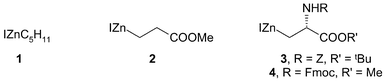
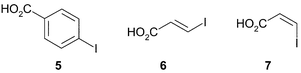
Cross coupling reactions of organozinc iodides with solid-supported electrophiles: synthesis of 4-substituted benzoic and 3-substituted (E)- and (Z)-propenoic acids and amides
Leslie J.
Oates
a,
Richard F. W.
Jackson†
*a and
Michael H.
Block
b
aDepartment of Chemistry, Bedson Building, The University of Newcastle, Newcastle upon Tyne, UK NE1 7RU. E-mail: r.f.w.jackson@shef.ac.uk
bAstra Zeneca Pharmaceuticals, Mereside, Alderley Park, Macclesfield, Cheshire, UK SK10 4TG
First published on 29th November 2002
Abstract
The solid-supported iodobenzoic acid derivatives 8–10 were coupled with a range of organozinc reagents 1–4 under palladium(0) catalysis. The coupled products released by acidic cleavage with TFA were obtained in high purities after recrystallisation. Analogous coupling of solid-supported (E)- and (Z)-3-iodoacrylic acids 18a, 18b, 19 and 20 gave (E)- and (Z)-α,β-unsaturated acids and amides 21–27 stereospecifically.
Introduction
The advantages of using solid-phase synthesis techniques for the rapid preparation of functionalised targets have been widely recognised. Early solid-phase work was concerned with the preparation of carbon–heteroatom bonds, and it is only relatively recently that methods for the formation of carbon–carbon bonds have been widely explored, and reviewed.1,2 The power of Pd-catalysed processes in the formation of carbon–carbon bonds has been recognised, and large sections of both reviews have been devoted to examples of the Stille, Heck and Suzuki reactions. It is quite striking that the Negishi reaction has been exploited to a much smaller extent, although the reaction of aryl and benzylic zinc halides with solid-supported aryl halides and triflates has been reported by Arlt3 and Knochel.4 The preparation of solid-supported zinc reagents has also been explored: Kondo has reported the preparation of solid-supported aryl zincates,5 and we have reported a method for the synthesis of solid-supported dialkylzinc reagents.6 Subsequently, the preparation of solid-supported imidazol-2-yl zinc halides has been described.7We now report the results of a study into the use of aliphatic zinc reagents, including those derived from amino acids, in cross coupling reactions with solid-supported 4-iodobenzoic acid, and also both (E)- and (Z)-3-iodopropenoic acid. This has resulted in the development of a straightforward solid-phase method for the synthesis of 4-substituted benzoic acids and 3-substituted (E)- and (Z)-propenoic acids, and the corresponding primary amides, following cleavage from the resin.
As representative organozinc iodides, we selected the simple pentylzinc iodide 1, the iodopropionate derivative 2 and the two serine-derived reagents 3 and 4.
In considering a solid support, we selected Wang resin,8 since it is widely used (including in Knochel's work) and easily prepared. We also chose to use a PEG-grafted polystyrene resin because the PEG graft confers wider solvent compatibility, and affects the solvent environment within the gel. In choosing the NovaSyn® TG HMP support we noted that the cleavage conditions were similar to those required for Wang resin. We also chose the more highly acid labile Rink acid and amide resins, so as to explore the compatibility of this chemistry with these linkers.
