Ion exchange chromatography and high precision isotopic measurements of zirconium by MC-ICP-MS
Maria
Schönbächler
*,
Mark
Rehkämper
,
Der-Chuen
Lee
and
Alex N.
Halliday
ETH Zürich, Institute of Isotope Geology and Mineral Resources, 8092 Zürich, Switzerland
First published on 3rd December 2003
Abstract
This paper presents a new technique for the precise and accurate determination of Zr isotopic compositions in geological samples. Following the separation of Zr from the geological matrix with a two-stage anion-exchange procedure the isotopic compositions are measured by multiple collector ICP-MS. Replicate dissolutions of the carbonaceous chondrite Allende with <100 ng Zr yield a long-term reproducibility of ±39 ppm for 91Zr/90Zr, ±25 ppm for 92Zr/90Zr, and ±82 ppm for 96Zr/90Zr. Analyses of synthetic standards solutions show that isobaric interferences of Mo and Ru can be adequately corrected for Mo/Zr ≤ 0.5 × 10−2 and Ru/Zr ≤ 1 × 10−2 and such elemental ratios are readily achieved for geological samples following the anion-exchange procedure. It is furthermore shown that the chemical separation technique effectively isolates Zr from Ti, Cr, and Fe. This is important because the Zr isotope data can be readily biased by the argides of these elements. The presented method has been successfully applied to terrestrial igneous rocks, meteorites and mineral separates including samples with high Ti contents.
1 Introduction
The element Zr is of special cosmochemical interest, mainly because of the production of 92Zr from the decay of the now-extinct radionuclide 92Nb, which has a half-life of 36 Myr.1 With this half-life, the 92Nb–92Zr system may be useful as a chronometer of early solar system development provided that (1) the initial abundance of 92Nb was sufficiently high and (2) there were processes that fractionated Nb/Zr. Moreover, Zr isotopes offer the opportunity to study nucleosynthetic processes,2 because they have different production mechanisms. The nuclide 96Zr is primarily produced by the r-process, which is thought to take place in a supernova environment. All other Zr isotopes (90Zr, 91Zr, 92Zr, 94Zr) are predominately formed by the s-process, presumably in AGB stars.With special focus on determining the initial abundance of 92Nb in the solar system, diverse efforts have been made to measure Zr isotopic compositions accurately and precisely during the past twenty years.3–12 The early studies, which were performed by thermal ionization mass spectrometry (TIMS), were hampered by the high first ionization potential of Zr. This problem was overcome by the development of multiple collector inductively coupled plasma mass spectrometry (MC-ICP-MS). The ICP ion source of these instruments produces ionization yields of >90% for most elements of the periodic table.13 This instrumental advance has renewed the search for anomalies in Zr isotopic compositions from the decay of extinct 92Nb. Although some recent studies found hints for formerly live 92Nb,6,9–11 they did not provide the most reliable evidence for its existence in the early solar system: an internal isochron from a meteorite containing a co-genetic suite of phases that remained undisturbed after their formation. Therefore, we developed a new analytical procedure for the determination of Zr isotopic compositions at high precision for various geological materials, with the ultimate aim of producing internal meteorite isochrons. This method includes, first, a selective dissolution procedure that is used in conjunction with conventional physical methods to obtain the required mineral separates. Second, we developed an optimized chemical separation procedure for Zr. It efficiently isolates Zr from matrix elements for different sample types, including chromite, ilmenite, terrestrial igneous whole rocks and stony meteorites. A clean separation of Zr from abundant elements such as Ti, Cr and Fe is particularly important for the MC-ICP-MS analyses, because these elements form argides that interfere with the Zr isotopes. Third, we present protocols for the precise and accurate determination of Zr isotopic compositions by MC-ICP-MS. A separate method that utilizes quadrupole ICP-MS was designed to determine Nb/Zr ratios, which are required for Nb–Zr chronology.14 The latter analyses are conducted with sample aliquots that do not undergo chemical separation in order to avoid any chemical fractionation of Nb from Zr during processing. Spectral interferences are minimized by using dynamic reaction cell technology with hydrogen as reactive gas.
