanium feedstock utilised in each process. Some of the commonly encountered by-product recycling/disposal options are also discussed.18,23 Waste acid can be re-concentrated for reuse in the process or neutralised with limestone to form gypsum, which is land-filled or marketed for use in products such as plasterboard and cement. The iron sulfate heptahydrate can be converted to iron(III) sulfate, where it is used in the water treatment industry.18 Metal impurity chlorides from the chloride process are mostly land-filled12 or, alternatively, after processing, can be used for water treatment.18 Lastly, the sub-group considers Life Cycle Assessment (LCA),12 a component of many contemporary TiO2 manufacturers' environmental strategy. In general LCA is a “cradle to grave” (extraction of raw materials, manufacture, use and eventual disposal) analysis of the environmental burdens associated with a particular product, in this case TiO2. Students then examine actual TiO2 industry LCA results for a sample of five distinct processing options described in Table 1.12 The results are based on energy consumption and waste. Fig. 10, for example, compares the liquid (acid and trace metals) discharges to water for the five alternatives. In particular, comparison between options A and B, both sulfate processes utilising ilmenite (see Table 1), demonstrates the dramatic positive effect of EU legislation in reducing effluent emanating from the industry. Caution, however, is advised against viewing LCA as an exact science, given that location specific factors such as sulfuric acid and chlorine availability, type of titanium feedstock, community environmental awareness and by-product outlets are all determinants.12 LCA is, therefore, only useful as part of a comprehensive environmental strategy. As for the other two sub-groups, oral presentations are used as the medium through which relevant information is distributed among the tutorial group as a whole.
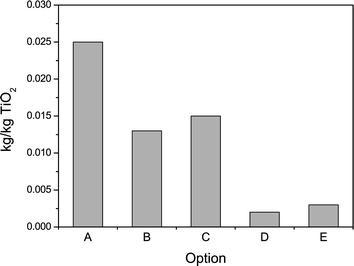 | ||
| Fig. 10 Liquid (waste acid and trace metals) discharges to water for five distinct TiO2 pigment processing options as outlined in Table 1. The figure is adapted from reference 12 with permission from the Oil and Colour Chemists Association. | ||
| Option | Feedstock | Process | Waste Treatment |
|---|---|---|---|
| A | Ilmenite | Sulfate | None |
| B | Ilmenite | Sulfate | Neutralisation to EU standard |
| C | Slag | Sulfate | Acid reconcentration |
| D | Slag | Chloride | Neutralisation |
| E | Synthetic Rutile | Chloride | Neutralisation |
Section C therefore aims to expose the tutorial group to the complexity of the industrial scale TiO2 manufacturing routes and the fact that different processes exhibit different attributes. Selection of any specific process is rarely a trivial matter. The environmental issues of operating such processes is considered by sub-group 3, who initially concentrate on the waste products and then examine the concept of LCA applied to both processes operating under specified conditions.
Section D. New production facilities?
Furnished with a broad insight into contemporary TiO2 manufacturing, the tutorial group is next confronted with a problem-based scenario. The tutorial group learns that the fictional manufacturing company, Titox Plc, intends to increase its global capacity by bringing on line a new 50,000 tonne per annum chloride processing plant, the location of the new site being either in Scotland or China. This is an example close to a real industrial case27 but with Scotland selected as the UK option to increase the significance to our students. Based on a range of important criteria (availability of chlorine, ease of imports/exports, energy costs, etc.) the UK site was initially favoured. The company, though, has since admitted that further penetration into the rapidly expanding Asia/Pacific market, particularly in China, remains very attractive. Students then find out that the proposed UK site is adjacent to Bankside, a fictitious town that is in serious economic decline, due to the recent demise of surrounding industry, particularly shipyards. Regrettably, this scenario has been common within regions of the west of Scotland and appears credible to the majority of our students. The provision of high value jobs associated with TiO2 manufacturing, via the Titox Plc investment, would undoubtedly improve the economic situation in the area. As such, Scottish Development International (SDI), a regional development agency, are very keen to attract the investment. SDI is associated with Scottish Enterprise, our local Regional Development Agency, which operates under the auspices of the Scottish Executive.15 There is, however, strong opposition to the site in the form of a fictional major UK environmental group, Greenwatch. To discuss the issues pertinent to the siting of the new facilities, the three previously established sub-groups adopt the respective positions of Titox Plc, SDI and Greenwatch.This arrangement, where sub-groups engage in a degree of role play, leads to an informed discussion within the tutorial group ultimately to approach a significant, technically-based decision. It is an important part of the ITU construction. Experience with the previous 2 ITU exercises4 has shown that the discussions will be active and informed only if the students have a reasonable technical exposure to ‘their’ topics, and that their interest levels/connectivity has been built up through prior exercises.
