Facilitated transport of sodium or potassium chloride across vesicle membranes using a ditopic salt-binding macrobicycle
Atanas V.
Koulov
,
Joseph M.
Mahoney
and
Bradley D.
Smith
*
Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN 46556-5670, USA. E-mail: smith.115@nd.edu; Fax: +1 (574) 631 6652; Tel: +1 (574) 631 8632
First published on 28th November 2002
Abstract
A synthetic receptor, with an ability to bind sodium or potassium chloride as a contact ion-pair, is shown to effectively transport either salt across vesicle membranes. Significant transport is observed even when the transporter ∶ phospholipid ratio is as low as 1 ∶ 2500. Chloride efflux from unilamellar vesicles is monitored using a chloride selective electrode. Mechanistic studies indicate that the facilitated efflux is due to the uncomplexed transporter diffusing into the vesicle and the transporter–salt complex diffusing out. Vesicle influx experiments are also reported, where the facilitated influx of chloride and sodium ions into vesicles is observed directly by 35Cl and 23Na NMR, respectively.
While nature has produced mobile carrier molecules, such as valinomycin and monensin, to transport metal cations across biological membranes, it is intriguing that there are no analogous biotic carriers that selectively transport anions, or salts.1 There are likely chemical and biological reasons for this situation. Chemically, anion transport across a bilayer membrane is less favorable than cation transport because anions are often more hydrophilic. In addition, most biological membranes have a high phospholipid content and the phosphate residues present in the phospholipid head groups compete strongly for anion binding sites. In fact our research group has recently demonstrated that various anionophores can bind and translocate certain types of phospholipids across bilayer membranes, a dynamic process also known as flip-flop.2 Nonetheless, facilitated transmembrane anion transport is a ubiquitous cellular process, and thus an interesting challenge for supramolecular chemists. Biologically, the most important target anion is Cl− since defective Cl− transport is related to a number of disease states, the most common being cystic fibrosis.3 It is thought by some researchers that synthetic Cl− transporters have potential as therapeutic agents.4 While the last few years have witnessed the first examples of synthetic Cl− channels,5 we know of no publication describing rationally-designed carrier-mediated transport of Cl− or chloride salts across phospholipid bilayer membranes.6 Recently, we reported the synthesis of macrocyclic receptor 1 (mp 121–123 °C)7 and described its ability to bind KCl or NaCl as contact ion-pairs in organic solution (Scheme 1). We now disclose that 1 is a very efficient transporter of these salts across vesicle membranes.
 | ||
| Scheme 1 Structures of 1·M+Cl− complex and partial ion receptors 2 and 3. | ||
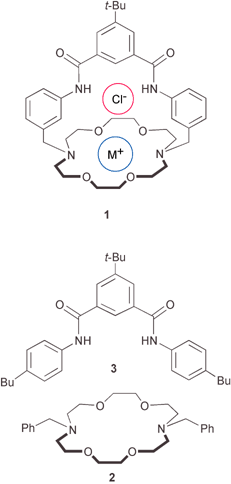
Cl− efflux from unilamellar 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) vesicles was monitored by a Cl− selective electrode.8 The Cl− efflux profiles in Fig. 1 show that 1 is a very effective transporter. For example, even a phospholipid–1 ratio of 2500 ∶ 1 leads to release of half of the vesicle Cl− content in about 300 s. Control experiments show that transporter 1 induces no leakage of entrapped fluorescent dyes or glucose-6-phosphate, reflecting the selectivity of the transport process.9The importance of the ditopic salt-binding ability of macrobicycle 1 is highlighted by the complete lack of Cl− efflux induced by high concentrations of a binary mixture of crown 2 and isophthalamide 3, the two ion-binding components of 1.