2 Sample preparation
2.1 Reagents and materials
All acids are purified once or twice (HF) by sub-boiling distillation in quartz or Teflon stills, except for the H2SO4, where Merck ultrapure sulfuric acid (96%) is employed. Reagent grade H2O2, CS2 and 18 MΩ grade water from a Millipore system is used. Saturated bromine water, which is required for sulfide digestion, is prepared by the equilibration of approximately 5 ml of high purity bromine (99.998%, Alfa-Aesar) with 100 ml of purified water in a 250 ml Teflon bottle.2.2 Dissolution of whole rocks and mineral separates
Whole rock samples of stony meteorites are crushed in a boron carbide mortar under a laminar flow of filtered air to avoid contamination. The terrestrial samples are processed in the same way, but in a different clean environment. Terrestrial and meteorite whole rock samples of up to 150 mg are digested in 16 ml Savillex® vials placed inside a 125 ml Parr® bomb. A mixture of 3 ml concentrated HF and 700 µl concentrated HNO3 is used for digestion during 4 ½ days at a temperature of 180 °C. Subsequently, the samples are dried down and completely re-dissolved in 6 ml 6 M HCl on a hot plate for 1 day. Instead of HCl, 4 ml of aqua regia is used, if the sample contains carbonaceous material. Traces of HF are added after this dissolution step. This ensures that all Zr and Nb is in solution and avoids possible fractionation between these two elements.Mineral separates of pyroxene, olivine and feldspar are obtained using a Frantz magnetic separator. Up to 1.5 g of these mineral separates are dissolved on a hotplate, first in 15 ml of concentrated HF and HNO3 (2:1), followed by 25 ml 7 M HNO3 and as a last step in 30 ml 6 M HCl and traces of HF. The samples are dried down between each step. For feldspar, some minor Ca and Mg precipitate may not completely dissolve in the last HCl step. However, this does not reduce the yield of the ion-exchange separation or fractionate Nb from Zr.
2.3 Selective dissolution
The determination of Nb–Zr isochrons requires mineral separates with different Nb/Zr ratios. However, most minerals do not significantly fractionate Nb from Zr. The most important exceptions are rutile (TiO2), zircon (ZrSiO4) and ilmenite (FeTiO3). Minerals such as ilmenite, however, are not easily separated by conventional methods (Frantz magnetic separator and heavy liquids) from bulk ordinary chondrites and, to a lesser extent, from eucrites. The separation is hampered by abundant inclusions, which render the magnetic separation ineffective, and by high contents of chromite (FeCr2O4) and troilite (FeS) with similar densities as ilmenite. Selective dissolution offers the possibility to overcome these problems.After crushing, the sample is washed and sieved under a laminar flow of filtered air. The metal is separated with a hand magnet, followed by a heavy liquid separation with Clerici solution. The metal needs to be removed prior to the heavy liquid separation to avoid corrosion of the metal. The extracted heavy mineral fraction consists mainly of troilite, chromite, ilmenite and possibly some zircons. This fraction is further cleaned by handpicking and sequentially treated with a series of reagents to selectively dissolve troilite, ilmenite and chromite.
In the first step, 2 M HNO3 and 25% bromine water are added to the heavy mineral fraction in a Savillex® vial to digest troilite. This needs to be done with caution due to the formation of toxic H2S. After 2½ h on a hot plate at ∼100 °C, the supernatant is decanted. The remaining grains and precipitated sulfur (from the oxidation of sulfide) are washed with water (which is combined with the decanted acid) and distilled ethanol. Then CS2 is added and the solution is left to stand for 1 h to dissolve precipitated sulfur. Following the removal of CS2, the residue is washed again with distilled ethanol and all steps starting with the addition of 2 M HNO3 and 25% bromine water are repeated.
This procedure leaves ilmenite and chromite unaltered, as was verified by optical examination under the binocular microscope and by weighing ilmenite and chromite before and after the procedure. Nevertheless, some leaching of the grain surfaces can not be excluded. Exposure of the minerals to the HNO3–bromine water mixture for one to several days on the hotplate, leads to etching of the ilmenite surfaces while chromite appears unaffected. Minor amounts of sulfur precipitate may remain with the chromite and ilmenite.
In the second step, ilmenite is dissolved by adding concentrated HCl and HF (1:1). After 3 h on a hot plate (∼130 °C), the ilmenite is completely dissolved. The supernatant is decanted and the residual chromite grains are washed with water. This fraction is also checked for the presence of zircons under a binocular microscope.
The third step comprises the digestion of chromite in concentrated HCl. Complete dissolution of the chromite is achieved after three days in a Savillex® vial placed inside a Parr® bomb at 180 °C.