In their capacity as board members of Titox Plc, the first sub-group finds out that the initial leaning towards the UK site has been heavily criticised. Superior market growth, cheaper labour costs and potentially relaxed environmental legislation are all cited as reasons by major investors as to why the company should locate in China. It is suggested, however, that the UK boasts an, historically, more stable and robust economy than that for the Asia/Pacific region. Moreover, the company has a well-established technical base in the UK, making installation, manufacture and maintenance at the UK site altogether easier tasks. Also concerning investors, is the negative publicity surrounding the proposed new investment thanks to the involvement of Greenwatch. In the Company's defence, the sub-group discuss and present results that outline recent positive strides taken in significantly reducing virtually all the environmental burdens arising from Titox Plc's operations.28 The tutor draws the students' attention to the fact that the constraints on global warming performance are limited by Equations (4) and (5) in Fig. 9, where every mole of TiO2 results in production of 1 mole of CO2, a potent greenhouse gas.29
The second sub-group, representing Greenwatch, are first reminded that, whilst the burdens associated with the sulfate route are of global concern, Titox Plc intend using the more sustainable chloride technology at their new facility. Concerns are nevertheless raised over two recent incidents of non-compliance, within the space of two years, at the Company's UK Deeside plant. Firstly, hazardous titanium tetrachloride was allowed to mix with a quantity of cooling water, sending a dense hydrochloric acid mist into the atmosphere. Evasive action had to be taken by residents in the area and an adjoining road was closed. The Environment Agency (EA) served the plant with a prohibition notice, which remained enforced for five weeks. Students are subsequently made aware of the second incident, where 8,000 tonnes of effluent, containing 37 tonnes of concentrated hydrochloric acid escaped onto protected marshland nearby. Affects to wildlife on the marsh was a subject of fierce debate, nonetheless, a court action brought against the company by the EA resulted in heavy fines. All the events outlined above are factual and have been reported elsewhere,30,31 and are associated with the UK operations of a major TiO2 manufacturer. The organisation actually associated with these incidents was Tioxide Europe Limited, although that fact is not revealed to the students. Tioxide Europe Limited was at that time part of the ICI Group of companies and is now under the ownership of the Huntsman Corporation.14Titox Plc is a fictitious organisation that is used as a convenient vehicle to represent milestones in the recent history of the UK titanium dioxide industry.
The remaining sub-group, role-playing SDI, has the task of conveying the benefits of locating in Scotland.15 Recognition is given to the significant number of chemical companies already operating successfully in Scotland. Next, the students look at the wide range of financial incentives available for inward investment, which include Property Support and Regional Selective Assistance grants.15 Scotland, in addition, boasts excellent transport and communication facilities, essential to the functioning of contemporary industry. Emphasis is also placed on the strong links existing between the chemical industry and educational institutions in Scotland, ultimately aiding the recruitment process for specialist positions. The suggestion is that, allied with moderate wage expenditures, inward investors would benefit from a highly trained, cost effective and flexible Scottish workforce.
Section E. Should Titox Plc locate in Bankside?