Results and discussion
Three acids, 4-iodobenzoic acid 5, (E)-3-iodoacrylic acid 6 and (Z)-3-iodoacrylic acid 7 were immobilised on the resins using DCC coupling conditions, with 3 molar equivalents being used relative to the resin. Although loading of the 4-iodobenzoic acid proceeded smoothly, careful control of the conditions was necessary for successful loading of the iodoacrylic acids. Treatment of 3-iodoacrylic acids with carbodiimides gives activated O-acyl intermediates which rearrange to the N-acyl species. As already noted in solution-phase studies,9,10 this unproductive process can be avoided by conducting the reaction at −20 °C in dichloromethane. The loading of the solid-supported iodo acids and amides was determined by cleavage of the acid/amide from a portion of resin with TFA, under conditions appropriate for the individual resin.Coupling of solid-supported iodobenzene derivatives
The resin-bound iodobenzene derivatives 8–11 (0.1–0.5 mmol) were treated with three molar equivalents of a representative selection of the organozinc reagents 1–4, under palladium catalysis. The organozinc reagents were prepared from the corresponding iodides using zinc dust in THF at ambient temperature. The organozinc solutions were decanted from excess zinc and added to the solid-supported partners and catalyst. The active catalyst was prepared in situ from Pd2(dba)3 and P(o-tolyl)3. Reactions were performed in THF at ambient temperature, overnight. The catalyst and by-products were removed from the solid-supported products by thorough washing, and the coupled products released by treatment with TFA. A 50% solution of TFA was used for Wang and Rink amide resins, and neat TFA was used for the NovaSyn® resin. The products from cleavage of the Wang and NovaSyn® TG HMP resin supported electrophiles were the free acids, whilst primary amides resulted from cleavage of the Rink amide-supported resin (Scheme 1, Table 1).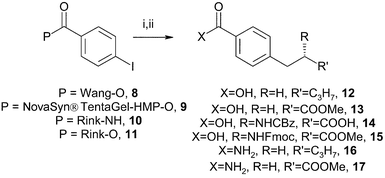 | ||
| Scheme 1 Reagents and conditions: i, organozincs 1–4 in THF (3 equiv.), Pd2(dba)3 (10 mol%), P(o-tolyl)3 (40 mol%), room temp., 16 h; ii, TFA–dichloromethane, 1 h. | ||
Coupling of Rink 4-iodobenzoate 11 with organozinc iodide 2 failed to give either the coupled product 13 or 4-iodobenzoic acid 5, after treatment with TFA. We reasoned that the acid-labile Rink ester might undergo cleavage under the conditions of the coupling reaction. Treatment of Rink 4-iodobenzoate resin 11 with 3 molar equivalents of zinc iodide in THF, at ambient temperature for 30 min, gave 4-iodobenzoic acid 5 in quantitative yield, supporting this proposal.
Synthesis of α,β-unsaturated acids and amides
Having established the scope and limitations of the process using supported aryl iodides, we now considered the coupling of organozinc reagents to (E)- and (Z)-iodoacrylic acid derivatives. The organozincs 1–4 were coupled with the resin-supported electrophiles 18a, 18b, 19 and 20 to give the α,β-unsaturated acids and amides 21–27 (Scheme 2, Table 2). The stereochemistry of the iodoacrylates 18a and 18b, and 19 and 20, was conserved during the coupling reactions. Cleavage of the products from the resin was again effected with TFA (50% for the Wang-supported products, neat for the NovaSyn® TG HMP derivatives and 1% for the Rink amide-supported product). The use of these much milder cleavage conditions for the Rink amide derivative allowed the isolation of the tert-butyl ester 27.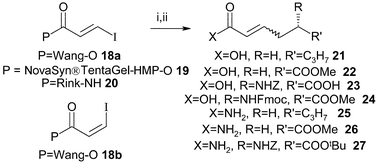 | ||
| Scheme 2 Reagents and conditions: i, organozincs 1–4 in THF (3 equiv.), Pd2(dba)3 (10 mol%), P(o-tolyl)3 (40 mol%), room temp., 16 h; ii, TFA–dichloromethane, 1 h. | ||
Conclusions
We have shown that aliphatic organozinc halides, including those derived from iodoalanine derivatives, can be effectively cross-coupled with a range of solid-supported 4-iodobenzoic acid derivatives, extending the work of Arlt3 on aryl zinc reagents and Knochel4 on aryl and benzylic zinc reagents. A limitation in the use of Rink esters has been uncovered. In addition, we have also demonstrated that solid-supported (E)- and (Z)-iodoacrylic acid derivatives may also be effectively employed in such reactions, leading to the stereospecific synthesis of 3-substituted (E)- and (Z)-propenoic acids. The removal of the need for chromatographic purification is a major advantage when compared to the use of solution phase electrophiles.Experimental
