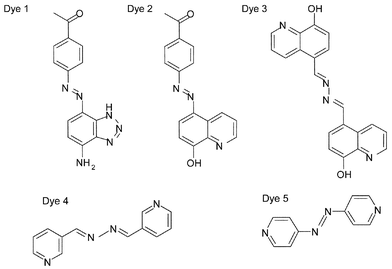
SERRS dyes
Part 2.† Syntheses and evaluation of dyes for multiple labelling for SERRS
C. J.
McHugh
,
F. T.
Docherty
,
D.
Graham
* and
W. E.
Smith
Department. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, UK G1 1XL. E-mail: duncan.graham@strath.ac.uk; Fax: 0141 552 0876; Tel: 0141 548 4701
First published on 11th December 2003
Abstract
The syntheses of a number of azo and azine dyes with various surface attachment groups is described. The dyes use different methods of achieving surface complexing and are evaluated for their suitability as multiple labels for SERRS. The surface complexing agents, 8-hydroxyquinoline, benzotriazole, and pyridine are both shown to form robust layers on the silver surface. The relative intensities of the SERRS signals from each dye were shown to be predictive by considering the molar absorption coefficient at the laser excitation frequency.
Introduction
Surface enhanced resonance Raman scattering (SERRS) has recently shown considerable potential as an extremely selective and sensitive technique for the detection and identification of a number of suitable molecules. Some analytical applications include the detection of labelled DNA1–4 and detection of explosives.5,6 The SERRS effect is obtained by adsorption of the analyte onto a suitable roughened metal surface, and by choosing an excitation frequency that is close to the frequency of a chromophore in the analyte. The sensitivity of this technique is comparable to fluorescence, but the advantage of SERRS is that the signals are much narrower and are more molecularly specific making them easier to discriminate in a mixture. This eliminates the requirement for separation and opens up the potential for simultaneous detection of multiple labels. Also, both fluorophores and nonfluorophores are suitable so a more extensive and simpler labelling chemistry can be employed.The introduction of a chromophore to a non-chromophoric analyte can be achieved by covalent attachment of functionalised dyes to create coloured molecules that can be easily detected. This can be done using extensive labelling chemistry already developed for techniques such as fluorescence, where the derivitisation of molecules containing the appropriate functional groups is relatively easy to achieve.7,8 For a dye to be an effective SERRS label it must be able to bind to the roughened metal surface and remain attached during the SERRS measurement. Although it is possible to adsorb the dye onto the surface by electrostatic attraction, the SERRS spectrum can be unreliable due to desorption, commonly observed below monolayer coverage, or orientational changes which can cause changes in intensity. Therefore for reproducible SERRS analysis and for coding nanoparticles the dyes must bind strongly to the silver surface. Here we report the syntheses of a number of new dyes for use as SERRS labels, which use different methods of surface complexing to form tightly bound layers on the surface and we evaluate their suitability for SERRS multiplexing.
Experimental
Dye synthesis
1H and 13C spectra were recorded on a Bruker DPX 400.13 MHz spectrometer or a Bruker WM 270.05MHz spectrometer. Mass spectra were recorded on a JEOL AX505 spectrometer using electron impact ionisation (EI) at 70 eV or Fast Atom Bombardment (FAB) using a 3-nitrobenzyl alcohol matrix. Reagent grade solvents were used throughout, thin layer chromatography was performed on aluminium sheets, silica gel 60 F254, 0.2 mm (Merck) and the products visualised by UV absorption at 254 nm. The following solvent system was used: (A) Dichloromethane:methanol 9:1.1-[4-(7-Amino-3H-benzotriazol-4-ylazo)-phenyl]-ethanone [1]
