Isolation and structural characterization of the first thermally robust and air stable Cr(4+) bent-metallocene complex†
Piet-Jan
Sinnema‡
,
Justin
Nairn
,
Ralph
Zehnder
,
Pamela J.
Shapiro
*,
Brendan
Twamley
and
Alex
Blumenfeld
Department of Chemistry, University of Idaho, Moscow, 83844-2343 ID, USA. E-mail: shapiro@uidaho.edu; Fax: +01 208 885 6173; Tel: +01 208 885 5785
First published on 24th November 2003
Abstract
The first thermally robust and air stable bent-sandwich chromocene complex with chromium in the +4 oxidation state has been isolated and fully characterized.
The chemistry of chromocene has remained elusive due to the metallocene's reluctance to adopt a bent-sandwich geometry and its susceptibility to ring loss. The strategy that we have employed to override these tendencies and gain access to bent-sandwich chromocene complexes is to introduce an interannular bridge between the cyclopentadienyl rings of the chromocene that is too short to span a parallel ring geometry.1,2 Brintzinger and coworkers demonstrated the effectiveness of this approach with their synthesis of Me4C2(η5-C5H4)2CrCO,3 which is thermally stable, unlike (η5-C5H4)2CrCO, which undergoes CO loss to form the more stable 16e- (η5-C5H5)2Cr.4 Recently we reported the first bent metallocene complexes of Cr(3+) and Cr(4+).5 Whereas the 3+ oxidation state is quite prevalent in organochromium chemistry, the 4+ oxidation state is rare and, prior to our examples, unknown for chromocene.6 The 18e-, diamagnetic Cr(4+) species 15a and 25b (Fig. 1) that we reported previously are only meta-stable, decomposing above −25 °C to form paramagnetic species, some of which have been structurally characterized. One decomposition product formed by 2 is the 15e-, zwitterionic complex [Me4C2(η5-C5H4){η5-C5H3B(C6F5)3}]Cr (3).5b Complex 3 reacts with various σ-donating ligands like CO, isocyanides and phosphines to give the 17e-complexes [Me4C2(η5-C5H4){η5-C5H3B(C6F5)3}]CrL. In this communication we highlight one of these species, the xylyl-isocyanide (CNXyl) complex [Me4C2(η5-C5H4){η5-C5H3B(C6F5)3}]CrCNXyl (4), which is oxidized by AgCN to form the first thermally robust and air-stable Cr(4+) bent-metallocene complex, [Me4C2(η5-C5H4){η5-C5H3B(C6F5)3}]Cr(CN)CNXyl (5).
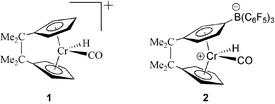 | ||
| Fig. 1 Meta-stable Cr(4+) ansa-metallocene complexes. | ||
The combination of green, high-spin (μeff = 3.85 μB) 3 with one equivalent of xylyl isocyanide produces brown, low-spin (μeff = 1.75 μB) 4 (Scheme 1). The X-ray crystal structure of 4 was determined. An ORTEP drawing of the complex is shown in Fig. 2.§ A particularly interesting feature of the structure is a π-stacking interaction between the isocyanide ligand and one of the C6F5 rings of the boryl group, with a distance of 3.331 Å from the centroid of the arene ring plane to the center of the CN bond. The C6F5 ring is practically coplanar with the xylyl ring, with a dihedral angle of 2.4° between the two ring planes. Although there are now several examples of early transition metal bent-metallocene complexes bearing an anionic B(C6F5)3 substituent on one of the cyclopentadienyl rings7 this is the first time an intramolecular π-stacking interaction between the B(C6F5)3 group and a ligand on the metal has been identified.