THF was distilled from potassium benzophenone ketyl. 1H NMR spectra were acquired at 500 MHz in CDCl3, referenced to TMS. 13C NMR spectra were acquired at 125 MHz, referenced to TMS. Chemical shifts are given in ppm, coupling constants are given in Hertz. Carboxylic acid protons were generally not observed. Wang resin was prepared from chloromethyl polystyrene (2.0 Meq g−1).11 All other resins were purchased from NovaBiochem. The following starting materials were prepared by literature methods: iodide 3,12 iodide 4,13 (E)-3-iodoacrylic acid 614 and (Z)-3-iodoacrylic acid 7.15Wang 4-iodobenzoate resin 8
Wang resin (5.03 g, 1.29 Meq g−1), 4-iodobenzoic acid (2.25 g, 11.1 mmol), DCC (2.59 g, 12.5 mmol) and DMAP (149 mg, 1.22 mmol) were combined in DMF (100 cm3) and stirred gently using a mechanical stirrer at 58–60 °C for 3 h. The solvent was removed by filtration and the resin washed with DMF (3 × 40 cm3), CH2Cl2 (3 × 40 cm3) and methanol (3 × 30 cm3). The resin was dried in air and in vacuo to constant weight 5.61 g. The loading was determined to be 1.1 Meq g−1 by cleavage of a sample using TFA–CH2Cl2 (1 : 1).NovaSyn® TG HMP 4-iodobenzoate resin 9
NovaSyn® TentaGel HMP resin (1.50 g, 0.38 Meq g−1), 4-iodobenzoic acid (282 mg, 1.14 mmol), DCC (255 mg, 1.24 mmol) and DMAP (15 mg, 0.12 mmol) were stirred in DMF (10 cm3) for 5 h at 60 °C. The mixture was cooled, and the resin was removed by filtration and washed with DMF (2 × 10 cm3), THF : water 1 : 1 (3 × 10 cm3), CH2Cl2 (3 × 10 cm3) and methanol (3 × 10 cm3). The resin was dried in air and in vacuo to constant weight 1.52 g. The loading was determined to be 0.16 Meq g−1 by cleavage of a sample using neat TFA.Rink 4-iodobenzamide resin 10
Fmoc Rink amide resin (0.47 Meq g−1) (995 mg, 0.47 mmol) was treated with piperidine in DMF (20% v/v, 10 cm3) for 30 min. The resin was washed with DMF (3 × 30 cm3), CH2Cl2 (3 × 10 cm3) and methanol (3 × 10 cm3) and dried in air and in vacuo. The resin, 4-iodobenzoic acid (352 mg, 1.42 mmol), DCC (309 mg, 1.50 mmol) and DMAP (17 mg, 0.14 mmol) were combined in DMF (10 cm3) and stirred gently at 58–60 °C for 3.5 h. The solvent was removed by filtration and the resin washed with THF (3 × 10 cm3), toluene–EtOH 1 : 1 (3 × 10 cm3), CH2Cl2 (3 × 10 cm3) and methanol (3 × 10 cm3). The resin was dried in air and in vacuo to constant weight 857 mg. The loading was determined to be 0.43 Meq g−1 by cleavage of a sample using 1% TFA in CH2Cl2.Preparation and coupling of alkylzinc iodides with solid-supported electrophiles. General procedure
Zinc dust (5.5 equiv.) was suspended in dry THF (1 cm3). 1,2-Dibromoethane (0.25 equiv.) was added and the slurry heated to ∼65 °C. After cooling to room temperature TMS-Cl (0.05 equiv.) was added and the slurry stirred for 10 min. The alkyl iodide was then added, and the zinc insertion process monitored by TLC. After insertion was judged to be complete, stirring was stopped and excess zinc was allowed to settle. The solution of alkylzinc iodide (3 equiv.) was transferred to a flask containing the solid-supported aryl or vinyl iodide, Pd2(dba)3 (0.05 equiv.) and P(o-tolyl)3 (0.2 equiv.) suspended in 2 cm3 solvent. The suspension was stirred gently overnight, then the solid support was washed with THF (4 × 10 cm3), THF–water (1 : 1, 3 × 10 cm3), THF (3 × 10 cm3), and CH2Cl2 (3 × 10 cm3). The coupled products were released by treatment of the resins with 50% TFA in dichloromethane (10 cm3) for 1 h. The resin was washed with dichloromethane (3 × 10 cm3) and the combined organic washings evaporated to give the products.4-n-Pentylbenzoic acid 12
Treatment of resin 8 (508 mg, 1.1 Meq g−1) with zinc reagent 1, according to the general procedure, followed by recrystallisation from EtOH–water, gave 12 (82 mg, 0.43 mmol, 78%). Mp 86–89 °C (lit.,16 86–88 °C), liq. cryst.-isotropic transition 124–126 °C (lit.,16 126–127 °C).Treatment of resin 9 (373 mg, 0.16 Meq g−1) with zinc reagent 1, according to the general procedure, gave 12 (10 mg, 87%).