3 Ion-exchange chromatography
The two-stage procedure for Zr separation (Table 1) applies anion exchange chromatography with Bio-Rad AG1-X8 resin (200–400 mesh, chloride form). The first step is adapted from the primary column of the Hf chemistry developed by Salters and Hart.15 The same method was previously used for the separation of W,16 but on a different scale. After the digestion, the sample solution is dried down at 100 °C. 2 ml of 4 M HF is added and allowed to sit for at least 2 h. Depending on the composition of the sample, some insoluble fluorides (mainly Ca–Mg fluorides) may form. This occurs for terrestrial and chondritic whole rock samples and mineral separates of chromite, feldspar and pyroxene. If fluorides precipitate, the sample is treated in an ultrasonic bath, centrifuged and decanted; the residue is subsequently treated twice with 1.5 ml 4 M HF, such that a total of 5 ml of acid is collected. This washing procedure enhances the total Zr yield by up to 7% for chromite separates. Ilmenite samples do not produce precipitates of Ca–Mg fluorides in HF and are directly loaded with 1 ml of 4 M HF. Ilmenite contains large amounts of Ti, which are adsorbed onto the resin in 4 M HF. Thus, each column is loaded with a maximum of ∼10 mg ilmenite to avoid overloading. For other sample types (whole rocks, silicates), the column has sufficient capacity to process about 130 mg of material.| Resin: Bio-Rad, AG1-X8, 200–400 mesh, chloride form (0.7 ml) | |||||
|---|---|---|---|---|---|
| 1. Column | 2. Column | ||||
| Step | Volume | Acid | Step | Volume | Acid |
| Cleaning | 8 ml | 3 M HCl–10 M HF | Cleaning | 8 ml | 3 M HCl–10 M HF |
| 5 ml | 6 M HCl–1 M HF | 5 ml | 6 M HCl–1 M HF | ||
| 5 ml | H2O | 5 ml | H2O | ||
| Preconditioning | 6 ml | 4 M HF | Preconditioning | 6 ml | 0.25 M H2SO4–1% H2O2 |
| Load sample | 1–5 ml | 4 M HF | Load sample | 1.5 ml | 0.25 M H2SO4–1% H2O2 |
| Rinse matrix | 8 ml | 4 M HF | Rinse matrix | 8 ml | 0.25 M H2SO4–1% H2O2 |
| Zr | 2 ml | 6 M HCl–1 M HF | Zr | 2 ml | 6 M HCl–1 M HF |
| W | 3 ml | 6 M HCl–1 M HF | |||
The first column with 0.7 ml of resin is preconditioned with 6 ml 4 M HF (Table 1). After loading of the sample dissolved in 1–5 ml 4 M HF, the matrix is eluted with 8 ml 4 M HF, while Zr, Hf, Ti, Mo, Te and W remain on the column. This behaviour is expected from the distribution coefficient for these elements.17 Zirconium is stripped from the column with 2 ml 6 M HCl–1 M HF together with Ti and Hf.18 Addition of further 3 ml 6 M HCl–1 M HF elutes W. There may be some minor W (<10% of the total W) in the Zr fraction, but this can be recovered in the following step.
The second separation step (Table 1) is based on the methods of Barovich et al.19 for the isolation of Hf from Ti-rich matrices. It was scaled down for use with 0.7 ml of resin and slightly modified to achieve a better separation of Ti from Zr. This separation is very important for Zr isotopic measurements because of the formation of Ti-argides, which may produce interferences for some Zr isotopes (Table 2). This is particularly true for ilmenite, which is an important mineral for Nb–Zr chronometry. Ilmenite can consist of more than 50 wt.% TiO2 and therefore, a reliable separation procedure, which can deal with high Ti contents, is required. The Zr fraction obtained from the first column is evaporated and taken up in 1.5 ml of freshly prepared 0.25 M H2SO4–1% H2O2. This solution is loaded onto the second column, which was previously preconditioned with 6 ml of 0.25 M H2SO4–1% H2O2. Titanium is stripped from the column with 8 ml 0.25 M H2SO4–1% H2O2, followed by the elution of Zr and Hf with 2 ml 6 M HCl–1 M HF. The separation of Ti is efficient, such that only traces of Ti elute in the Zr fraction, which displays Ti/Zr ≪ 1. A further separation of Zr from Hf is not necessary prior to the MC-ICP-MS measurements. Tungsten can be eluted, if this is desired, by addition of 3 ml 6 M HCl–1 M HF.
| Isotope | 90Zr | 91Zr | 92Zr | 94Zr | 96Zr |
|---|---|---|---|---|---|
| a The upper limit indicates the maximum elemental contents that are permissible for the Zr solutions. Higher concentrations lead to erroneous Zr isotope data. b An interference correction is applied to account for the isobaric interferences from Mo and Ru. The Mo and Ru interferences can be adequately corrected, if the concentrations do not exceed the indicated limits. c The actual upper limit may be higher, but sample solutions are kept cleaner than this by the ion exchange chemistry. | |||||
| Interference: | |||||
| Isobaric | 92Mo | 94Mo | 96Mo | ||
| 96Ru | |||||
| Double charge | 180Hf++ | 182W++ | 184W++ | ||
| Argides | 50Ti40Ar | 51V40Ar | 52Cr40Ar | 54Fe40Ar | 56Fe40Ar |
| 40Ar214N | 40Ar216O | ||||
| upper limit:a | |||||
| Mo/Zrb | 0.01 | 0.005 | |||
| Ru/Zrb | 0.01 | ||||
| Ti/Zr | 1c | ||||
| V/Zr | 0.3c | ||||
| Cr/Zr | 0.3 | ||||
| Fe/Zr | 0.9 | ||||
The yields for the two-stage procedure have been measured semi-quantitatively. To this end, the sample solutions and Zr standards of known concentration were spiked with 84Sr. The Zr abundances were then determined by the comparison of the 90Zr/84Sr and 91Zr/84Sr ratios obtained for samples and the Zr standards. The yields are estimated to be between 70 and 100%. Total procedural chemistry blanks, including sample dissolution and ion exchange separation are typically <150 pg. If Teflon bombs are used for the digestion of the sample, the blanks can be as high as 1000 pg.
