Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Murray H. G.
Munro
a,
Peter T.
Northcote
c and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: j.blunt@chem.canterbury.ac.nz
bDepartment of Chemistry, University of Auckland, Auckland, New Zealand
cSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 19th December 2002
Abstract
This review covers the literature published in 2001 for marine natural products, with 497 citations (373 for the period January to December 2001) and includes 793 compounds isolated from marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates and echinoderms. The emphasis is on new compounds and new stereochemical assignments (683 for 2001), together with relevant biological activities, source organisms and country of origin. Syntheses that confirm or revise structures or stereochemistries have been included (95), including any first total synthesis of a marine natural product.
Covering: 2001. Previous review: Nat. Prod. Rep., 2002, 19, 1.
 John W. Blunt | John Blunt obtained his BSc (Hons) and PhD degrees from the University of Canterbury, followed by postdoctoral appointments in Biochemistry at the University of Wisconsin-Madison, and with Sir Ewart Jones at Oxford University. He took up a lectureship at the University of Canterbury in 1970, where he is now a Professor. His research interests are with natural products, and the application of NMR techniques to structural problems. |
 Brent R. Copp | Brent Copp received his BSc (Hons) and PhD degrees from the University of Canterbury, where he studied the isolation, structure elucidation and structure–activity relationships of biologically active marine natural products under the guidance of Professors Blunt and Munro. He undertook postdoctoral research with Jon Clardy at Cornell and Chris Ireland at the University of Utah. 1992–93 was spent working in industry as an isolation chemist with Xenova Plc, before returning to New Zealand to take a lectureship at the University of Auckland, where he is currently a Senior Lecturer. |
 Murray H. G. Munro | Murray Munro, a Professor in Chemistry at the University of Canterbury, Christchurch New Zealand, has worked on New Zealand natural products all of his professional career. His interest in marine natural products was stimulated by a study-leave period spent with Professor Kenneth Rinehart, University of Illinois, in 1973. A marine chemistry group at Canterbury followed soon after and has been active ever since. In more recent years his research interests have widened to include marine fungi and actinomycetes and drug delivery systems based on polymer therapeutics. |
 Peter T. Northcote | Peter Northcote, received his BSc and PhD degrees from the University of British Columbia, Canada where he was a member of R. J. Andersen's marine natural products research group. He carried out postdoctoral research with Professors Blunt and Munro at the University of Canterbury before taking a position as a senior research scientist at Lederle Laboratories, American Cyanamid Co. He joined the faculty of the Victoria University of Wellington in 1994 where he is currently a Senior Lecturer in organic chemistry. |
 Michèle R. Prinsep | Michèle Prinsep received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied the isolation and structural elucidation of biologically active secondary metabolites from sponges and bryozoans under the supervision of Professors Blunt and Munro. She undertook postdoctoral research on cyanobacteria with Richard Moore at the University of Hawaii before returning to New Zealand to take up a lectureship at the University of Waikato, where she is currently a Senior Lecturer. |
1 Introduction
The previous author of these reviews, Professor D. John Faulkner, died on November 23, 2002. For over 30 years John had been a constant source of inspiration and insights that totally changed the perspective of marine natural products. He was both an exceptional researcher and an inspirational teacher who constantly exhorted his students to greater heights by his own example. From his research he will be remembered for the precision and conciseness he brought to bear and for the wonderful results that unfolded. This review is dedicated to John’s memory.These reviews were initiated by Faulkner in 19841 following his original 1977 overview in Tetrahedron.2 Since then 17 further reviews have appeared, with 13 annually since 1990. We are all indebted to Professor Faulkner for this amazing volume of work. These meticulously prepared reviews, notable for their comprehensiveness, accuracy and readability, have become the most highly cited articles for this journal. Earlier in 2002 John relinquished the task of preparing these reviews. With trepidation we agreed to take over the task. Foremost in our minds was the need to maintain the high ‘Faulknerian’ standards.
For this review we follow the same style and layout seen previously. There has been continued growth in the reports from studies on microorganisms, and on synthetic efforts on marine natural products. We have included papers on syntheses if they provide new information on the stereochemistry of previously reported compounds, or provide for a revision of structure. We also report the first total synthesis of any marine natural product. In view of the rapid growth in this area, we believe that it would be timely to dedicate a companion review to this topic.