4-(2′-Methoxycarbonylethyl)benzoic acid 13
Treatment of resin 8 (496 mg, 1.1 Meq g−1) with zinc reagent 2, according to the general procedure, followed by recrystallisation from CH2Cl2–petrol, gave 13 (113 mg, 0.54 mmol, 99%). Mp 155–157 °C; (Found M+ 208.0739; C11H12O4 requires 208.0736); IR (KBr disc) 3442, 3000, 2954, 2850, 1725, 1687, 1428, and 1346; NMR δH 2.67 (2H, t, J 7.5), 3.03 (2H, t, J 7.5), 3.67 (3H, s), 7.31 (2H, d, J 8), and 8.04 (2H, d, J 8); δC 30.9, 35.1, 51.8, 127.5, 128.5, 130.5, 146.9, 172.0, and 173.0; m/z (EI) 208 (M+, 58%), 148 (100), 135 (64), 103 (36), 77 (49), and 69 (82).Treatment of resin 9 (384 mg, 0.16 Meq g−1) with zinc reagent 2, according to the general procedure, gave 13 (12 mg, 95%).
4-(2′-Benzyloxycarbonylamino-2′-carboxyethyl)benzoic acid 14
Treatment of resin 8 (502 mg, 1.1 Meq g−1) with zinc reagent 3, according to the general procedure, followed by recrystallisation from EtOH–water, gave 14 (156 mg, 0.46 mmol, 83%). Mp 200–202 °C (Found M+ − ZHNCHCO2H 135.0439; C8H7O2 requires 135.0446); IR (KBr disc). 3309, 3031, 2960, 2673, 2551, 1689, 1539, 1425, 1286, and 1268; [α]D 14.2 (c 1.5, EtOH); NMR δH 3.12 (1H, dd, J 14, 9.5), 3.34 (1H, dd, J 14, 5), 4.54–4.59 (1H, m), 5.02 (2H, s), 6.61 (1H, d, J 8.5), 7.26–7.35 (5H, m), 7.44 (2H, d, J 8.5), and 7.96 (2H, d, J 8.5); δC (125 MHz, acetone-d6) 38.1, 55.7, 66.6, 128.5, 128.6, 129.1, 129.8, 130.3, 130.5, 138.1, 143.9, 156.8, 167.5, and 173.0; m/z (EI) 135 (CH2C6H4COOH+, 100%), 107 (62), 91 (89, C7H7+), and 79 (82).Treatment of resin 9 (370 mg, 0.16 Meq g−1) with zinc reagent 3, according to the general procedure, gave 14 (19 mg, 93%).
4-[2′-(Fluoren-9″-ylmethoxycarbonylamino)-2′-(methoxycarbonyl)ethyl]benzoic acid 15
Treatment of resin 8 (202 mg, 1.1 Meq g−1) with zinc reagent 4, according to the general procedure, followed by recrystallisation from CH2Cl2–petrol, gave 15 (48 mg, 0.11 mmol, 69%). Mp 172–174 °C; IR (KBr disc) 3315, 3065, 3018, 2672, 2554, 1735, 1695, 1533, 1287, and 740; [α]D 7.8 (c 1.0 in EtOH); NMR δH 3.15 (1H, dd, J 6, 14), 3.22 (1H, dd, J 6, 14), 3.74 (3H, s), 4.21 (1H, t, J 7), 4.39 (1H, dd, J 7, 10.5), 4.48 (1H, dd, J 7, 10.5), 4.72 (1H, q, J 6), 5.44 (1H, d, J 8), 7.18 (2H, d, J 8), 7.29–7.34 (2H, m), 7.40 (2H, t, J 7.5), 7.58 (2H, t, J 6.5), 7.77 (2H, d, J 7.5), and 8.03 (2H, d, J 8); δC 38.3, 47.2, 52.6, 54.6, 67.0, 120.0, 125.0, 127.1, 127.8, 128.3, 129.5, 130.5, 141.4, 142.1, 143.7, 155.6, 171.1, and 171.8; m/z (EI) 368 (58%), 206 (69), 178 (100), and 88 (61).4-n-Pentylbenzamide 16
Treatment of resin 10 (176 mg, 0.43 Meq g−1) with zinc reagent 1, according to the general procedure, followed by recrystallisation from petrol–ether, gave 16 (8 mg, 55%). Mp 149–150 °C (lit.,17 147–148 °C, aq. EtOH); (Found M+ 191.1304, C12H17NO requires 191.1310); IR (KBr disc) 3386, 3173, 2927, 2854, 1649, 1617, 1567, 1417, and 1398; NMR δH 0.89 (3H, t, J 7), 1.25–1.36 (4H, m), 1.59–1.66 (2H, m), 2.65 (2H, t, J 7.5), 6.14 (2H, br s), 7.25 (2H, d, J 8.5), and 8.00 (2H, d, J 8.5); δC 14.0, 22.5, 30.8, 31.4, 35.8, 127.5, 128.7, 130.4, 147.8, and 169.9; m/z (EI) 191 (M+, 61%), 175 (100), 134 (49) and 91 (39).4-(2′-Methoxycarbonylethyl)benzamide 17