 | ||
| Fig. 2 Molecular structure of 4. Selected bond lengths (Å) and angles (°). Cr1–Cent(C1-5) 1.834(3), Cr1–Cent(C8-12) 1.830(3), Cr1–C17 1.944(3), C17–N1 1.171(4), C2–B1 1.635(4), Cp<Cp 43.5, Cent-Cr1-Cent 142.7, Cr–C17–N1 178.6(3). | ||
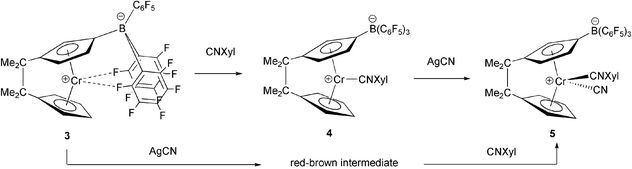 | ||
| Scheme 1 Preparation of complexes 4 and 5 from complex 3. | ||
A dramatic effect of the borate substituent on the redox properties of the ansa-chromocene complex is revealed in cylic voltammetry measurements on 4. The complex exhibits a reversible Cr2+/3+ couple at −1523 mV, which, significantly, is 350 mV lower than that of [Me2C4(η5-C5H4)2CrCNtBu,2 a non-borylated analog. Complex 4 also exhibits a reversible Cr3+/4+ couple at +24 mV. [Me4C4(η5-C5H4)2CrCNtBu, by contrast, exhibits an irreversible anodic peak at +200 mV. This prompted us to investigate the chemical oxidation of 4. Refluxing a solution of 4 in THF with excess AgCN for 24 h resulted in the deposition of metallic silver and the formation of complex 5, which was isolated as a dark orange-red solid in 73% yield (Scheme 1). Alternatively, 3 reacts readily with AgCN at room temperature to form a paramagnetic, reddish brown species, which reacts slowly with CNXyl to form 5 in 54% yield. Efforts to identify the paramagnetic intermediate formed in the reaction between 3 and AgCN are in progress. We presume it to be a dimer or higher aggregate of the 16e-ansa-chromocene cyanide derivative.
An 18e-complex, 5 is diamagnetic, thermally robust (stable when heated for 16 h at 70 °C in THF) and stable in air for at least 1 h. We have characterized it thoroughly by 1H, 13C, 19F, and 11B NMR spectroscopy and determined its molecular structure by X-ray crystallography. An ORTEP drawing of the molecule is shown in Fig. 3.§ Significantly, the boryl group is positioned over the much bulkier xylyl isocyanide ligand, with which it retains its π-stacking interaction. The same arrangement of ligands results when the CN− and CNXyl ligands are introduced in reverse order onto chromium (i.e. by reacting 3 with AgCN prior to introducing CNXyl). A comparison of the Cp–Cp dihedral angles (5: 44.6° 4: 43.5°; 2: 42.5°; 3: 36.9°) and Cp–Cr–Cp angles (5: 133.6° 4: 142.7°; 2: 140.0°; 3: 149.3°) shows that introducing more ligands on chromium results in greater tilting of the cyclopentadienyl rings away from the equatorial wedge.
 | ||
| Fig. 3 Molecular structure of 5. Selected bond lengths (Å) and angles (°). Cr1–Cent(C1-5) 1.853(2), Cr1–Cent(C8-12) 1.864(2), Cr1–C44 2.0136(16), C35–N1 1.1556(19), C10–B1 1.649(2), Cp<Cp 44.6, Cent-Cr1-Cent 133.6, Cr–C35–N1 171.66(13). | ||
1H and 13C NMR spectra of 5 reflect the lack of symmetry in the compound; all of the cyclopentadienyl ring carbons and hydrogens are inequivalent, as are all four methyl groups along the ethanediyl bridge. A variable temperature 19F NMR study revealed restricted rotation of the –B(C6F5)3 substituent in the complex (ESI†). At 25 °C the three C6F5 rings appear equivalent, showing only three resonances for the o, m and p-fluorines. At −34 °C, the resonance for the p-fluorines at −163 ppm decoalesces into three resonances. Line shape analysis gave ΔH‡ = 7.6 ± 0.8 kcal mol−1 and ΔS‡ = 11 ± 3 eu for the three site exchange. The decoalescence patterns of the o- and m-fluorines are more complex since they are affected by two processes, the Cp-Boryl rotation and the rotation of the individual C6F5 rings. Ultimately, at −94 °C all 15 inequivalent fluorine atoms are distinguishable.