A number of reviews on a variety of topics appeared in 2001. One group covered natural products from many sources including marine organisms: “Natural products in anticancer therapy”,3
“Endophytes: a rich source of functional metabolites”,4
“Simple indole alkaloids and those with nonrearranged monoterpenoid unit”,5
“Diterpenoids”,6
“Biologically active proteins from natural product extracts”7 and “Natural product-based anti-HIV drug discovery and development facilitated by the NCI Developmental Therapeutics Program”.8 Cyanobacteria have been well covered by “Marine cyanobacteria – a prolific source of natural products”,9
“The toxins of cyanobacteria”,10 and “Nitrogen-containing metabolites from marine cyanobacteria”.11 Ascidian-derived compounds are partially reviewed in “Recent advances on the research of natural products of ascidians”.12 Specific compounds have been reviewed in “Chemistry of potent anticancer compounds, amphidinolides”,13
“Distribution and origin of tetrodotoxin”,14 the mini-review “Domoic acid: a fascinating marine toxin”,15 and “Pectenotoxins – an issue for public health”,16 while specific compound classes have been covered in “Recent advances in study on cyclic peptides from marine sponges”,17
“Aquatic animal carotenoids”,18
“Advances in marine natural products of the indole and annelated indole series: chemical and biological aspects”,19 and “Marine sulfur-containing natural products”20 which describes the 482 sulfur-containing compounds (excluding sulfates) reported from 1985–1999. Some ecological and taxonomic aspects are reviewed in “Secondary metabolites from Antarctic marine organisms and their ecological implications”,21
“Marine chemical ecology: applications in marine biomedical prospecting”,22 and “Marine natural products chemistry as an evolutionary narrative”23 which includes a taxonomic survey. Other reviews include “Biologically active compounds from marine organisms”,24
“Marine bioprospecting – trawling for treasure and pleasure”25 which highlights the molecular diversity seen in the results obtained by the University of Melbourne's marine natural product group, “Marine pharmacology in 1999: antitumor and cytotoxic compounds”26 which describes the structures reported in 1999 for 30 antitumour and cytotoxic compounds, and “Marine organisms as a source of new anticancer agents”27 which summarises current preclinical and clinical trial data for a range of marine natural products. Volume 6 of “Recent Advances in Marine Biotechnology” contains a series of reviews: “Novel pharmaceutical compounds from marine bacteria”,28
“Recent developments on antimicrobial metabolites from marine sponges”,29
“Bioactive compounds from hard corals”,30
“Novel bioactive compounds from the soft corals: Chemistry and biomedical applications”,31
“Pore-forming proteins from sea anemones and the construction of immunotoxins for selective killing of harmful cells”,32
“Bioactive compounds from bryozoans”,33
“Novel alkaloids from marine bryozoans”,34
“Ion channel toxins as molecular models for the design of new drugs”,35
“Proteinases from marine organisms”36 and “Cooperative antifoulant testing: A novel multisector approach”.37 A new database of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 natural products has been introduced in “Using XML in the marine natural products database”,38 while the MarinLit database39 continues to be updated, and has been used for the preparation of this review.
000 natural products has been introduced in “Using XML in the marine natural products database”,38 while the MarinLit database39 continues to be updated, and has been used for the preparation of this review.