Treatment of resin 10 (205 mg, 0.43 Meq g−1) with zinc reagent 2, according to the general procedure, gave 17 (56 mg, 0.27 mmol, 49%); (Found M+ 207.0887, C11H13NO3+ requires 207.0895); IR (KBr disc) 3375, 3003, 2998, 2959, 2924, 1725, 1672, 1414, 1402, 1287, 969 and 771; NMR δH 2.66 (2H, t, J 7.5), 3.01 (2H, t, J 7.5), 3.67 (3H, s), 6.40 (1H, br s), 6.55 (1H, br s), 7.30 (2H, d, J 8.5), and 7.76 (2H, d, J 8.5); δC 30.7, 35.2, 51.7, 127.7, 128.6, 130.9, 145.3, 169.4, and 172.9; m/z (EI) 207 (M+, 94%), 191 (54), 149 (78), 148 (94), 147 (100), 131 (95), 103 (44), and 91 (44).Wang (E)-3-iodoacrylate resin 18a
Wang resin (2.00 g, 1.29 Meq g−1), (E)-3-iodoacrylic acid (1.633 mg, 8.25 mmol) and DMAP (107 mg, 0.88 mmol) were suspended in CH2Cl2 (20 cm3) and cooled to −20 °C. DCC (1.706 mg, 8.27 mmol) was added as a solution in CH2Cl2 (1 cm3) and the suspension stirred gently for 3.5 h at −20 °C. The solvent was removed by filtration and the resin washed with CH2Cl2 (3 × 10 cm3), EtOH–toluene (1 : 1, 4 × 10 cm3), CH2Cl2 (3 × 10 cm3), and methanol (3 × 5 cm3). The resin was dried in air and in vacuo to constant weight, 2.595 g. The loading was determined to be 1.22 Meq g−1 by cleavage of a sample using 1 : 1 (TFA–CH2Cl2). Another sample with loading 1.00 Meq g−1 was also prepared.Wang (Z)-3-iodoacrylate resin 18b
Wang resin (2.00 g, 1.29 Meq g−1), (Z)-3-iodoacrylic acid (1.62 g, 8.25 mmol) and DMAP (104 mg, 0.88 mmol) were suspended in CH2Cl2 (30 cm3) and cooled to −20 °C. DCC (1.70 g, 8.27 mmol) was added as a solution in CH2Cl2 (2 cm3) and the suspension stirred gently overnight at −20 °C. The solvent was removed by filtration and the resin washed with CH2Cl2 (3 × 50 cm3), toluene–EtOH 1 : 1 (5 × 50 cm3), DMF (3 × 30 cm3), CH2Cl2 (3 × 30 cm3) and methanol (3 × 30 cm3). The resin was dried in air and in vacuo to constant weight, 2.51 g. The loading was determined to be 1.28 Meq g−1 by cleavage of a sample using 1 : 1 (TFA–CH2Cl2).NovaSyn® TG HMP (E)-3-iodoacrylate resin 19
NovaSyn® TentaGel HMP resin (1.52 g, 0.38 Meq g−1), (E)-3-iodoacrylic acid (249 mg, 1.21 mmol) and DMAP (15 mg, 0.12 mmol) were suspended in CH2Cl2 (10 cm3) and cooled to −20 °C. DCC (263 mg, 1.27 mmol) was added as a solution in CH2Cl2 (1 cm3) and the suspension stirred gently for 19 h at −20 °C. The resin was removed by filtration and washed with CH2Cl2 (3 × 10 cm3), toluene–EtOH 1 : 1 (3 × 10 cm3), CH2Cl2 (3 × 10 cm3) and methanol (3 × 10 cm3). The resin was dried in air and in vacuo to constant weight 1.574 g. The loading was determined to be 0.26 Meq g−1 by cleavage of a sample using neat TFA.Rink (E)-3-iodoacrylamide resin 20
Fmoc Rink amide resin (1.00 g, 0.47 Meq g−1) was treated with piperidine in DMF (20% v/v, 10 cm3) for 30 min. The resin was washed with DMF (3 × 10 cm3), CH2Cl2 (3 × 10 cm3) and methanol (3 × 10 cm3) and dried in air and in vacuo to give 969 mg of resin. The resin, (E)-3-iodoacrylic acid (280 mg, 1.41 mmol) and DMAP (17 mg, 0.14 mmol) were suspended in CH2Cl2 (10 cm3) and cooled to −20 °C. DCC (300 mg, 1.45 mmol) was added as a solution in CH2Cl2 (1 cm3) and the suspension stirred gently for 19 h at −20 °C. The resin was removed by filtration and washed with CH2Cl2 (4 × 10 cm3) and methanol (3 × 5 cm3). The resin was dried in air and in vacuo to constant weight 847 mg. The loading was determined to be 0.47 Meq g−1 by cleavage of a sample using 1% TFA in CH2Cl2.(E)-Oct-2-enoic acid 21a
Treatment of resin 18a (476 mg, 1.22 Meq g−1) with zinc reagent 1, according to the general procedure, gave 21a (80 mg, 97%), which exhibited identical spectroscopic properties to those in the literature.18Treatment of resin 19 (374 mg, 0.26 Meq g−1) with zinc reagent 1, according to the general procedure, gave 21a (13 mg, 94%).