In summary, we have prepared and fully characterized the first thermally robust, air stable bent metallocene complex of Cr(4+). The electron releasing properties of the anionic –B(C6F5)3 substituent was key to achieving this result by lowering the redox potential of the chromium and stabilizing the unusual Cr(4+) oxidation state. A similar effect of the anionic boryl group on the redox properties of metallocene complexes of other early transition metals may be expected.
The authors are grateful to the National Science Foundation (grant no. CHE-9816730) for its generous financial support.
Notes and references
- D. M. J. Foo and P. J. Shapiro, Organometallics, 1995, 14, 4957–4959 CrossRef CAS.
- G. J. Matare, D. M. Foo, K. M. Kane, R. Zehnder, M. Wagener and P. J. Shapiro, Organometallics, 2000, 19, 1534–1539 CrossRef CAS.
- H. Schwemlein, L. Zsolnai, G. Huttner and H. H. Brintzinger, J. Organomet. Chem., 1983, 256, 285–289 CrossRef CAS.
- K. L. Tang Wong and H. H. Brintzinger, J. Am. Chem. Soc., 1975, 97, 5143–5146 CrossRef CAS.
- (a) D. M. J. Foo, P.-J. Sinnema, B. Twamley and P. J. Shapiro, Organometallics, 2002, 21, 1005–1007 CrossRef CAS; (b) P.-J. Sinnema, D. M. J. Foo, B. Twamley and P. J. Shapiro, J. Am. Chem. Soc., 2002, 124, 10996–10997 CrossRef CAS.
- (a) M. J. Morris, in Comprehensive Organometallic Chemistry, eds. E. W. Abel, F. G. A. Stone and G. Wilkinson, Pergamon, Oxford, 1995, Vol. 5, Ch. 7 Search PubMed; (b) R. Davis and L. A. P. Kane-Maguire, in Comprehensive Organometallic Chemistry, eds. E. W. Abel, F. G. A. Stone and G. Wilkinson, Pergamon, Oxford, 1982, Vol. 3, Ch. 26.2 Search PubMed.
- (a) S. Liu, F.-C. Liu, G. Renkes and S. G. Shore, Organometallics, 2001, 20, 5717–5723 CrossRef CAS; (b) V. V. Burlakov, P.-M. Pellny, P. Arndt, W. Baumann, A. Spannenberg, V. B. Shur and U. Rosenthal, Chem. Commun., 2000, 241–242 RSC; (c) V. V. Burlakov, S. I. Troyanov, A. V. Letov, L. I. Strunkina, M. K. Minacheva, G. G. Furin, U. Rosenthal and V. B. Shur, J. Organomet. Chem., 2000, 598, 243–247 CrossRef CAS; (d) L. H. Doerrer, A. J. Graham, D. Haussinger and M. L. H. Green, J. Chem. Soc., Dalton Trans., 2000, 813–820 RSC; (e) J. Ruwwe, G. Erker and R. Frölich, Angew. Chem., Int. Ed. Engl., 1996, 35, 80–82 CrossRef CAS; (f) X. Song and M. Bochmann, J. Organomet. Chem., 1997, 545–546, 597–600 CrossRef CAS; (g) S. J. Lancaster, M. Thornton-Pett, D. M. Dawson and M. Bochmann, Organometallics, 1998, 17, 3829–3831 CrossRef CAS; (h) M. Bochmann, S. J. Lancaster and O. B. Robinson, J. Chem. Soc., Chem. Commun., 1995, 2081–2082 RSC; (i) Y. Sun, R. E. v. H. Spence, W. E. Piers, M. Parvez and G. P. A. Yap, J. Am. Chem. Soc., 1997, 119, 5132–5143 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: details of synthesis and characterization for 4 and 5. See http://www.rsc.org/suppdata/cc/b3/b311352c/ |