 | ||
| Fig. 1 Cl− efflux upon addition of 1 (0.4 µM, green ○; 4.0 µM, red ▽; 40.0 µM, blue □) or a 1 ∶ 1 molar mixture of 2 and 3 (40 µM each, blue ■) to unilamellar POPC vesicles (200 nm mean diameter, 1 mM phospholipid) containing 500 mM NaCl and dispersed in 500 mM NaHCO3. | ||
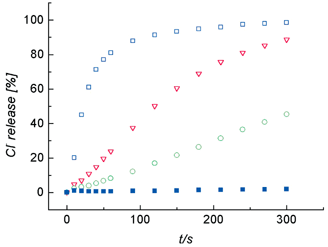
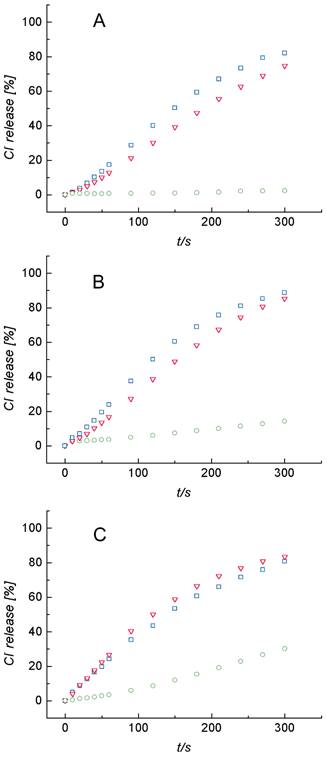
Mechanistic insight was gained by monitoring transporter-promoted Cl− efflux from POPC vesicles containing NaCl, KCl or CsCl. Furthermore, the Cl− efflux was monitored with three different extravesicle solutions, Na2SO4, NaHCO3 and NaNO3 (Fig. 2). Previously, we have shown that receptor 1 has a much weaker affinity for CsCl than NaCl or KCl,10 and as shown in Fig. 2 the rates of Cl− efflux from vesicles containing CsCl are considerably lower than the efflux from vesicles containing NaCl or KCl. This is strong evidence that the Cl− is transported from the vesicles as a 1–salt complex.11 A related mechanistic question is whether transporter 1 enters the membrane as an uncomplexed receptor, or as a salt complex. The data in Fig. 3 shows that the rate of Cl− efflux from vesicles containing NaCl is unaltered if the extravesicle solution is changed from Na2SO4 to Cs2SO4. The fact that Cl− efflux rates are independent of external metal cation identity (and external anion identity, see Fig. 2) indicates that transporter 1 enters the vesicle as an uncomplexed receptor. The proposed major transport pathway for facilitated Cl− efflux from vesicles in shown in Scheme 2.
 | ||
| Fig. 2 Cl− efflux upon addition of 1 (4.0 µM) to unilamellar POPC vesicles (200 nm mean diameter, 1 mM phospholipid) containing 500 mM of NaCl (blue □), KCl (red ▽) or CsCl (green ○) and dispersed in: (A) 375 mM Na2SO4, (B) 500 mM NaHCO3, or (C) 500 mM NaNO3. | ||
 | ||
| Fig. 3 Cl− efflux upon addition of 1 (4.0 µM) to unilamellar POPC vesicles (200 nm mean diameter, 1 mM phospholipid) containing 500 mM of NaCl and dispersed in 375 mM Na2SO4 (blue □) or 375 mM Cs2SO4 (red ▽). | ||
 | ||
| Scheme 2 Proposed mechanism for Cl− efflux mediated by transporter 1. | ||
In addition to Cl− efflux, established 23Na and 35Cl NMR transport assays were employed to directly observe facilitated influx of Na+ and Cl− ions into vesicles.12 The influx experiments started with vesicles containing Cs2SO4 which were dispersed in NaCl. Both NMR assays used the same principle, that is, a membrane impermeable shift reagent was added to the vesicle solutions which allowed internalized Na+ (or Cl−) to be distinguished from externalized ion. The shift reagent for the 23Na NMR was a DyCl3–sodium tripolyphosphate mixture which moves the 23Na resonance upfield.13 As shown in Fig. 4A and B, an unshifted peak, corresponding to internalized Na+, appeared after addition of 1 to the vesicles. The 35Cl NMR shift reagent was CoCl2 which moves the broadened 35Cl resonance downfield.14,15 As shown in Fig. 4C and D, an unshifted peak, corresponding to internalized Cl−, appeared after addition of 1 to the vesicles.
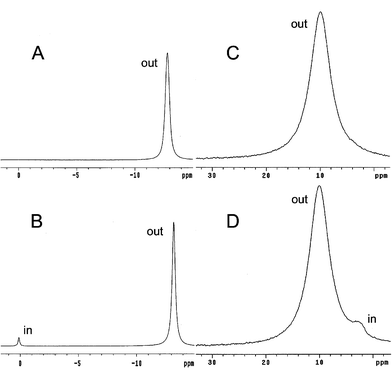 | ||
| Fig. 4 Na+ and Cl− influx into vesicles (egg-PC ∶ cholesterol, 7 ∶ 3). (A) 23Na NMR spectrum of vesicles containing 150 mM Cs2SO4 and dispersed in 20 mM Na5P3O6–100 mM NaCl–5.5 mM DyCl3. (B) 23Na NMR spectrum one hour after addition of 1 (lipid ∶ 1, 250 ∶ 1). (C) 35Cl NMR spectrum of vesicles containing 225 mM Cs2SO4 and dispersed in 300 mM NaCl–15 mM CoCl2. (D) 35Cl NMR spectrum one hour after addition of 1 (lipid ∶ 1, 250 ∶ 1). | ||
In summary, a salt-binding macrobicycle is shown for the first time to transport NaCl or KCl across vesicle membranes. The ditopic receptor 1 is an extremely effective transporter, whereas a binary mixture of crown 2 and isophthalamide 3, the two ion-binding components of 1, has essentially no transport activity. Our results suggest that salt transporters, such as 1, are likely to induce interesting biological effects.
Acknowledgements
This work was supported by the National Science Foundation (USA).References
- A very rare group of chloride transporters are the prodigiosins. See for example: K. Tanigaki, T. Sato, Y. Tanaka, T. Ochi, A. Nishikawa, K. Nagai, H. Kawashima and S. Ohkuma, FEBS Lett., 2002, 524, 37–42 Search PubMed.
