Preparation and structure of 2-iodoxybenzoate esters: soluble and stable periodinane oxidizing reagents†
Viktor V.
Zhdankin
*a,
Dmitry N.
Litvinov
a,
Alexey Y.
Koposov
a,
Thanh
Luu
b,
Michael J.
Ferguson
b,
Robert
McDonald
b and
Rik R.
Tykwinski
*b
aDepartment of Chemistry, University of Minnesota Duluth, Duluth, Minnesota 55812, USA. E-mail: vzhdanki@d.umn.edu
bDepartment of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2, Canada. E-mail: rik.tykwinski@ualberta.ca
First published on 18th November 2003
Abstract
Esters of 2-iodoxybenzoic acid (IBX-esters) are potentially valuable oxidizing reagents belonging to a new class of pentavalent iodine compounds with a pseudo benziodoxole structure.
Organic derivatives of pentavalent iodine (λ5-iodanes) have found wide applications as versatile oxidizing reagents in the synthesis of complex organic molecules.1 Dess–Martin periodinane (DMP, 1) and its precursor 1-hydroxy-1,2-benziodoxol-3-(1H)-one 1-oxide (IBX, 2) are the most recognizable members of this class of compounds.1,2 Solid state analysis of 2 shows an extended series of intermolecular secondary bonding interactions, dominated by two I⋯O contacts, an O–H⋯O hydrogen bond, and π-stacking. This affords a three dimensional polymeric network in the solid state that substantially limits the solubility of IBX in common organic solvents.3 We reasoned that a partial disruption of the polymeric nature of IBX could be achieved through manipulation of the intramolecular I⋯O bonding interaction of the benziodoxole substructure, and this would be a mechanism toward enhancing reagent solubility. This concept has been initially explored with 2-iodoxybenzamides, which do indeed show improved solubility and stability in comparison to IBX.4 We now report on the facile preparation of a new class of I(V) oxidants, the IBX-esters 4. These stable, soluble reagents show oxidizing properties similar to IBX and DMP. X-Ray crystallographic analyses show that 4a·DMSO, 4c and 4d share a common, pseudocyclic benziodoxole configuration and suggest that this structural characteristic is sufficient for increasing the solubility of I(V) oxidants.
Iodoxyarenes 4 were prepared from the esters of 2-iodobenzoic acid 3 using hypochlorite oxidation5 (Scheme 1), and the products are isolated by filtration in the form of stable, white, microcrystalline solids. This facile procedure allows for the preparation of reagents 4 derived from a wide variety of precursors, including primary, secondary, and tertiary alcohols, adamantanols, as well as optically active menthols and borneol.
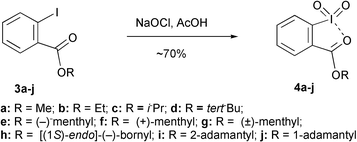 | ||
| Scheme 1 | ||
The structure and constitution of compounds 4a–j were established through a combination of elemental analysis, spectroscopic data, ESI mass spectrometry, and single crystal X-ray analysis.6 All products have moderate to good solubility in common organic solvents, such as chloroform, dichloromethane, and acetonitrile. In CH2Cl2, for example, the solubilities of 4c, 4d and 4e are 2.9, 0.2, and 1.7 M, respectively. The solubility of crystalline 4a at 0.003 M, however, is considerably less.