After giving oral presentations to demonstrate their initial viewpoints, the sub-groups amalgamate for a tutor-led discussion and debate on the issue of whether Titox Plc should locate in Scotland or, alternatively, China. Students who are against location in Scotland are asked to consider the implications of such a decision for the local and national economy. They must be made aware of the long-term and high value jobs a modern chemical plant, with ancillary research and development facilities, offers to a region. With respect to the environmental concerns, the attitude of many students may be ‘not in my back yard’. However, the demand for TiO2 is such that production will undoubtedly continue, if not in the UK, then elsewhere in the world. Assuming a global perspective, it is likely that environmental burdens would be better managed in areas of the planet (i.e. the UK) with the infrastructure in place to deal effectively with such problems. It is also possible that the group discussion can swing strongly in favour of a purely environmental perspective, and reject the premise that new TiO2 plants should be constructed anywhere. The tutors are instructed to tolerate any perspective from their groups, as long as the students can reasonably defend the stance adopted.Section F. Plenary session
The three-hour ITU concludes with a plenary session involving the whole class and led by a senior tutor. Each tutorial group elects a spokesperson to deliver its conclusions on the location of the new TiO2 facility. As regards the actual scenario at the time the ITU was constructed,27 the class are informed that no decision has yet been reached on whether to locate in the UK or China. However, a senior company executive has been quoted as saying “Its not if but when” in relation to developing in China.32 Finally, the students are provided with some documentation demonstrating that TiO2 has uses other than just as a pigment. A brief review of photocatalysis reminds the students of material encountered within their solid state chemistry lectures. They are then given short briefing notes on aspects of water treatment,33 air purification34 and solar energy.35 This brief and broad overview is meant to demonstrate the wide range of applications to which this important and interesting chemical compound can be utilised.5 Assessment
Experience has shown that a post-ITU assessment requirement, that carries a substantial contribution to the overall class mark, enhances student participation in the whole ITU exercise. The ITU format forces the students to work in small groups, give modest oral presentations and to work through technically-based issues with some recognition of the associated economic, environmental and/or political issues. To further supplement their communication skills, the students are then asked to prepare a short technical report that is connected to the overall ITU. For example, a representative assignment would require the students to provide a brief development plan that identifies the main issues for a chemical manufacturer considering entering the TiO2 pigment manufacturing business. The students would be expected to cover the following points: (i) scale of TiO2 demand, (ii) structural aspects of TiO2 crystals, (iii) the chemical manufacturing process, (iv) customer base and (v) how LCA can be applied to evaluate the environmental burden of the process selected. Full ITU documentation is accessible to the students via the Chemistry Branch Library. Written communication skills are an essential prerequisite for a successful chemist and the students, generally, rise to the occasion to produce well-rounded reports.6 Student evaluation
To gauge the effectiveness of this teaching initiative, a pre/post evaluation exercise was implemented for ITU 3. Representative results from student evaluation of ITU 3 in 2001 are shown in Table 2. Prior to the unit, less than half of the ITU class felt they could attain job satisfaction working in the chemical industry. However, after exposure to the dynamics of the TiO2 industry, this increased to over two-thirds of the student group. The result suggests that a significant proportion of the class became more aware and also, more receptive to the rewards of working in the industry. Also interesting was the significant polarising effect the unit had on student attitudes towards environmental performance within the TiO2 industry. A significant percentage of student opinion, unsure of whether or not the industry deserved criticism over its environmental performance, was seen to diverge equally towards agreement and disagreement after completing the unit. It could be argued that the result reflects the particular viewpoints adopted by the different sub-groups. However, it is just as likely that the high level of interaction and debate between the groups provided students with the opportunity to make an informed decision. ITU 3 ultimately aims to present the facts, with no bias intended, in relation to TiO2 manufacture and the environment. An overwhelming majority of students also registered enjoyment in participating in the unit. This is all the more encouraging in view of the demand on every student to give an oral presentation at some stage during the exercise.| Sample Evaluation Questions | Response | |||||||