4-Aminoacetophenone (0.270 g, 2.0 mmol) was dissolved in 50% HCl/H2O (5 mL) with heating and then cooled to 0 °C in an icebath to precipitate the hydrochloride salt as a white paste. Addition of a chilled solution of sodium nitrite (0.152 g, 2.2 mmol) in water (2 mL) afforded a clear solution, which was added dropwise to a stirred solution of 4-amino-1H-benzotriazole (0.268 g, 2.0 mmol) dissolved in sodium acetate buffer (1 M, 100 mL, pH 6) and methanol (10 mL) to give an orange precipitate. The solids were collected by filtration, washed with water and cold methanol and then dried under vacuum to afford [1] as a red powder (0.482 g, 86%). Rf (A) 0.54. λmax (MeOH)/nm 398. 1H NMR (DMSO-d6) δ 2.62 (3H, s, CH3), 6.62 (1H, d, J = 8.40 Hz, ArH), 7.62 (2H, br s, NH2), 7.95 (1H, d, J = 8.30 Hz, ArH), 8.06–8.14 (4H, m, ArH), 15.68 (1H, br s, NH). m/z (EI) 280.10735 [C14H12N6O (M)+ < 0.3 ppm].1-[4-(8-Hydroxy-quinolin-5-ylazo)-phenyl]-ethanone [2]
4-Aminoacetophenone (1.080 g, 8.0 mmol) was dissolved in 50% HCl/H2O (5 mL) with heating then cooled to 0 °C in an icebath to precipitate the hydrochloride salt as a white paste. Addition of sodium nitrite (0.607 g, 8.8 mmol) in water (2 mL) afforded a clear solution of the diazonium salt, which was added dropwise to 8-hydroxyquinoline (1.160 g, 8.0 mmol) in 10% sodium hydroxide solution (100 mL) and methanol (30 mL). After stirring for 30 min the violet solution was acidified with 50% HCl/H2O to precipitate a dark purple solid which was collected by filtration and washed with water and cold methanol. The crude dye was recrystallised from ethanol and dried under vacuum to afford [2] as a purple powder (2.328 g, 100%). Rf (A) 0.66. λmax (MeOH)/nm 403, 477. 1H NMR (DMSO-d6) δ 2.64 (3H, s, CH3), 7.32 (1H, br s, ArH), 7.83 (1H, br s, ArH), 8.03–8.14 (5H, m, ArH), 9.00 (1H, br s, ArH), 9.35 (1H, br s, ArH). m/z (EI) 291.10057 [C17H13N3O2 (M)+ < 0.7 ppm].N,N′-Bis-quinolin-8-ol-5-ylmethylene-hydrazine [3]
To a solution of 8-hydroxyquinoline-5-carbaldehyde (0.100 g, 0.58 mmol) in ethanol (15 mL) was added dropwise hydrazine hydrate (1.032 g, 21 mmol) with vigorous stirring. After 20 min the pale milky pink precipitate was collected by filtration, washed with cold ethanol and dried to afford the azine [3] as a yellow powder (0.084 g, 84 %). λmax (DMSO)/nm 383 (ε = 29560). 1H NMR (DMSO) δ 7.21 (2H, d, J = 7.88 Hz, ArH), 7.74 (2H, dd, J = 4.04, 3.92 Hz, ArH), 8.05 (2H, d, J = 8.00 Hz, ArH), 8.95 (2H, d, J = 3.96 Hz, ArH), 9.22 (2H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.76 (2H, d, J
= 8.60 Hz, ArH). m/z
(EI) 342.11136 [C20H14N4O2(M)+ < 0.9 ppm]. Anal. Calcd for C20H14N4O2:C, 70.17; H, 4.09; N, 16.37. Found C, 69.88; H, 4.10; N, 15.56.
N), 9.76 (2H, d, J
= 8.60 Hz, ArH). m/z
(EI) 342.11136 [C20H14N4O2(M)+ < 0.9 ppm]. Anal. Calcd for C20H14N4O2:C, 70.17; H, 4.09; N, 16.37. Found C, 69.88; H, 4.10; N, 15.56.
N,N′-Bis-pyridin-3-ylmethylene-hydrazine [4]
To a solution of pyridine-3-carbaldehyde (4.494 g, 42 mmol) in ethanol (15 mL) was added dropwise hydrazine hydrate (1.032 g, 21 mmol) with vigorous stirring. After 20 min the pale precipitate was collected by filtration, washed with cold ethanol and dried to afford the azine [4] as yellow microcrystals (2.688 g, 64%). λmax (MeOH)/nm 297 (ε = 33010). 1H NMR (DMSO) δ 7.55 (2H, dd, J = 4.80, 4.80 Hz, ArH), 8.27 (2H, d, J = 7.88 Hz, ArH), 8.70 (2H, d, J = 4.64 Hz, ArH), 8.79 (2H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.02 (2H, s, ArH). m/z
(EI) 210.09184 [C12H10N4(M)+ < 6.2 ppm]. Anal. Calcd for C12H10N4: C, 68.57; H, 4.76; N, 26.66. Found C, 68.48; H, 4.71; N, 26.32.