4 Mass spectrometry
4.1 Instrumentation and data collection protocols
All measurements have been performed with a Nu Plasma MC-ICP-MS at ETH Zürich. Like other MC-ICP-MS instruments, the Nu Plasma combines an ICP ion source with a double focusing magnetic sector mass spectrometer. Its characteristic feature is the combination of fixed Faraday cups with variable dispersion zoom optics.20 The instrument is used in conjunction with a Cetac MCN 6000 desolvating nebuliser at uptake rates between 80 and 120 µl min−1. The masses from 90 to 97 amu are measured with Faraday cups and 1011 Ω resistors in a single cycle. A second cycle, which immediately follows the first, is required for the measurement of mass 99, which is needed for the Ru correction. The zoom optics and the laminated magnet of the Nu Plasma allow for fast switching between two different collector configurations. The Ru correction is only applied if necessary. This is checked on a small sample aliquot prior to the actual Zr isotopic measurement.In each measurement, 80 ratios (5 s integrations) are collected in blocks of 20. On-peak baselines are measured for 15 s prior to each block, while the ion beam is deflected by the electrostatic analyzer. A measurement that includes one cycle requires ∼10 min and consumes 100 ng Zr for a Zr solution of 100 ng ml−1. Such solutions yield total Zr ion beams intensities of 4 × 10−11–6 × 10−11 A. Before analyzing samples, the performance of the instrument is checked with several runs of Zr standard solutions. The samples are always measured interspersed between runs of Zr standards at concentrations that are adjusted to match the samples to within 20%. Between the analyses, the sample introduction system is cleaned with 2% HNO3–0.01% HF. Washout of Zr usually requires <5 min.
4.2 Data processing and interference correction
Instrumental mass fractionation is internally normalized to 94Zr/90Zr = 0.33813 with the exponential law. The 94Zr/90Zr ratio is used for normalization because this yielded the most precise and reproducible Zr isotope data. The interference of 94Mo on 94Zr (Table 2) is corrected with the following procedure. First, the mass fractionation of all relevant ratios is corrected by normalization to 91Zr/90Zr = 0.21798,3 because both 90Zr and 91Zr are free of isobaric interferences. Then the Mo interference on mass 94 is estimated based on the measured intensity at mass 95 using the Mo isotopic abundances of Lee and Halliday.21 The obtained 94Mo/90Zr ratio is subtracted from the measured 94(Zr+Mo)/90Zr ratio. The resulting 94Zr/90Zr ratio is corrected for both Mo and mass fractionation. The mass fractionation correction is back calculated to obtain the true measured 94Zr/90Zr ratio, which in turn can be used to determine the mass fractionation based on 94Zr/90Zr. This correction procedure is repeated and the newly obtained true measured 94Zr/90Zr ratio is used to correct for mass fractionation in the next iteration step until the calculated true measured 94Zr/90Zr ratios converge.Molybdenum interferences on 92Zr and 96Zr also are corrected using 95Mo as the interference monitor and 94Zr/90Zr for normalization. The Ru interference on 96Zr is estimated based on 99Ru and the Ru isotopic abundances of De Bièvre et al.22
5 Results and discussion
5.1 Precision and accuracy of standard solutions
Four Zr standard solutions were analyzed in this study. These are NIST SRM 3169, ICP standard solutions from Aldrich and Alfa-Aesar, and a solution made of the JMC (Johnson Matthey Company) Zr metal. All four solutions have the same Zr isotopic composition, within the analytical uncertainty (Fig. 1). This result does not agree with the TIMS data of Sahoo and Masuda.7 These workers analyzed various terrestrial Zr standards and they found significant differences in the Zr isotopic composition. The most striking feature of their data, which displays a precision similar to that obtained in the present study, is a difference in 96Zr/90Zr of ∼0.86‰ between an Aldrich and a JMC Zr standard. Our data, however, do not display such variations (Fig. 1). This uniformity in the Zr isotopic composition for terrestrial materials is supported by the results of other workers, who analyzed Zr isotopes in various terrestrial samples.8,10,12![Zirconium isotopic compositions for Zr standard solutions (NIST SRM 3169, Aldrich and Alfa-Aesar ICP standards, solution of JMC Zr metal). Each point reflects the mean of at least 6 runs. Error are 2σ standard deviations. ε9xZr =
{[(9xZr/90Zr)meas
−
(9xZr/90Zr)std]/(9xZr/90Zr)std}
× 104, x
= 1,2,6.](/image/article/2004/AN/b310766c/b310766c-f1.gif) | ||
| Fig. 1 Zirconium isotopic compositions for Zr standard solutions (NIST SRM 3169, Aldrich and Alfa-Aesar ICP standards, solution of JMC Zr metal). Each point reflects the mean of at least 6 runs. Error are 2σ standard deviations. ε9xZr = {[(9xZr/90Zr)meas − (9xZr/90Zr)std]/(9xZr/90Zr)std} × 104, x = 1,2,6. | ||
In the present study, long-term averages of 91Zr/90Zr = 0.217926 ± 23, 92Zr/90Zr = 0.333376 ± 25, 96Zr/90Zr = 0.054371 ± 8 (all 2σ standard deviations from the mean) were obtained for analyses conducted on 40 separate measurement sessions over a period of 2 years. These results lie in the range of previously published data acquired by both TIMS and MC-ICPMS (Rehkämper et al.,20 see Münker et al.23 for compilation). The external precision for standard measurements conducted on a single day is always better than ±40 ppm for 91Zr/90Zr, ±30 ppm for 92Zr/90Zr, and ±120 ppm for 96Zr/90Zr. These are maximum values, however, and the typical external precision is ±30 ppm, ±20 ppm and ±80 ppm, for 91Zr/90Zr, 92Zr/90Zr and 96Zr/90Zr, respectively. The maximum values are used as a conservative estimate of the analytical uncertainty for the Zr standard measurements.