A broad range of solubility, i.e., 4cvs.4a, suggested that molecular structure was not the only factor, but that secondary bonding interactions in the solid-state (i.e., polymeric vs. nonpolymeric) might also play a significant role. Several derivatives 4 have therefore been characterized by X-ray crystallography (Fig. 1). The structure of 4c shows a unit cell consisting of two crystallographically independent molecules (Fig. 1A). Strong secondary I⋯O bonding interactions between neighboring molecules afford dimeric pairs (dashed lines). These dimers are then linked together by a combination of strong and weak (dotted lines) interactions, forming a polymeric motif. Within each molecule, an intramolecular close contact between the I(V) center and the oxygen atom of the ester group (I1–O13 2.8185(17) and I2–O23 2.6979(16) Å) affords the pseudo-benziodoxole ring.3,7
![ORTEP representations of molecules 4c
(top), 4d
(middle) and 4a
(bottom). Selected distances [Å] for 4c: I1–O11 1.8170(15), I1–O12 1.8033(17), I1–O13 2.8185(17), I1–O11′ 2.6285(16), I1–O22 2.6711(17), I2–O21 1.8164(16), I2–O22 1.8088(16), I2–O23 2.6979(16), I2–O12′ 3.0510(17), I2–O21″ 2.5597(17); 4d: I1–O11 1.804(2), I1–O12 1.816(2), I1–O12′ 2.698(2), I1–O13 2.688(2), I1–O21′ 2.710(3), I2–O11 2.838(3), I2–O12′ 3.021(3), I2–O21 1.811(3), I2–O22 1.781(3), I2–O23 2.673(3); 4a: I–O1 2.6979(15), I–O3 1.8061(14), I–O3′ 2.7805(15), I–O4 1.7940(15), I–O1S 2.7560(16). Primed atoms are related to unprimed ones via crystallographic inversion centers at the following positions: 4c
(1/2, 0, 0), 4d
(0, 1/2, 1/2) and 4a
(1/2, 0, 0). Thermal ellipsoids shown at the 20% probability level.](/image/article/2004/CC/b312961f/b312961f-f1.gif) | ||
| Fig. 1 ORTEP representations of molecules 4c (top), 4d (middle) and 4a (bottom). Selected distances [Å] for 4c: I1–O11 1.8170(15), I1–O12 1.8033(17), I1–O13 2.8185(17), I1–O11′ 2.6285(16), I1–O22 2.6711(17), I2–O21 1.8164(16), I2–O22 1.8088(16), I2–O23 2.6979(16), I2–O12′ 3.0510(17), I2–O21″ 2.5597(17); 4d: I1–O11 1.804(2), I1–O12 1.816(2), I1–O12′ 2.698(2), I1–O13 2.688(2), I1–O21′ 2.710(3), I2–O11 2.838(3), I2–O12′ 3.021(3), I2–O21 1.811(3), I2–O22 1.781(3), I2–O23 2.673(3); 4a: I–O1 2.6979(15), I–O3 1.8061(14), I–O3′ 2.7805(15), I–O4 1.7940(15), I–O1S 2.7560(16). Primed atoms are related to unprimed ones via crystallographic inversion centers at the following positions: 4c (1/2, 0, 0), 4d (0, 1/2, 1/2) and 4a (1/2, 0, 0). Thermal ellipsoids shown at the 20% probability level. | ||
X-Ray crystallographic analysis of 4d shows a centrosymmetric arrangement of four molecules (Fig. 1B). As observed for 4c, the secondary I⋯O bonding interactions that link neighboring molecules are present, as is the intramolecular close contact between the iodine center and the oxygen atom of the ester group (I1–O13 2.688(2) and I2–O23 2.673(3) Å). Unlike 4c, however, there are no additional interactions present to connect discrete tetramers into a polymeric structure, and as a result O22 remains uncoordinated.
Whereas crystals of 4c and 4d could be grown from CH3CN, the more sparingly soluble 4a provided X-ray quality crystals only from DMSO.8 Analysis of the crystals shows a dimeric structure between two inversion related molecules. The remaining close contact to the I(V) center, however, occurs with oxygen from a DMSO molecule. As found in the structures of 4c and 4d, the intramolecular I⋯O interaction center involving the iodonium center and ester oxygen is again significant, with I–O1 2.6979(15) Å. Like 4d, there are no repeating, polymeric interactions, and O4 is uncoordinated.