N), 9.02 (2H, s, ArH). m/z
(EI) 210.09184 [C12H10N4(M)+ < 6.2 ppm]. Anal. Calcd for C12H10N4: C, 68.57; H, 4.76; N, 26.66. Found C, 68.48; H, 4.71; N, 26.32.
4,4′-Azopyridine [5]
The above compound was prepared according to the procedure of Schalley et al.9 and gave identical analytical data.Spectroscopy
All spectra were acquired using a Renishaw 2000 Raman Microprobe with a charge-coupled device (CCD) spectrometer. The excitation was provided by a Spectra-Physics Model 2020 argon-ion laser with a wavelength of 514.5 nm and 3 mW of power at the source. Samples were analysed in a plastic microtitre plate using a ×50 objective. The acquisition time for all spectra was 10 s and the grating was centred at 1350 cm−1.The metal used for surface enhancement was a citrate reduced silver colloid, prepared by a modified Lee and Meisel method.10,11 For each sample 250 µL of silver colloid suspension, 250 µL of distilled water, 30 µL of analyte and 10 µL of 0.067 M spermine were mixed together and the SERRS signal was immediately acquired. Some of the dyes had limited solubility in water so dimethyl sulfoxide (DMSO) was used to make dye solutions of 1 × 10−3 M and they were diluted to lower concentrations using water. All spectra have been normalised so that the most intense feature in the spectrum has an intensity of one thousand counts. For each dye, five consecutive spectra were acquired to check the spectra were reproducible. With the exception of dye 4, which has a poor signal to noise ratio, the peak positions and relative intensities were reproducible for all of the dyes.
Results and discussion
The structures of the dyes synthesised are shown in Fig. 1.12 Highly coloured 1H-benzotriazole azo dyes have been reported to give strong characteristic and reproducible SERRS signals when adsorbed on silver colloid.13 Benzotriazole is believed to adhere to silver by complexing to more than one silver ion to form a polymeric species coated on the surface and is commonly used as an anti-corrosion agent for silver due to the strong complexing properties of the triazole functionality.14 It has been shown that 1H-benzotriazole is adsorbed strongly at the surface of silver colloids and is therefore an excellent complexing group to incorporate into a dye for SERRS.15,16 Irreversible attachment to the surface is possible and the orientation of the dye will be fixed and remain in place over a wide range of experimental conditions, making it a good SERRS label. Dye 1, 1-[4-(7-amino-3H-benzotriazol-4-ylazo)-phenyl]-ethanone has been designed to include a benzotriazole surface attachment group. However, for the first time a ketone functional group has also been incorporated into the molecule. This means that the dye is reactive towards amino containing analytes and on condensation this will provide new molecular species and hence different SERRS spectra.Dye 2, 1-[4-(8-hydroxy-quinolin-5-ylazo)-phenyl]-ethanone was synthesised to investigate the use of alternative surface complexing groups. This dye has the same keto function for attachment to the analyte molecule, but the benzotriazole surface attachment group has been replaced by 8-hydroxyquinoline. Of the seven possible hydroxyquinolines, only 8-hydroxyquinoline forms chelate compounds with metals.17 The use of these chelate compounds for the detection of magnesium and aluminium is well known, but it has also been used to form chelates with silver.
Many of the dyes previously synthesised for use specifically as SERRS labels are azo dyes. In order to investigate the effect of the azo chromophore, an azine linkage has been used in place of the azo. Dye 3, N,N′-bis-quinolin-8-ol-5-ylmethylenehydrazine has the same metal attachment group as dye 2 to allow the effect of the azine group to be compared with the azo. The azo–azine comparison was further investigated by comparing dyes 4, N,N′-bis-pyridin-3-ylmethylenehydrazine and 5, 4,4′-azopyridine. Both of these complex to the metal surface through pyridine. The use of pyridine to complex with silver is synonomous with the first SERS experiments, being the first molecule to exhibit surface-enhanced Raman scattering through adsorption at the metal surface.18 Here, both ends of the molecule are expected to complex to silver, thus forming a polymeric layer on the surface. Crystal structures of two silver complexes obtained from dyes 4 and 5 indicate that this is correct.19 This is expected to provide stability to the surface species.