| ‡ Current address: Molecular Inorganic Chemistry, University of Groningen, Neijenborgh 4, 9747 AG, Groningen. The Netherlands. |
§ Crystal data:4
+ C6H6: C49H34BCrF15N, M
= 984.58, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2), a
= 11.864(3), b
= 12.943(3), c
= 15.545(2)
Å, α
= 72.61, β
= 73.68(1), γ
= 71.90(2)°, V
= 2118.1(8)
Å3, Z
= 2, Dc
= 1.544 g cm−3, μ(Mo–Kα)
= 37.4 cm−1, F(000)
= 998. An orange parallelpiped of dimensions 0.19 × 0.11 × 0.07 mm3 was used. 5
+ 1.5C6H6: C53H37BCrF15N2, M
= 1049.66, monoclinic, space group P2(1)/n
(#14), a
= 13.975(3), b
= 19.331(4), c
= 16.878(3)
Å, β
= 90.30(3), V
= 4559.6(16)
Å3, Z
= 4, Dc
= 1.529 g cm−3, μ(Mo–Kα)
= 35.4 cm−1, F(000)
= 2132. A red block of dimensions 0.44 × 0.33 × 0.20 mm3 was used. 27874 (60130) reflections were collected at 203(2) K (83(2) K) on a Bruker SMART APEX diffractometer with Mo–Kα radiation using ω scans for 4
(5), respectively. The structures were solved by direct methods. All atoms were refined anisotropically using full materix least squares based on F2 to give R1
= 0.0645 (0.0445), wR2
= 0.1205 (0.1030) for 9713 (13043) independent reflections [|Fo| > 2σ(|Fo|), 2θ
≤ 55(60)°] and 610 (655) parameters for 4
(5), respectively. CCDC reference numbers 219696 and 219697. See http://www.rsc.org/suppdata/cc/b3/b311352c/ for crystallographic files in .cif format.
(#2), a
= 11.864(3), b
= 12.943(3), c
= 15.545(2)
Å, α
= 72.61, β
= 73.68(1), γ
= 71.90(2)°, V
= 2118.1(8)
Å3, Z
= 2, Dc
= 1.544 g cm−3, μ(Mo–Kα)
= 37.4 cm−1, F(000)
= 998. An orange parallelpiped of dimensions 0.19 × 0.11 × 0.07 mm3 was used. 5
+ 1.5C6H6: C53H37BCrF15N2, M
= 1049.66, monoclinic, space group P2(1)/n
(#14), a
= 13.975(3), b
= 19.331(4), c
= 16.878(3)
Å, β
= 90.30(3), V
= 4559.6(16)
Å3, Z
= 4, Dc
= 1.529 g cm−3, μ(Mo–Kα)
= 35.4 cm−1, F(000)
= 2132. A red block of dimensions 0.44 × 0.33 × 0.20 mm3 was used. 27874 (60130) reflections were collected at 203(2) K (83(2) K) on a Bruker SMART APEX diffractometer with Mo–Kα radiation using ω scans for 4
(5), respectively. The structures were solved by direct methods. All atoms were refined anisotropically using full materix least squares based on F2 to give R1
= 0.0645 (0.0445), wR2
= 0.1205 (0.1030) for 9713 (13043) independent reflections [|Fo| > 2σ(|Fo|), 2θ
≤ 55(60)°] and 610 (655) parameters for 4
(5), respectively. CCDC reference numbers 219696 and 219697. See http://www.rsc.org/suppdata/cc/b3/b311352c/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2004 |
