Photomodulated molecular recognition of the guanidinium cation†
Christopher A.
Hunter
*a,
Mahmut
Togrul
b and
Salvador
Tomas
a
aCentre for Chemical Biology, Krebs Institute for Biomolecular Science, Department of Chemistry, University of Sheffield, Sheffield, UK S3 7HF
bUniversity of Dicle, Faculty of Science and Art, Chemistry Department, TR 21280 Diyarbak, Turkey
First published on 7th November 2003
Abstract
Azobenzene moieties were incorporated into a synthetic receptor allowing its affinity for the guanidinium cation to be modulated ten-fold by photoirradiation and/or heating.
The search for new ways to manipulate matter at the molecular level has resulted in an increase in systems that can act as molecular switches in response to a variety of stimuli. The potential applications range from information technology,1 to the design of complex molecular systems that reproduce some aspects of the action of macroscopic motors or machines.2,3 Molecular switches that respond to light are most interesting, because photochemical reactions present distinct advantages over other forms of transformation, that may depend on the accessibility of reactants to the reaction centre and will in most cases produce secondary waste products.4–6 Photoisomerisation of azobenzene has been thoroughly studied and is specially suited for such applications.7–10
Here we use the photochemical properties of azobenzene to modulate host–guest recognition by H-bonding interactions. Using molecular models, we designed the guanidinium receptor shown in Fig. 1.11,12 There are two photoisomerisable azobenzene moieties each bearing a carboxylate group as the recognition motif. The isophthaloyl spacer was chosen so that in the Z,Z form of the receptor, 1Z, both carboxylate groups should be able to make simultaneous H-bonds to the guest. This is not possible in either the E,Z mixed form, 1M or the E,E form, 1E, and so these should display a lower affinity for guanidinium (Fig. 1).
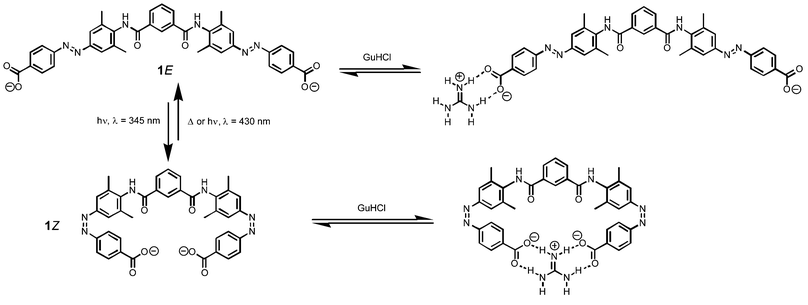 | ||
| Fig. 1 Photomodulated recognition of the guanidinium cation. Two-dimensional structures are used for the purposes of illustration, because the detailed conformational properties of the system have not been investigated. Although the isophthalamide moiety probably populates other conformations, the carboxylate groups are always remote in 1E and can only be close in 1Z. | ||
Synthesis of the receptor was carried out as follows. Azobenzene 2 was obtained by reaction of methyl 4-aminobenzoate with sodium nitrite, followed by addition of 2,6-dimethylaniline to the resulting diazonium salt. Reaction of an excess of 2 with isophthaloyl acid dichloride, followed by hydrolysis with KOH in EtOH–H2O gave the desired compound 1 in 20% overall yield. 1H NMR spectroscopy showed that the major isomer obtained is 1E (> 95%). Model compound 3 was synthesised in the same way: condensation of 2 with 4-t-butylbenzoyl chloride, followed by hydrolysis with KOH in EtOH–H2O (Scheme 1). Again, exclusively the trans isomer of 3 was obtained (3E).
 | ||
| Scheme 1 | ||
UV/visible absorption spectra of 1 in DMSO show one band with a maximum at 345 nm and a second much less intense band at 454 nm. Upon irradiation at 345 nm, the 345 nm band decreases in intensity, whilst the minor band becomes more intense and experiences a bathochromic shift. When the sample is stored in the dark at room temperature, the original spectrum is eventually restored (see supplementary material). This behaviour is indicative of an E–Z photoisomerisation followed by thermal back conversion.8 Irradiation of a more concentrated sample in a photoreactor allowed us to corroborate this observation by 1H NMR spectroscopy (Fig. 2).‡
 | ||
| Fig. 2 Aliphatic region of the 1H NMR spectrum of 1 in DMSO-d6, showing how the composition changes on irradiation or heating. | ||
The molecular recognition properties of the system were investigated using 1H NMR titrations. Guanidinium hydrochloride (GuHCl) was titrated into model compound 3E in DMSO-d6, and the data were fit to a 1 : 1 binding isotherm, giving an association constant of 1700 ± 200 M−1. Then the titration was carried out with 1E, and the data could again be fit to a 1 : 1 model giving an association constant of 2200 ± 250 M−1. Although more complex equilibria are possible in this system (GuHCl has three binding sites and 1 has two), if all of the association constants are comparable, a simple 1 : 1 model should behave well, and the similarity in the values of the association constants obtained for 1E and 3E suggests that this is the case.