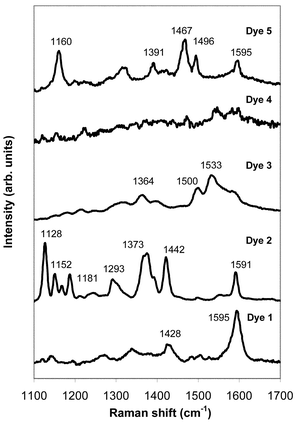
Fig. 2 shows the spectra acquired from each of the individual dyes. It can be seen from Fig. 2 that good SERRS signals are obtained from all of the dyes except dye 4. Some common features are observed in dyes that contain the same functional group. For example, the peaks observed between 1420 and 1470 cm−1 are due to azo stretches, and the peaks around 1600 cm−1 can be assigned to phenyl ring quadrant stretches. The intensities of these features compared to other features in the spectrum vary for the different dyes. This means that changes in relative intensity, as well as changes in peak position can be used to distinguish between different components in multiplex analysis using SERRS.
One area of particular interest for SERRS multiplexing is the detection of target DNA sequences which are each labelled with a unique SERRS dye. The correlation of an individual dye to a particular DNA sequence will allow detection of a number of disease states simultaneously. Therefore, to test the suitability of these dyes for multiplex analysis an attempt was made to simultaneously detect a commercially available dye-labelled oligonucleotide and dyes 1, 2 and 3. The oligonucleotide examined contained a basic priming sequence of 5′ GTG CTG CAG GTG TAA ACT TGT ACC AG 3′. The visible chromophore used was the fluorophore 2,5,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein (HEX), which was attached at the 5′ terminus. HEX is negatively charged and therefore repels the negatively charged metal surface. Surface attachment was achieved by the incorporation of positively charged modified nucleobases at the 5′-terminus next to the HEX label.20 Spermine was again used as the aggregating agent since it is known to neutralise the negatively charged phosphate backbone of the oligonucletide,21 therefore removing any repulsion between the analyte and metal surface.
To investigate the effect of using mixtures of dyes to create specific coding on the particle surfaces a similar SERRS experiment to that used for single dyes but using 30 µL of each dye solution was carried out. To ease the interpretation of the spectrum from all four analytes it is desirable that the peaks from each of the dyes have similar intensities. When the spectra from the individual dyes were acquired at the same concentration there were large variations in the intensities of the signals collected from each dye, even though the same acquisition time was always used. This suggested that the dyes had to be used in different concentrations to have similar intensities in the multiplex spectrum. The optimal concentrations were obtained by optimising the concentration in a two-dye mixture to obtain the desired intensities, then repeating the experiment to incorporate a third dye, and finally the fourth.
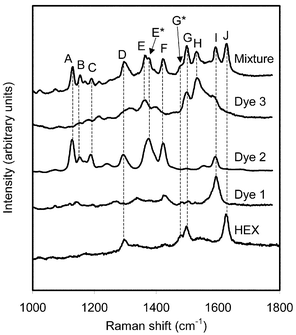
Fig. 3 shows the spectra recorded from the dye mixture and from the individual dyes at the concentrations used in the mixture. All spectra have been normalised to have the same maximum intensity in the highest peak in each spectra. In this figure dashed lines have been added linking features in the spectrum from the mixture with the spectrum from the pure sample from which they originate. Table 1 summarises the marker bands for each dye in the spectrum from the mixture and states the volumes and concentrations of the dyes used in the mixture.