The intramolecular I⋯O bonding motif with the carbonyl oxygen is common to the structures of IBX-esters 4a, 4c and 4·DMSO, and suggests that the presence of an ester moiety in the ortho-position is vital for improved solubility.9,10 This results from a disruption of intermolecular I⋯O bonds and a concurrent reduction of the polymeric interactions found in some iodyl and iodosyl derivatives.10 In comparison to the IBX-esters, IBX shows a stronger intramolecular I–O interaction with the carboxylate oxygen (2.263(2) Å),3 as well as a three-dimensional network based on a combination of intermolecular O–H⋯O and I⋯O secondary bonding. It is, therefore, the absence of the hydrogen bonding interactions in the IBX-esters that appears sufficient to enhance solubility vs. IBX. The consequence of this effect can be appreciated by considering the structure of 4c, which shows that even with partial oligomeric nature on the basis of I⋯O bonding, considerable solubility can be maintained.
According to literature data,1 iodylbenzene (PhIO2) as well as other non-cyclic iodylarenes are not effective oxidants toward alcohols, due in part to decreased solubility. In agreement with their structural features, the oxidizing reactivity of IBX-esters 4 is closer to the benziodoxole-based pentavalent iodine reagents such as IBX, in contrast to the non-cyclic iodylarenes.
A range of alcohols can be oxidized by reagents 4 to the respective carbonyl compounds under mild conditions in the presence of trifluoroacetic acid, BF3-etherate, or KBr as a catalyst. For example, oxidation of benzyl alcohol in the presence of KBr11 in chloroform at 50 °C cleanly gives benzaldehyde as the only product detected by 1H NMR spectroscopy. A variety of secondary alcohols, such as cyclohexanol and cycloheptanol, are converted to the corresponding ketones in 95–98% yields as determined by GC analysis. Furthermore, the increased solubility of, e.g., 4c greatly expands the range of solvents that can be utilized in comparison to IBX. The oxidation of 1-phenylethanol to acetophenone, for example, proceeds in good yield (>65%) using CH3CN, CHCl3, CH2Cl2, and benzene.
This work was supported by a research grant from the National Institutes of Health (R15 GM065148-01), the Natural Sciences and Engineering Research Council of Canada, and the University of Alberta.
Notes and references
- A. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic Press, London, 1997 Search PubMed; G. F. Koser, in The Chemistry of Halides, Pseudo-Halides and Azides, Suppl. D2, ed. S. Patai and Z. Rappoport, Wiley-Interscience, Chichester, 1995, ch. 21, pp. 1173–1274 Search PubMed; V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 Search PubMed; T. Wirth, Angew. Chem., Int. Ed., 2001, 40, 2812 Search PubMed.
- K. C. Nicolaou, P. S. Baran, Y.-L. Zhong and K. Sugita, J. Am. Chem. Soc., 2002, 124, 2212 CrossRef CAS and refs therein.
- P. J. Stevenson, A. B. Treacy and M. Nieuwenhuyzen, J. Chem. Soc., Perkin Trans. 2, 1997, 589 RSC; J. Z. Gougoutas, Cryst. Struct. Commun., 1981, 10, 489 Search PubMed.
- V. V. Zhdankin, A. Y. Koposov, B. C. Netzel, N. V. Yashin, B. P. Rempel, M. J. Ferguson and R. R. Tykwinski, Angew. Chem., Int. Ed., 2003, 42, 2194 CrossRef CAS.
- D. Barton, C. Godfrey, J. Morzycki and W. Motherwell, J. Chem. Soc., Perkin Trans. 1, 1982, 1947 RSC.