|---|---|---|---|---|---|---|---|---|
| Do you think working within the chemical industry would provide you with a varied and stimulating career? | Strongly disagree | Disagree | Not sure | Agree | ||||
| Pre 0 (0.0%) | Post 0 (0.0%) | Pre 4 (4.9%) | Post 2 (2.3%) | Pre 38 (46.3%) | Post 25 (28.4%) | Pre 40 (48.8%) | Post 61 (69.3%) | |
| ITU3 investigates issues in the manufacture of TiO2. Environmental pollution attributed to the industry has resulted in accusations of placing profit before environmental issues. To what extent would you agree, or disagree, with that accusation? | Strongly disagree | Disagree | Not sure | Agree | ||||
| Pre 4 (4.9%) | Post 4 (4.9%) | Pre 16 (19.5%) | Post 38 (44.2%) | Pre 59 (71.9%) | Post 17 (19.8%) | Pre 3 (3.7%) | Post 27 (31.4%) | |
| Did you enjoy participating in ITU 3? | Yes 76 (87.3%) | No 11 (12.7%) | ||||||
6 Concluding remarks
Sustainable Development is at the heart of Green Chemistry. It is important that chemistry graduates realise the vocational nature of their discipline but also that successful operation of large-scale industrial facilities requires an awareness of a broad range of issues, not always chemical in nature. The TiO2 manufacturing industry is well developed and its operation represents a good example of the dynamics generally active in the chemical manufacturing industry. Although undergraduate teaching packages highlighting aspects of TiO2 production are already available,5 the concepts of problem based learning incorporated into the interactive teaching format presented in ITU 3 has been shown to enhance the students' awareness of sustainable development. It is important that undergraduate chemistry courses are occasionally supplemented with such exercises. They illustrate to the students that the chemical industry exists to meet the requirements of a complex consumer society and that chemists play a vital role in ensuring the safe operation of the, often complex, manufacturing units required to meet that consumer demand. Presently, the Chemistry Department of the University of Glasgow operate four ITU exercises. All four teaching packages will shortly be available via the Royal Society of Chemistry website (http://www.rsc.org/lap/educatio/rsedhome.htm).8 Abbreviations and glossary of terms
| ITU | Interactive teaching unit. |
| ITU 1 | The Age of Refrigeration, University of Glasgow, 1997. |
| ITU 2 | Mercury, Membrane or Diaphragm, University of Glasgow, 1998. |
| ITU 3 | Industrial Chemistry: Titanium Dioxide, University of Glasgow, 2002. |
| GDP | Gross domestic product. |
| EU | European Union. |
| LCA | Life cycle assessment. |
| SDI | Scottish Development International. |
| EA | Environmental Agency. |
| Full class | 45 students + 5 tutors. |
| Tutorial group | 9 students + 1 tutor. |
| Sub-group | 3 students. |
Acknowledgements
This paper is dedicated to Dr Craig Gray, who tragically died in March 2003. Craig demonstrated considerable wisdom, patience and foresight that ensured that this ITU made the transition from an initial concept to an active part of our undergraduate teaching programme. He is sorely missed.The authors acknowledge assistance from two organisations indirectly featured in the ITU ‘game’ presented to the students. Firstly, Huntsman Tioxide have demonstrated a commitment to chemical education and public awareness of chemical processing by providing some technical guidance. Secondly, Scottish Enterprise are thanked for providing information on the range of options typically offered by competitive Regional Development Organisations. Assistance from these two organisations has permitted a more realistic scenario to be presented to our students. Finally, the authors wish to acknowledge the assistance of the ITU team. Dr John Dymond, Dr Louis Farrugia, Dr Justin Hargreaves, Professor Neil Issacs, Dr Malcolm Kadodwala, Dr Adrain Lapthorn, Dr David McComb, Dr Ken Muir, Dr David Procter, Dr David Rycroft and Dr David White have provided wonderful support as tutors. Dr Ken Muir is also thanked for the preparation of structural diagrams. Mr Bob Munro and Mrs Lesley Bell are thanked for skilful document preparation and administrative support respectively.
References
- T. Platt, E. Barber, A. Yoshinaka and V. Roth, Biochem. Mol. Biol. Educ., 2003, 31, 132 Search PubMed.
- E. T. Washington, J. W. Tysinger, L. M. Snell and L. R. Palmer, Medical Teacher, 2003, 25, 136 Search PubMed.
- D. Raine and J. Collett, Eur. J. Phys., 2003, 24, 541 CrossRef.
- D. Lennon, A. A. Freer, J. M. Winfield, P. Landon and N. Reid, Green Chem., 2002, 4, 181 RSC.