(Z)-Oct-2-enoic acid 21b
Treatment of resin 18b (414 mg, 1.28 Meq g−1) with zinc reagent 1, according to the general procedure, gave 21b (70 mg, 94%). NMR δH 0.90 (3H, t, J 5), 1.27–1.49 (6H, m), 2.68 (2H, dq, J 7.5, 1.5), 5.79 (1H, dt, J 11.5, 1.5), 6.36 (1H, dt, J 11.5, 6); Lit.1,19(E)-Hex-2-enedioic acid 6-methyl ester 22a
Treatment of resin 18a (485 mg, 1.22 Meq g−1) with zinc reagent 2, according to the general procedure, followed by recrystallisation from CH2Cl2–ether, gave 22a (107 mg, 100%). Mp 56–58 °C (lit.,20 58–59 °C, hexane–CHCl3); (Found M+ 158.0579, C7H10O4 requires 158.0574); IR (KBr disc) 3001, 2954, 2852, 2687, 2589, 1727, 1701, 1273, and 1158; NMR δH 2.46–2.61 (4H, m), 3.69 (3H, s), 5.86 (1H, dt, J 15.5, 1.5), and 7.06 (1H, dt, J 15.5, 6.5); δC 27.3, 32.1, 51.9, 121.4, 149.4, 171.3, and 172.6; m/z (EI) 158 (M+, 4%), 140 (45), 127 (39), 108 (100), and 81 (66).Treatment of resin 19 (353 mg, 0.26 Meq g−1) with zinc reagent 2, according to the general procedure, gave 22a (14 mg, 97%).
(Z)-Hex-2-enedioic acid 6-methyl ester 22b
Treatment of resin 18b (400 mg, 1.28 Meq g−1) with zinc reagent 2, according to the general procedure, gave 22b (50 mg, 63%). (Found M+ 158.0577, C7H10O4 requires 158.0574); IR (KBr disc) 3001, 2954, 2852, 2687, 2589, 1727, 1701, 1273, and 1158; NMR δH 2.49 (2H, t, J 7), 2.47–2.51 (2H, m), 3.69 (3H, s), 5.86 (1H, d, J 11), and 6.35 (1H, dt, J 11, 7.5); δC (125 MHz, CDCl3) 24.5, 33.1, 51.8, 121.0, 149.8, 171.9, and 173.4; m/z (EI) 158 (M+, 6%), 140 (53), 127 (46), 108 (100), and 81 (61).(E)-(2S)-5-(Benzyloxycarbonylamino)hex-2-enedioic acid 23a
Treatment of resin 18a (469 mg, 1.22 Meq g−1) with zinc reagent 3, according to the general procedure, followed by recrystallisation from EtOH–water, gave 23a (175 mg, 90%). Mp 153–156 °C (Found M+ − C7H7 − CO2, 293.0894, C14H15NO6 requires 293.0899); IR (KBr disc) 3337, 3035, 2671, 2581, 2487, 1695, 1534, 1421, and 1283; [α]D +20 (c 1.9 in EtOH); NMR δH 2.68–2.75 (1H, m), 2.81–2.87 (1H, m), 4.41–4.46 (1H, m), 5.09 (2H, s), 5.96 (1H, dt, J 15.5, 1.5), 6.72 (1H, d, J 8), 6.92–6.99 (1H, m), and 7.27–7.42 (5H, m); δC 34.8, 66.7, 96.6, 125.1, 128.5, 128.6, 129.2, 138.1, 144.7, 156.9, 166.8, and 172.7; m/z (EI) 293 (M+ − C7H7 − CO2, 89%), 267 (36), 202 (50, M+ − C7H7), 163 (68), 151 (75), 108 (79), 91 (100, C7H7+), and 79 (49).Treatment of resin 19 (374 mg, 0.26 Meq g−1) with zinc reagent 3, according to the general procedure, gave 23a (22 mg, 78%).