- Crystal data for 4a: C10H13IO5S, M
= 372.16, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2), a
= 8.0364(4), b
= 9.0124(5), c
= 10.0969(6)
Å; α
= 81.281(1), β
= 76.595(1), γ
= 67.422(1)°; V
= 655.25(6)
Å3, T
= 193 K; µ
= 2.609 mm−1, Z
= 2, 4571 reflections measured, 2632 unique (Rint
= 0.0140); final R1
= 0.0160. For 4c: C10H11IO4, M
= 322.09, triclinic, space group P
(No. 2), a
= 8.0364(4), b
= 9.0124(5), c
= 10.0969(6)
Å; α
= 81.281(1), β
= 76.595(1), γ
= 67.422(1)°; V
= 655.25(6)
Å3, T
= 193 K; µ
= 2.609 mm−1, Z
= 2, 4571 reflections measured, 2632 unique (Rint
= 0.0140); final R1
= 0.0160. For 4c: C10H11IO4, M
= 322.09, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2); a
= 9.6939(6), b
= 10.5225(7), c
= 11.2574(7)
Å; α
= 96.658(1), β
= 94.913(1), γ
= 105.585(1)°; V
= 1090.40(12)
Å3, T
= 193 K; µ
= 2.928 mm−1, Z
= 4, 8154 reflections measured, 4428 unique (Rint
= 0.0160); final R1
= 0.0199. For 4d: C11H13IO4, M
= 336.11, triclinic, space group P
(No. 2); a
= 9.6939(6), b
= 10.5225(7), c
= 11.2574(7)
Å; α
= 96.658(1), β
= 94.913(1), γ
= 105.585(1)°; V
= 1090.40(12)
Å3, T
= 193 K; µ
= 2.928 mm−1, Z
= 4, 8154 reflections measured, 4428 unique (Rint
= 0.0160); final R1
= 0.0199. For 4d: C11H13IO4, M
= 336.11, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (No. 2), a
= 10.8981(9), b
= 11.3827(10), c
= 11.5247(10)
Å; α
= 83.3624(17), β
= 62.3591(16), γ
= 89.6303(17)°; V
= 1256.18(19)
Å3, T
= 193 K; µ
= 2.546 mm−1, Z
= 4, 7149 reflections measured, 5063 unique (Rint
= 0.0198); final R1
= 0.0324. CCDC 222124–222126. See http://www.rsc.org/suppdata/cc/b3/b312961f/ for crystallographic data in .cif or other electronic format.
(No. 2), a
= 10.8981(9), b
= 11.3827(10), c
= 11.5247(10)
Å; α
= 83.3624(17), β
= 62.3591(16), γ
= 89.6303(17)°; V
= 1256.18(19)
Å3, T
= 193 K; µ
= 2.546 mm−1, Z
= 4, 7149 reflections measured, 5063 unique (Rint
= 0.0198); final R1
= 0.0324. CCDC 222124–222126. See http://www.rsc.org/suppdata/cc/b3/b312961f/ for crystallographic data in .cif or other electronic format. - V. V. Zhdankin, Rev. Heteroatom Chem., 1997, 17, 133 Search PubMed.
- 4a·DMSO is considerably more soluble in other solvents than pure 4a.
- (a) N. W. Alcock and J. F. Sawyer, J. Chem. Soc., Dalton Trans., 1980, 115 RSC; (b) A. R. Katritzky, G. P. Savage, G. J. Palenik, K. Qian, Z. Zhang and H. D. Durst, J. Chem. Soc., Perkin 2., 1990, 1657 RSC; (c) D. G. Naae and J. Z. Gougoutas, J. Org. Chem., 1975, 40, 2129 CrossRef CAS.
- (a) D. Macikenas, E. Skrzypczak-Jankun and J. D. Protasiewicz, Angew. Chem., Int. Ed., 2000, 39, 2007 CrossRef CAS; (b) D. Macikenas, E. Skrzypczak-Jankun and J. D. Protasiewicz, J. Am. Chem. Soc., 1999, 121, 7164 CrossRef CAS; (c) U. H. Hirt, M. F. H. Schuster, A. N. French, O. G. Wiest and T. Wirth, Eur. J. Org. Chem., 2001, 1569 CrossRef CAS; (d) U. H. Hirt, B. Spingler and T. Wirth, J. Org. Chem., 1998, 63, 7674 CrossRef CAS.
- KBr has been previously used as effective catalyst in oxidation of alcohols with PhIO: H. Tohma, S. Takizawa, T. Maegawa and Y. Kita, Angew. Chem., Int. Ed., 2000, 39, 1306 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: synthetic and characterization data for all new compounds; general procedures for the oxidation of alcohols with reagent 4c. See http://www.rsc.org/suppdata/cc/b3/b312961f/ |
| This journal is © The Royal Society of Chemistry 2004 |