5.2 Spectral interferences and ion-exchange chemistry
For precise and accurate isotopic measurements by MC-ICP-MS, it is crucial to pay attention to spectral interferences. Isobaric interferences from Mo and Ru (Table 2) are particularly problematic for Zr. Our ion-exchange procedure ensures a good separation of Zr from Mo, because Mo remains on the column in both the first and the second stage. For the terrestrial and extraterrestrial samples, the purified fractions have Mo/Zr ratios that are always less than 1 × 10−3. Moreover, they also possess Ru/Zr ratios that are generally less than 1 × 10−3 even for Ru-rich samples, such as primitive chondrites. However, despite the good chemical separation, a correction for the isobaric interferences of Mo and Ru is necessary. Analyses of Zr standard solutions, which were doped with Mo and Ru, were used to investigate the reliability of the correction procedures for these elements. The Mo correction is accurate up to a Mo/Zr ratio of 1 × 10−2 for 92Zr and ∼0.5 × 10−2 for 96Zr (Fig. 2a, Table 2). Larger amounts of Mo result in over- or undercorrected Zr isotopic ratios (Fig. 2a). Interferences from Ru can be adequately corrected for Ru/Zr up to 1 × 10−2 (Fig. 2b). These results indicate that our chemical separation procedure yields Zr fractions with Mo and Ru concentrations that are low enough, such that the isobaric interferences of these elements can be adequately corrected during the analyses.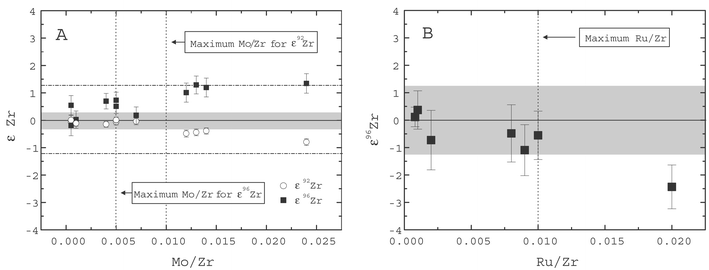 | ||
| Fig. 2 Zr isotopic compositions obtained for Alfa Zr standard solutions doped with (A) Mo and (B) Ru. (a) An accurate correction for 92Zr is possible up to Mo/Zr of 1 × 10−2, whereas for 96Zr the correction breaks down above ∼0.5 × 10−2. The gray bar shows the external reproducibility of the standard (2σ) for ε92Zr, the dashed horizontal lines for ε96Zr. Errors on data points are 2σ in-run precisions. (b) The Ru interference on 96Zr can be adequately corrected for Ru/Zr up to ∼1 × 10−2. The correction is usually negligible for terrestrial samples, while it is crucial for most of the primitive meteorites, because of their high Ru contents. | ||
A particular difficulty of Zr isotopic measurements is that elements such as Ti, Cr and Fe are present at high concentration in many geological samples and they form argides that interfere with the masses of Zr (Table 2). This problem is circumvented by the ion-exchange procedure, because this efficiently separates Zr from these elements. The small residual amounts that are present in the Zr fraction, do not disturb the Zr isotopic measurements. No corrections are, therefore, needed for these interferences and this is ascertained by checking the concentrations of these elements on a small aliquot prior to the isotopic measurement.