| Sample | Concentration/mol L−1 | Volume/µL | Marker bands |
|---|---|---|---|
| HEX | 1 × 10−7 | 30 | D, G, G*, J |
| Dye 1 | 1 × 10−5 | 30 | I |
| Dye 2 | 1 × 10−5 | 30 | A, B, C, D, E*, F |
| Dye 3 | 1 × 10−4 | 30 | E, G, H |
It is clear that the peaks labelled A, B and C are due to the presence of dye 2. Peak D has contributions from both dye 2 and HEX. The relative intensity of this peak is higher in the spectrum from the mixture than in either of the individual spectra from HEX or dye 2 showing that the presence of both samples have contributed to this feature. A shoulder, E*, is visible on peak E. It is likely that E and E* originate from contributions from dye 3 and dye 2 respectively, which have peaks at similar positions. Feature F can be attributed to dye 2 and the peak G, and its shoulder G* look similar to the feature in HEX at that wavenumber. However, dye 3 also has a peak at the position of peak G. The relative intensity of G* compared to G has decreased in the spectrum from the mixture compared to that of pure HEX, indicating that the contribution from dye 3 has increased the intensity of peak G in the mixture. In the pure HEX spectrum peak G is less intense than the peak at higher wavenumber. The difference in intensities between these peaks is not so large in the spectrum from the mixture, providing further evidence for a contribution from dye 3. Peak H is due to the presence of dye 3 and I can be attributed to dye 1. Dye 2 also has a peak at the position of peak I. However, examination of the spectrum from dye 2 shows that this peak is less intense than the group of peaks responsible for features A–C in that spectrum. That group of peaks has a low relative intensity in the spectrum from the mixture, and the peak at 1590 cm−1 will have an even smaller contribution to the spectrum from the mixture. Therefore peak I will be dominated by the contribution from dye 1 and the effect of dye 2 can be taken to be minor. Finally, peak J can be assigned to HEX.
Table 1 shows that a wide range of concentrations are required for the dyes to give spectral features of similar intensities. If dyes of this type are to be used for multiplexing it is essential that they are used at the correct concentrations, so that the absence of the SERRS signal from an analyte is due to it not being present, rather than the SERRS intensity from it being so small that it is swamped by the signals from other compounds. Therefore it would be useful to be able to predict the concentrations of the dyes required to generate SERRS marker bands of the correct intensity. Two factors which could affect this intensity are the strength of the dye adsorption onto the metal surface, and the absorption of the molecule at the laser excitation frequency. Both of these factors are now considered.
One aim of this work was to investigate the use of 8-hydroxyquinoline as the surface attachment group, compared to the more commonly used benzotriazole group. Dyes 1 and 2 have the same structure, apart from the different surface attachment group. Table 2 lists the wavelength of the absorption maxima and the molar absorption coefficient, ε,at this wavelength and at the laser excitation wavelength for all of the dyes studied. It is clear that both dyes have similar values of ε at the laser wavelength so any differences in their SERRS signals would be due to change in the surface complexing group. However, Table 1 shows that they are used in the same concentrations to get similar intensities in the dye mixture. This indicates that the 8-hydroxyquinoline group can be used instead of benzotriazole for surface attachment without lowering the intensities of the SERRS signals attained.
| Dye | λ max/nm | ε/dm3 mol−1 cm−1 at λmax | ε/dm3 mol−1 cm−1 at 514.5 nm |
|---|---|---|---|
| 1 | 442 | 44480 | 1632 |
| 2 | 403, 477 | 21624, 6772 | 1556 |
| 3 | 410 | 32820 | 1217 |
| 4 | 297 | 33010 | 0 |
| 5 | 460 | 26250 | 31 |
| HEX | 535 | 73000 | 9882 |
The other 8-hydroxyquinoline containing dye is dye 3. In the dye mixture it has to be used at a higher concentration to give a similar SERRS intensity as dye 2. Since they have the same surface attachment group it is likely that differences in their absorption at the laser frequency is responsible for dye 3 having to be used at a higher concentration. This is confirmed in Table 2 since the value of ε is around 20% lower for dye 3 than dye 2. The large effect on the SERRS signal of the value of ε at the excitation frequency is clearly demonstrated by considering HEX, which is used at the lowest concentration in the dye mixture and has a substantially larger value of ε at 514.5 nm than for any of the other dyes.
One major difference between dyes 2 and 3 is that one is an azo and one is an azine. However, they also have other structural differences so a better understanding of the effect of the azo/azine group on ε can be gained by comparing dyes 4 and 5, which are structurally more similar than dyes 2 and 3. Both complex to the silver through pyridine to form linear polymers, so surface attachment is not the reason for the poor spectrum from dye 4 since a good signal to noise ratio is obtained from dye 5. Table 2 shows that dye 5 only absorbs very weakly at the laser frequency and no signal could be detected from dye 4 which would explain the poor SERRS signal from it. This provides further evidence that absorption from an azine is not as good as from an azo at this excitation frequency and that the value of ε at the laser frequency is a good indicator of the magnitude of the SERRS signal.