(Z)-(2S)-5-(Benzyloxycarbonylamino)hex-2-enedioic acid 23b
Treatment of resin 18b (414 mg, 1.28 Meq g−1) with zinc reagent 3, according to the general procedure, followed by recrystallisation from CH2Cl2–petrol, gave 23b (72 mg, 37%). Mp 130–133 °C (Found M+ − C7H7 − CO2, 293.0903; C14H15NO6 requires 293.0899); IR (KBr disc) 3323, 3036, 2621, 2592, 1701, 1689, 1537, 1271, and 1241; [α]D +27 (c 0.92, EtOH); NMR δH 3.08–3.26 (2H, m), 4.33–4.44 (1H, m), 5.09 (2H, s), 5.97 (1H, dt, J 11.5, 1.5), 6.39 (1H, d, J 11.5, 7), 6.72 (1H, d, J 7.5), 7.30–7.44 (5H, m); δC 32.6, 67.6, 96.6, 124.8, 128.5, 128.6, 129.2, 138.0, 144.4, 156.8, 166.8, and 172.7; m/z (EI) 293 (M+ − C7H7 − CO2, 29%), 220 (35), 158 (37), 138 (52), 108 (38), 91 (100, C7H7+), and 79 (16, C6H5+).(E)-(2S)-5-(Fluoren-9′-ylmethoxycarbonylamino)hex-2-enedioic acid 6-methyl ester 24
Treatment of resin 18a (156 mg, 1.00 Meq g−1) with zinc reagent 4, according to the general procedure, followed by recrystallisation from ether–petrol, gave 24 (27 mg, 46%). Mp 152–153 °C (Found MH+ 396.1427, C22H22NO6 requires 396.1447); IR (KBr disc) 3321, 3065, 2952, 1741, 1700, 1538, 1278, and 739; [α]D + 19 (c 2.7, EtOH); NMR δH 2.65–2.76 (1H, m), 2.77–2.86 (1H, m), 3.78 (3H, s), 4.22 (1H, t, J 7), 4.38–4.47 (2H, m), 4.53–4.69 (1H, m), 5.33 (1H, d, J 7), 5.91 (1H, d, J 15), 6.88–6.97 (1H, m), 7.25–7.34 (2H, m), 7.40 (2H, t, J 7.5), 7.59 (2H, t, J 7.5), and 7.76 (2H, d, J 7.5); δC (125 MHz, CDCl3) 35.1, 47.1, 52.8, 67.2, 120.0, 124.4, 125.0, 127.1, 127.8, 143.6, 143.7, 144.5, 144.6, 155.7, 170.1, and 171.5; m/z (EI) 396 (MH+, 5%), 395 (24), 304 (54), 289 (84), 178 (100, C14H10+), and 165 (31).(E)-Oct-2-enoic amide 25
Treatment of resin 20 (204 mg, 0.47 Meq g−1) with zinc reagent 1, according to the general procedure, gave 25 (11 mg, 81%). Mp 125–129 °C (lit.,21 129 °C); NMR δH 0.89 (3H, t, J 6.5), 1.23–1.53 (6H, m), 2.14–2.25 (2H, m), 5.70 (2H, s), 5.84 (1H, dt, J 15.5, 1.5), 6.88 (1H, dt, J 15.5, 7.0).(E)-5-Carbamoylpent-4-enoic acid methyl ester 26
Treatment of resin 20 (178 mg, 0.47 Meq g−1) with zinc reagent 2, according to the general procedure, gave 26 (13 mg, 99%). Mp 129–131 °C (Found M+ − NH3 140.0473, C7H8O3 requires 140.0473); IR (KBr disc) 3328, 3159, 1733, 1676, 1611, 1439, and 1166; NMR δH 2.43–2.60 (4H, m), 3.69 (3H, s), 5.79 (2H, s), 5.90 (1H, d, J 15.5), and 6.78–6.93 (1H, m); δC 25.4, 34.2, 51.9, 126.4, 129.3, 167.3, and 172.6; m/z (EI) 140 (M+ − NH3, 100%), 125 (47), 108 (91), 98 (88), 91 (77), and 81 (75).(E)-(2S)-2-(Benzyloxycarbonylamino)-5-carbamoylpent-4-enoic acid tert-butyl ester 27