Titanium, V, Cr and Fe are efficiently separated from Zr and the Zr fractions generally display lower element/Zr ratios than required for accurate data acquisition (Table 2). Particular care is needed, if Cr or V is enriched in samples, which is the case for chromite. Passing V-rich samples through the second column twice optimizes the V/Zr ratio. Following loading of the secondary columns with Cr-rich samples, the exposition of the resin to air for more than ∼5 min should be avoided. Otherwise, some Cr is eluted in the Zr fraction. This results in a Cr/Zr ratio >0.3, which may lead to erroneous Zr isotopic data. The elution of Cr is probably due to the oxidation of Cr3+ to Cr6+ by air on the surface of the ion-exchange resin. Hexavalent Cr is adsorbed onto the resin in 4 M HF, but partially released by 6 M HCl–1 M HF. The maximum contents of Ti, V, Cr and Fe that can be tolerated in the sample solutions were determined by analyzing Zr standards doped with these elements. The limits for Cr (Fig. 3) and the other elements (Table 2) are set conservatively to account for variations in the argide formation rates. Such variations are observed between different measurement session. A Fe/Zr ratio of 0.9 in the sample solutions does not compromise the Zr isotopic measurement, while the Cr/Zr ratio needs to be less than ∼0.3. Therefore, more Fe can be tolerated in the sample solution than Cr. This is surprising, because 52Cr and 56Fe have similar isotopic abundances and because 96Zr is much less abundant than 92Zr (2.8% vs. 17.1%). This indicates that Cr-argides have a higher formation rate than Fe-argides.
 | ||
| Fig. 3 Zr isotopic compositions obtained for the Alfa Zr standard solution doped with variable amounts of Cr. Chromium/Zr ratios of up to 0.3 are acceptable, but higher amounts of Cr may significantly influence ε92Zr. The Cr/Zr ratios are therefore checked prior to the measurements. The gray bar denotes the external reproducibility of the standard (2σ). | ||
Large amounts of Nb may generate peak-tailing effects on 92Zr and 94Zr. However, only high Nb/Zr ratios of >6 start to influence the Zr isotopic measurements. Our chemical separation produces Zr fractions that have much lower Nb contents.
Repeated analyses of Zr standards with different concentrations reveal an unidentified interference on 91Zr (Fig. 4), which is common to all standard and sample solutions. The interference is of identical magnitude for samples and standards and this indicates that it is not due to the contamination of solutions with V, Mn, or As, which may form 51V40Ar+, 55Mn36Ar+, or 75As16O+, respectively. No other Zr isotope displays such an interference, and this implies that it is unrelated to the presence of 53Cr38Ar+, 77Se14N+, or doubly charged 182W2+. The interference appears to be present as a low-level background during all analyses. Our results demonstrate that the interference is insignificant when large Zr ion beams are monitored (total ion beam ≥2.5 × 10−11 A). It can furthermore be compensated at lower ion beam intensities by analyzing samples relative to standard solutions that have similar (±20%) Zr concentrations.
 | ||
| Fig. 4 Ratios of 91Zr/90Zr for variably diluted solutions of NIST SRM 3169 Zr. The different total ion beam intensities correspond to Zr solutions with concentrations of 200 ppb, 70 ppb, 50 ppb and 20 ppb. Errors are 2σ in-run precisions. The results indicate the presence of an unknown interference on 91Zr. The ε91Zr data of samples are therefore determined relative to standards that are analyzed using the same or similar (±20%) ion beam intensities. | ||
5.3 Reproducibility and accuracy of sample measurement
The external reproducibility for sample measurements was estimated from 12 analyses of bulk Allende, a carbonaceous chondrite (Table 3). Most of the Allende data are for separate dissolutions of a homogenous sample powder. The external reproducibility is ±39 ppm for 91Zr/90Zr, ±25 ppm for 92Zr/90Zr and ±82 ppm for 96Zr/90Zr (Table 3). This is only slightly worse than the typical precision achieved for pure standard solutions. The external reproducibility estimated in this way, accounts for the total uncertainty introduced through sample digestion, ion exchange chemistry and mass spectrometry. Furthermore, the Allende data are in excellent agreement with results of previous isotopic studies (Table 3) and this demonstrates that our measurements are both precise and accurate.| Sample | ε 92Zr | ±2σ | ε 91Zr | ±2σ | ε 96Zr | ±2σ |
|---|---|---|---|---|---|---|
| a The data were acquired over a period of 2 years. Allende 1–4 are repeat measurements of the same sample solution; all other data are for separate dissolutions. Errors are 2σ in-run precision, except for those of the average composition, which represent the 2σ standard deviation. b Data from Schönbächler et al.2 c Münker et al.10 d Sanloup et al.11 | ||||||