- The Titanium Dioxide Project, Project Improve, Faculty of Science & The Environment, University of Hull, 1998 Search PubMed.
- W. Jucker, Chimica, 2002, 54, 500 Search PubMed.
- C. Brandt, Chem. Unserer Zeit, 2002, 36, 214 Search PubMed.
- J. Fiksel, ACS Symp. Ser, 2002, 823, 13 CAS.
- P. T. Anatas and R. L. Lankey, ACS Sym. Ser, 2002, 823, 1 Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry, ACS Symposium Series, 1996, 626, 1 Search PubMed.
- P. T. Anastas and T. C. Williamson, Green Chemistry: Theory and Practice, OUP, Oxford, 1998 Search PubMed.
- E. Reck and M. Richards, Jocca Surface Coatings, 1997, 80(12), 568 Search PubMed.
- Council Directive 78/176/EEC of 20 Feb 1978 on Waste From the Titanium Dioxide Industry, Official Journal L 054, 1978, p. 24 Search PubMed.
- Huntsman Tioxide, http://www.huntsman.com/tioxide/ (Accessed Oct 2001).
- Scottish Development International (SDI), http://www.scottishdevelopment international.com/ (Accessed Oct 2001).
- David R. Lide, Handbook of Chemistry and Physics, 81st Ed., 2000–2001, 4, 32 Search PubMed.
- Austpac Resources N. L., http://www.austpacresources.com/titanium /default.htm (Accessed Oct 2001).
- K. H. Büchel, H.-H. Moretto and P. Woditsch, Industrial Inorganic Chemistry, Wiley-VCH, Weinheim, 2000, p. 552 Search PubMed.
- Millennium Chemicals, TiO2 crystal types, http://www.millenniumchem.com/Channels_EN (Accessed Oct 2001).
- R. E. Day, Polymer Degradation and Stability, 1990, 29, 73 Search PubMed.
- M. Diebold, Unconventional Effects of TiO2 on Paint Durability, 5th Nürnberg Congress, Germany, 1999 Search PubMed.
- M. Thayer, Chem. Eng. News, 1998, 76(10), 10.
- D. V. Borst, Jocca Surface Coatings, 1997, 80(2), 60 Search PubMed.
- R. Thompson, The Modern Inorganic Chemicals Industry, 1977, p. 354 Search PubMed.
- W. Faith, D. Keys, R. Clark, Industrial Chemicals, Wiley, New York, 3rd ed., 1965, p. 762 Search PubMed.
- J. R. Fisher, J. Coat. Technol., 1993, 65(820), 67 Search PubMed.
- N. Alperowicz, Chem. Week, 2001, 163, 21, p. 18 Search PubMed.
- Huntsman Tioxide, Environmental Performance Report 2000, http://www.huntsman.com/tioxide/environmentalreport2000/index.htm (Accessed Nov 2001).
- The Green House Effect, Climatic Change, and Ecosystems, Eds. B. Bolin, B. R. Döös, J. Jäger and R. A. Warrick, Wiley, Chichester, 1989 Search PubMed.
- News Review, Chem. Br., 1997, 33(7), 5 Search PubMed.
- The Chemical Engineer (London), 1999, vol. 675, issue 3p. 3 Search PubMed.
- A Bright Outlook, Paints & Coatings Industry, 2001, http://www.pcimag.com/cda/articleinformation/features/bnp__features__item/0,1846,62585,00.html (Accessed Nov 2001).
- University of Ulster Scientists Fight for Safer Water, University of Ulster News Release, 1999, http://www.ulst.ac.uk/news/releases/1999/112.html (Accessed Dec 2001).
- Y. Goswami, Air Cleaning System Destroys Anthrax, Other Pathogens, 2001 http://www.napa.ufl.edu/2001news/anthrax.htm (Accessed Dec 2001)..
- Titania Solar Tremendous Promise, Australian Energy News, 2001, Issue 20, http://www.isr.gov.au/resources/netenergy/aen/aen20/10titania.html (Accessed Dec 2001)..
| This journal is © The Royal Society of Chemistry 2004 |