Treatment of resin 20 (175 mg, 0.47 Meq g−1) with zinc reagent 3, according to the general procedure, using 1% TFA in CH2Cl2, gave 27 (28 mg, 98%). Mp 111–116 °C (Found M+ − C4H8 292.1066, C14H16N2O5 requires 292.1059); IR (KBr disc) 3404, 3340, 2853, 1726, 1676, 1647, and 1534; [α]D +6.0 (c 1.7, EtOH); NMR δH 1.46 (9H, s), 2.61–2.65 (1H, m), 2.72–2.76 (1H, m), 4.38–4.40 (1H, m), 5.10 (2H, s), 5.46 (1H, s), 5.73 (2H, s), 5.89 (1H, d, J 15.5), 6.69–6.76 (1H, m), and 7.29–7.38 (5H, m); δC 28.0, 35.4, 53.4, 67.0, 82.9, 125.9, 128.2, 128.2, 128.6, 132.3, 139.8, 155.6, 167.4, and 170.1; m/z (EI) 292 (M+ − C4H8, 4%), 264 (1.5), 203 (46), 164 (45), and 91 (100, C7H7+).Acknowledgements
We thank AstraZeneca for support (studentship to LJO).References
- B. A. Lorsbach and M. J. Kurth, Chem. Rev., 1999, 99, 1549 CrossRef CAS.
- R. E. Sammelson and M. J. Kurth, Chem. Rev., 2001, 101, 137 CrossRef CAS.
- S. Marquais and M. Arlt, Tetrahedron Lett., 1996, 37, 5491 CrossRef CAS.
- M. Rottlander and P. Knochel, Synlett, 1997, 1084 CAS.
- Y. Kondo, T. Komine, M. Fujinami, M. Uchiyama and T. Sakamoto, J. Comb. Chem., 1999, 1, 123 CrossRef CAS.
- R. F. W. Jackson, L. J. Oates and M. H. Block, Chem. Commun., 2000, 1401 RSC.
- S. Havez, M. Begtrup, P. Vedso, K. Andersen and T. Ruhland, Synthesis, 2001, 909 CrossRef CAS.
- S. Wang, J. Am. Chem. Soc., 1973, 95, 1328 CrossRef CAS.
- R. J. Boyce and G. Pattenden, Tetrahedron Lett., 1996, 37, 3501 CrossRef CAS.
- I. Paterson and J. Man, Tetrahedron Lett., 1997, 38, 695 CrossRef CAS.
- G. Lu, S. Mojsov, J. P. Tam and R. B. Merrifield, J. Org. Chem., 1981, 46, 3433 CrossRef CAS.
- E. Dumez, J. S. Snaith, R. F. W. Jackson, A. B. McElroy, J. Overington, M. J. Wythes, J. M. Withka and T. J. McLellan, J. Org. Chem., 2002, 67, 4882 CrossRef CAS.
- M. S. Smyth and T. R. Burke, Jr., Tetrahedron Lett., 1994, 35, 551 CrossRef CAS.
- T. Zoller and D. Uguen, Tetrahedron Lett., 1998, 39, 6719 CrossRef CAS.
- E. Piers, T. Wong, P. D. Coish and C. Rogers, Can. J. Chem., 1994, 72, 1816 CAS.
- P. L. Creger, J. Am. Chem. Soc., 1970, 92, 1396 CrossRef CAS.
- H. A. Fahim and A. M. Fleifel, J. Chem. Soc., 1952, 4519 Search PubMed.
- J. L. Brevet and K. Mori, Synthesis, 1992, 1007 CrossRef CAS.
- C. Rappe, Org. Synth., 1973, 53, 123 CAS.
- F. Foubelo, F. Lloret and M. Yus, Tetrahedron, 1994, 50, 6715 CrossRef CAS.
- D. Elad and G. Friedman, J. Chem. Soc. C, 1970, 893 RSC.
Footnote |
| † Present address: Department of Chemistry, Dainton Building, The University of Sheffield, Sheffield, UK S3 7HF. |
| This journal is © The Royal Society of Chemistry 2003 |