Irradiation of a sample of 1E gave a mixture of all three azobenzene isomers 1Z, 1M and 1E in a 1 : 1 : 1 ratio, but it proved difficult to isolate the pure 1Z and 1M forms. However, the 1H NMR signals due to the three compounds are well-resolved, so it is possible to independently monitor the interactions of all three isomers with GuHCl in the mixture (Fig. 2). Representative data for the signals due to the aromatic proton labelled * in Scheme 1 are shown in Fig. 3 together with the corresponding fits to 1 : 1 binding isotherms. The association constant for 1E is 1.5 ± 0.5 × 103 M−1, in good agreement with the value obtained for pure 1E. The association constant for 1M is similar (1.5 ± 0.5 × 103 M−1), but the value obtained for 1Z is significantly larger (2 ± 1 × 104 M−1) as expected from the shape of the titration curve shown in Fig. 3. Given the complexity of the system, these association constants should be taken as approximations. The guanidinium cation has three binding sites and 1 has two, so a range of higher order open and closed assemblies are possible. However, the behaviour of 1Z is clearly qualitatively different from the other two isomers. It has a significantly higher affinity for GuHCl, and the increase in the association constant is good evidence for the simultaneous cooperative interaction of both carboxylate groups with the guanidinium cation in the 1Z complex illustrated in Fig. 1.
 | ||
| Fig. 3 Titration of a 0.5 mM mixture 1E : 1M : 1Z (1 : 1 : 1) with GuHCl in DMSO-d6. The lines represent fits to a 1 : 1 binding isotherm. | ||
This result shows that it is possible to use light to modulate the binding of 1 to GuHCl by approximately one order of magnitude by irradiation at the appropriate wavelength. This approach can now be incorporated into more complicated systems, where control of the interaction with guanidinium provides us with new possibilities for manipulating structures at molecular level.
Notes and references
- C. P. Collier, J. O. Jeppesen, Y. Luo, J. Perkins, E. W. Wong, J. R. Heath and J. F. Stoddart, J. Am. Chem. Soc., 2001, 123, 12632 CrossRef CAS.
- J.-P. Collin, C. Dietrich-Buchecker, P. Gavina, M. C. Jimenez-Molero and J.-P. Sauvage, Acc. Chem. Res., 2001, 34, 477 CrossRef CAS.
- D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 175 CrossRef CAS.
- R. Ballardini, V. Balzani, A. Credi, M. T. Gandolfi and M. Venturi, Acc. Chem. Res., 2001, 34, 445 CrossRef CAS.
- N. Koumura, R. W. J. Zijistra, R. A. Van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS.
- T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150 CrossRef CAS.
- F. Würthner and J. Rebek, Jr, Angew. Chem., Int. Ed., 1995, 34, 446 CrossRef.
- S. Shinkai, in Comprehensive Supramolecular Chemistry, Elsevier, Oxford, UK, 1996, Vol. 38, pp. 671–700 Search PubMed.
- T. Schultz, J. Quenneville, B. Levine, A. Toniolo, T. J. Martinez, S. Lochbrunner, M. Schmitt, J. P. Shaffer, M. Z. Zgierski and A. Stolow, J. Am. Chem. Soc., 2003, 125, 8098 CrossRef CAS.
- I. A. Banerjee, L. Yu and H. Matsui, J. Am. Chem. Soc., 2003, 125, 9542 CrossRef CAS.
- E. Fan, S. A. Van Arman, S. Kincaid and A. D. Hamilton, J. Am. Chem. Soc., 1993, 115, 369 CrossRef CAS.
- A. Echavarren, A. Galan, J.-M. Lehn and J. De Mendoza, J. Am. Chem. Soc., 1989, 111, 4994 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: UV/visible absorption spectra of 1, showing changes observed on irradiation at 345 nm and thermal recovery of the original spectrum. See http://www.rsc.org/suppdata/cc/b3/b311060e/ |
| ‡ A Hitachi F-4500 fluorimeter was used for the irradiation of samples at µM concentrations. A photochemical reactor equipped with Rayonet 3500A lamps (max. at 350 nm) was used for the irradiation of samples at mM concentrations. |
| This journal is © The Royal Society of Chemistry 2004 |