- J. M. Boon and B. D. Smith, J. Am. Chem. Soc., 2001, 123, 6221–6226 CrossRef CAS.
- B. J. Rosenstein and P. L. Zeitlin, Lancet, 1998, 351, 277–282 CrossRef CAS.
- (a) L. Gao, J. R. Broughman, T. Iwamoto, J. M. Tomich, C. J. Venglarik and H. J. Forman, Am. J. Physiol. Lung Cell Mol. Physiol., 2001, 281, L24–L30 Search PubMed; (b) C. Jiang, E. R. Lee, M. B. Lane, X.-F. Xiao, D. J. Harris and S. H. Cheng, Am. J. Physiol. Lung Cell Mol. Physiol., 2001, 281, L1164–L1172 Search PubMed; (c) H. C. Rodgers and A. J. Knox, Eur. Respir. J., 2001, 17, 1314–1321 CrossRef CAS.
- (a) L. A. Weiss, N. Sakai, B. Ghebremariam, C. Ni and S. Matile, J. Am. Chem. Soc., 1997, 119, 12142–12149 CrossRef CAS; (b) M. Merritt, M. Lanier, G. Deng and S. L. Regen, J. Am. Chem. Soc., 1998, 120, 8494–8501 CrossRef CAS; (c) V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267–2278 CrossRef CAS; (d) P. H. Schlesinger, R. Ferdani, J. Liu, J. Pajewska, R. Pajewski, M. Saito, H. Shabany and G. W. Gokel, J. Am. Chem. Soc., 2002, 124, 1848–1849 CrossRef CAS.
- There are a number of papers in the literature describing facilitated anion or salt transport across liquid organic membranes. See, for example: (a) J. L. Sessler, P. I. Sansom, A. Andrievsky and V. Kral, in Supramolecular Chemsitry of Anions, eds. A. Bianchi, K. Bowman-James and E Garcia-Espana, Wiley, NewYork, 1997 Search PubMed; (b) L. A. J. Chrisstoffels, D. N. Reinhoudt, S. Sivelli, L. Gazzola, A. Casnati and R. Ungaro, J. Am. Chem. Soc., 1999, 121, 10142–10151 CrossRef CAS; (c) P. K. Beer and J. D. McKinney, Chem. Commun., 1999, 1253–1254 RSC; (d) D. M. Rudkevich, W. Verboom, R. Ungaro, F. de Jong and D. N. Reinhoudt, J. Am. Chem. Soc., 1995, 117, 6124–6125 CrossRef CAS; (e) H. Tsukube, Tetrahedron Lett., 1981, 22, 3981–3984 CrossRef CAS.
- J. M. Mahoney, A. M. Beatty and B. D. Smith, J. Am. Chem. Soc., 2001, 123, 5847–5848 CrossRef CAS.
- Briefly, unilamellar vesicles (mean diameter of 200 nm) containing 500 mM of salt were prepared by freeze–thawing, followed by extrusion through polycarbonate membranes. The non-encapsulated salt was removed by dialysis. The chloride efflux experiments used a chloride selective electrode (Accumet) and 5 mL vesicle solutions (1 mM phospholipid). The initial chloride reading was considered as 0% release and the reading after detergent addition at 300 s considered as 100% release. Typical chloride concentrations were 6.0 × 10−5 M in the beginning and 6.0 × 10−4 M after 100% release.
- P. R. Westmark, S. J. Gardiner and B. D. Smith, J. Am. Chem. Soc., 1996, 118, 11093–11100 CrossRef CAS.
- J. M. Mahoney and B. D. Smith, unpublished results.
- Additional experiments show that Cl− efflux is very slow from vesicles containing 500 mM CsCl and dispersed in 500 mM CsNO3 (e.g., 4.0 µM of 1 induces 10% Cl− release after 300 s) which is strong evidence against a simple anion antiport mechanism.
- (a) F. G. Riddell in Encyclopedia of Spectroscopy and Spectrometry, eds. J. C. Lindon, G. E. Tranter and J. L. Holmes, Academic Press, London, 1999, 1584–1593 Search PubMed; (b) P. W. Kuchel, B. E. Chapman, and A. S. L. Xu, in Progress in Cell Research, vol. 2, eds. E. Bamberg and H. Passow, Elsevier, Amsterdam, 1992 Search PubMed.
- Q. Xie, Y. Li, G. Gokel, J. Hernandez and L. Echegoyen, J. Am. Chem. Soc., 1994, 116, 690–696 CrossRef CAS.
- Y. Shachar-Hill and R. Shulman, Biochemistry, 1992, 31, 6272–6278 CrossRef CAS.
- In our hands, CoCl2 disrupts vesicles composed of only POPC. Therefore, the NMR-based transport assays used vesicles composed of egg-PC ∶ cholesterol (7 ∶ 3) which were mechanically stable in the presence of CoCl2.
| This journal is © The Royal Society of Chemistry 2003 |