Conclusion
In conclusion, we have synthesised a number of novel dyes for use as labels for SERRS. All except one are shown to give good SERRS signals. The intensity of the SERRS signal is comparable when the surface complexing group 8-hydroxyquinoline replaces benzotriazole, which has previously been shown to be a good attachment group to silver for SERRS. The differences in peak positions and relative intensities between the dyes means that they can be easily distinguished when the SERRS is acquired from a mixture, allowing for the development of multiplexing. However, they have to be used in different concentrations to be simultaneously observed with similar intensities. The concentrations required can be predicted by examination of the molar absorption coefficient of the dye at the laser excitation frequency. We have shown that azine dyes give poorer SERRS signals than azos with 514.5 nm laser excitation and have to be used in higher concentrations to give similar SERRS intensities. The ability to predict the concentrations of dyes required will aid their use in SERRS multiplexing and the use of 8-hydroxyquinoline as a new robust surface attachment group will expand the range of dye labels available for SERRS.Acknowledgements
The authors would like to thank the RSC Analytical Trust Fund for the award of their Analytical Grand Prix to DG.References
- D. Graham, L. Fruk and W. E. Smith, Analyst, 2003, 128, 692 RSC
.
- L. Fruk, A. Grondin, W. E. Smith and D. Graham, Chem. Commun., 2002, 18, 2100 RSC
.
- D. Graham, B. J. Mallinder and W. E. Smith, Biopolymers (Biospec.), 2000, 57, 85 Search PubMed
.
- D. Graham, W. E. Smith, A. M. T. Linacre, C. H. Munro, N. D. Watson and P. C. White, Anal. Chem., 1997, 69, 4703 CrossRef CAS
.
- C. J. McHugh, W. E. Smith, R. Lacey and D. Graham, Chem. Commun., 2002, 21, 2514 RSC
.
- C. J. McHugh, R. Keir, D. Graham and W. E. Smith, Chem. Commun., 2002, 6, 580 RSC
.
- Z. R. Zhu, J. Chao, H. Yu and A. S. Waggoner, Nucleic Acids Res., 1994, 22, 3418 CAS
.
- C. Wojczewski, K. Stolze and J. W. Engels, Synlett, 1999, 1667 CAS
.
- C. A. Schalley, T. Muller, P. Linnartz, M. Witt, M. Schafer and A. Lutzen, Chem. Eur. J., 2002, 8(15), 3538 CrossRef CAS
.
- C. Munro, W. E. Smith, M. Garner, J. Clarkson and P. C. White, Langmuir, 1995, 11, 3712 CrossRef CAS
.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS
.
- G. McAnally, C. McLaughlin, R. Brown, D. C. Robson, K. Faulds, D. R. Takely, W. E. Smith and D. Graham, Analyst, 2002, 127(6), 838 RSC
.
- D. Graham, C. McLaughlin, G. McAnally, J. C. Jones, P. C. White and W. E. Smith, Chem. Commun., 1998, 11, 1187 RSC
.
- Y. C. Wu, P. Zhang, H. W. Pickering and D. L. Allara, J. Electrochem. Soc., 1993, 140, 2791 CAS
.
- M. M. Muisiani, G. B. Mengoli, M. Fleischmann and R. B. Lowry, J. Electroanal. Chem., 1987, 217, 187 CrossRef
.
- H. Yeung, H. Chan and M. J. Weaver, Langmuir, 1999, 15, 3348 CrossRef
.
- J. P. Phillips, Chem. Rev., 1956, 56, 271 CrossRef CAS
.
- M. Fleischmann, J. Phys. Lett., 1974, 26, 163 Search PubMed
.
-
A. R. Kennedy, P. C. Andrikopoulos, D. R. Armstrong, K. G. Brown, M. Clarke, D. Graham, J. B. Kirkhouse, C. Major, C. J. McHugh, P. Murdoch, S. J. Teat and W. E. Smith, unpublished
.
- D. Graham, B. J. Mallinder and W. E. Smith, Angew. Chem. Int. Ed., 2000, 39, 1061 CrossRef CAS
.
- H. S. Basu and L. J. Marton, Biochem. J., 1987, 244, 243 CAS
.
Footnote |
| † For Part 1 see ref. 12 |
| This journal is © The Royal Society of Chemistry 2004 |