| Allendeb | 0.22 | 0.12 | 0.51 | 0.12 | 1.18 | 0.31 |
| Allendeb | −0.09 | 0.20 | 0.15 | 0.23 | 0.95 | 0.66 |
| Allendeb | 0.04 | 0.12 | −0.04 | 0.17 | 1.08 | 0.37 |
| Allendeb | 0.07 | 0.29 | 0.46 | 0.26 | 0.66 | 0.74 |
| Allendeb | −0.07 | 0.14 | 0.10 | 0.20 | 1.27 | 0.47 |
| Allende 1 | −0.08 | 0.15 | −0.07 | 0.21 | 0.24 | 0.59 |
| Allende 2 | −0.09 | 0.13 | 0.19 | 0.19 | 0.40 | 0.52 |
| Allende 3 | 0.14 | 0.15 | 0.16 | 0.16 | 0.68 | 0.48 |
| Allende 4 | 0.01 | 0.15 | 0.01 | 0.18 | 1.29 | 0.45 |
| Allende | 0.08 | 0.14 | −0.05 | 0.16 | 1.39 | 0.43 |
| Allende | 0.27 | 0.16 | 0.03 | 0.20 | 1.43 | 0.59 |
| Allende | −0.10 | 0.17 | −0.04 | 0.22 | 1.40 | 0.65 |
| Average | 0.04 | 0.25 | 0.12 | 0.39 | 1.00 | 0.82 |
| Allendec | −0.30 | 0.50 | ||||
| Allended | −0.09 | 0.15 | 0.18 | 0.30 | ||
The reliability of the method was further tested by analyzing various terrestrial whole rocks and minerals, including ilmenite and chromite (Table 4). For all samples, the measured isotopic ratios are identical to the terrestrial standard given the analytical uncertainty. The Zr isotopic composition of the Earth is expected to be homogenous because extensive mixing should have destroyed any early reservoir with distinct Zr isotopic compositions and there are no known terrestrial processes that can produce mass-independent Zr isotopic anomalies. The uniformity of our results (Table 4) demonstrates that the analytical method does not suffer from spectral interferences or matrix effects. The procedure is, therefore, suitable for the accurate and precise determination of Zr isotopic compositions in a wide range of geological samples with different compositions including meteorites and minerals such as ilmenite and chromite.
| Sample | ε 92Zr | ±2σ | ε 91Zr | ±2σ | ε 96Zr | ±2σ | |
|---|---|---|---|---|---|---|---|
| a The errors (2σ) include the external reproducibility of the sample and the standard. Those are combined as: σ2total = σ2std + σ2sam, where σ is the external reproducibility of the standard (std) or the sample (sam). b Data from Schönbächler et al.24 | |||||||
| ATHO rhyoliteb | Iceland | −0.29 | 0.37 | 0.10 | 0.54 | 0.79 | 1.36 |
| ATHO rhyolite | Iceland | 0.11 | 0.40 | 0.26 | 0.55 | −0.27 | 1.44 |
| BTHO basaltb | Iceland | −0.15 | 0.38 | 0.12 | 0.53 | −0.31 | 1.34 |
| BIR-1 basaltb | Iceland | −0.04 | 0.36 | −0.05 | 0.52 | 0.30 | 1.35 |
| C235A lherzoliteb | Cameroon line | 0.20 | 0.37 | −0.27 | 0.52 | 0.13 | 1.30 |
| Ilmeniteb | Russia | 0.01 | 0.36 | −0.30 | 0.51 | 0.01 | 1.31 |
| Chromite | South Africa | −0.09 | 0.38 | 0.01 | 0.56 | 1.02 | 1.40 |
| Zirconb | Jack Hills, Aus, 4.01 Ga | 0.13 | 0.36 | 0.25 | 0.52 | −0.06 | 1.31 |
| Zirconb | Jack Hills, Aus, 3.5 Ga | 0.29 | 0.44 | 0.27 | 0.53 | −0.57 | 1.32 |
| Zirconb | Jack Hills, Aus, 4.1 Ga | 0.01 | 0.41 | 0.20 | 0.51 | 0.55 | 1.29 |
| Zirconb | Jack Hills, Aus, 3.8–4.1 Ga | 0.22 | 0.44 | 0.24 | 0.53 | 0.04 | 1.33 |
| Zirconb | Jack Hills, Aus, 3.7 Ga | −0.12 | 0.42 | 0.04 | 0.51 | −0.15 | 1.31 |
| Zirconb | Jack Hills, Aus, 4.0 Ga | −0.12 | 0.40 | 0.02 | 0.51 | 0.45 | 1.31 |
| Zirconb | Jack Hills, Aus, 3.4 Ga | 0.02 | 0.40 | 0.07 | 0.51 | 0.27 | 1.30 |
| Zirconb | Jack Hills, Aus, 3.4 Ga | −0.13 | 0.43 | 0.07 | 0.52 | −0.07 | 1.32 |
| Zirconb | Jack Hills, Aus, 4.0 Ga | 0.00 | 0.38 | 0.13 | 0.51 | 0.18 | 1.30 |
| Zirconb | Jack Hills, Aus, 3.4 Ga | −0.01 | 0.41 | −0.02 | 0.52 | 0.63 | 1.32 |
| Zirconb | Acasta Gneisses, Canada, 3.6 Ga | −0.08 | 0.42 | −0.13 | 0.52 | 0.61 | 1.31 |
| Zirconb | Acasta Gneisses, Canada, 3.5 Ga | 0.15 | 0.42 | −0.14 | 0.52 | 0.03 | 1.31 |
6 Conclusion
A new method is presented for the determination of Zr isotopic composition in geological samples at high precision. Following the chemical separation of Zr by ion exchange chromatography, the isotopic measurements are conducted by MC-ICP-MS. The technique is suitable for analyses of terrestrial and extraterrestrial rocks, including samples with high contents of Ti and Cr. This renders the procedure particularly useful for Zr isotopic measurements of various mineral separates. The method has been successfully applied to terrestrial samples and bulk meteorites ranging from carbonaceous chondrites to differentiated meteorites such as eucrites.2 Furthermore, the first internal isochrons for the 92Nb–92Zr system were produced with this analytical procedure.24Acknowledgements
We thank the Swiss National Science Foundation and ETH for support of this research, G. L. MacPherson for providing the Allende meteorite sample (USNW-6159) and the two anonymous referees for their helpful comments.References
- D. R. Nethaway, A. L. Prindle and R. A. Van Konynenburg, Phys. Rev. C, 1978, 17, 1409 CrossRef CAS.
- M. Schönbächler, D.-C. Lee, M. Rehkämper, A. N. Halliday, M. A. Fehr, B. Hattendorf and D. Günther, Earth Planet. Sci. Lett., 2003, 216, 467 CrossRef CAS.
- J. F. Minster and L. P. Ricard, Int. J. Mass Spectrom. Ion Phys., 1981, 37, 259 CrossRef CAS.
- J. F. Minster and C. J. Allègre, Geochim. Cosmochim. Acta, 1982, 46, 565 CAS.
- M. Nomura, K. Kogure and M. Okamoto, Int. J. Mass Spectrom. Ion Phys., 1983, 50, 219 CrossRef CAS.
- C. L. Harper, Jr, Astrophys. J., 1996, 466, 437 CrossRef CAS.
- S. K. Sahoo and A. Masuda, Chem. Geol., 1997, 141, 117 CrossRef CAS.
- T. Hirata and T. Yamaguchi, J. Anal. At. Spectrom., 1999, 14, 1455 RSC.
- Q. Z. Yin, S. B. Jacobsen, W. F. McDonough, I. Horn, M. I. Petaev and J. Zipfel, Astrophys. J., 2000, 535, L49.
- C. Münker, S. Weyer, K. Mezger, M. Rehkämper, F. Wombacher and A. Bischoff, Science, 2000, 289, 1538 CrossRef CAS.
- C. Sanloup, J. Blichert-Toft, P. Télouk, P. Gillet and F. Albarède, Earth Planet. Sci. Lett., 2000, 184, 75 CrossRef CAS.
- T. Hirata, Chem. Geol., 2001, 176, 323 CrossRef CAS.
- K. E. Jarvis, A. L. Gray and R. S. Houk, Handbook of Inductively Coupled Plasma-Mass Spectrometry, Blackie, London, 1992 Search PubMed.
- B. Hattendorf, D. Günther, M. Schönbächler and A. N. Halliday, Anal. Chem., 2001, 73, 5494 CrossRef CAS.
- V. J. M. Salters and S. R. Hart, Earth Planet. Sci. Lett., 1991, 104, 364 CrossRef CAS.
- D. C. Lee and A. N. Halliday, Nature, 1997, 388, 854 CrossRef CAS.
- W. G. Faix, R. Caletka and V. Krivan, Anal. Chem., 1981, 53, 1719 CrossRef CAS.
- F. Nelson, R. M. Rush and K. A. Kraus, J. Am. Chem. Soc., 1960, 82, 339 CrossRef CAS.
- K. M. Barovich, B. L. Beard, J. B. Cappel, C. M. Johnson, T. K. Kyser and B. E. Morgan, Chem. Geol., 1995, 121, 1 CrossRef CAS.
- M. Rehkämper, M. Schönbächler and C. H. Stirling, Geostand. Newslett., 2001, 25, 23 CrossRef CAS.
- D.-C. Lee and A. N. Halliday, Int. J. Mass Spectrom. Ion Proc., 1995, 146/147, 35 CrossRef.
- P. De Bièvre, M. Gallet, N. E. Holden and I. L. Barnes, J. Phys. Chem. Ref. Data, 1984, 13, 809 CAS.
- C. Münker, S. Weyer, E. Scherer and K. Mezger, Geochem., Geophys., Geosyst., 2001, 2, 2001GC000183 Search PubMed.
- M. Schönbächler, M. Rehkämper, A. N. Halliday, D.-C. Lee, M. Bourot-Denise, B. Zanda, B. Hattendorf and D. Günther, Science, 2002, 295, 1705 CrossRef.
| This journal is © The Royal Society of Chemistry 2004 |