2 Marine microorganisms and phytoplankton
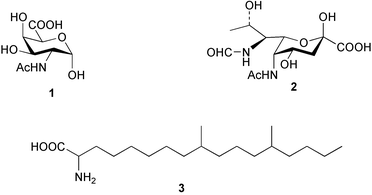
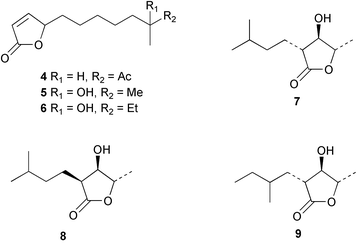
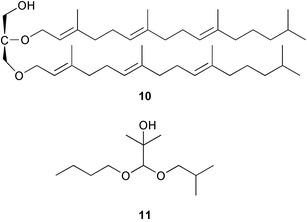
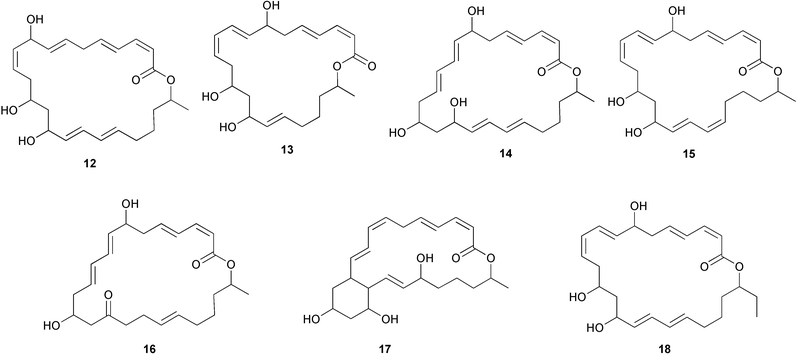
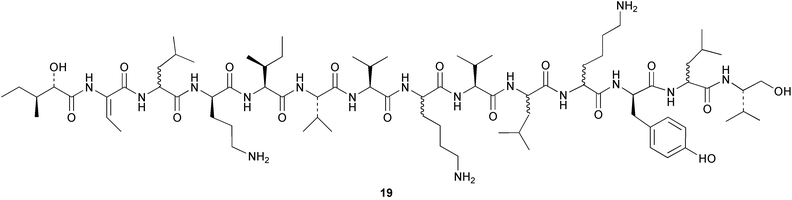
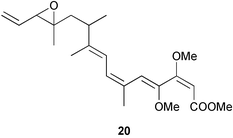
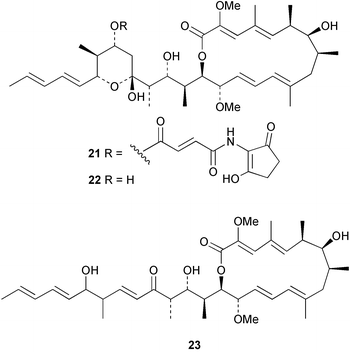
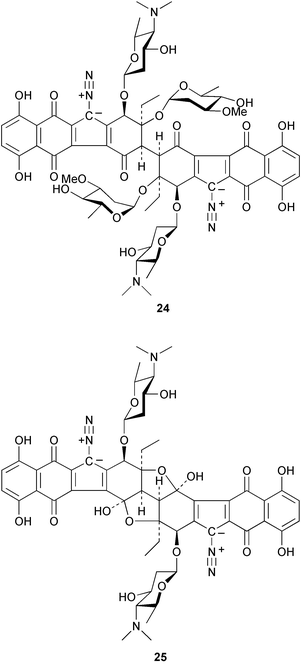
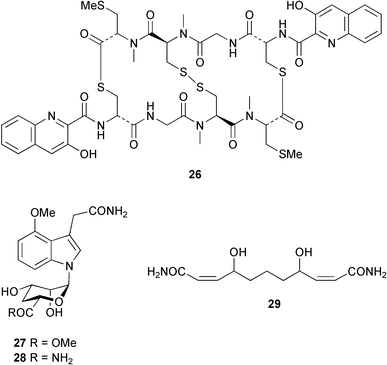
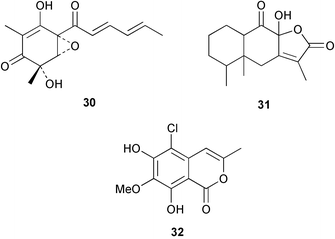
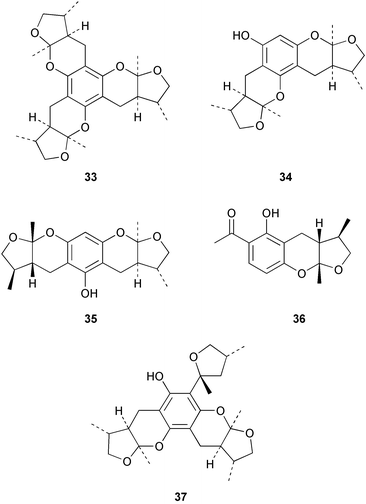
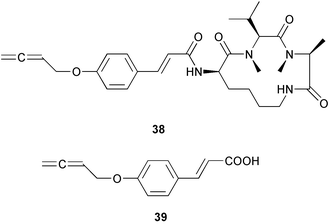
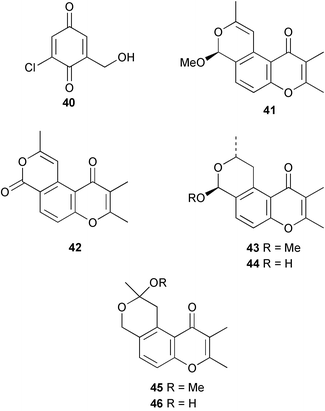
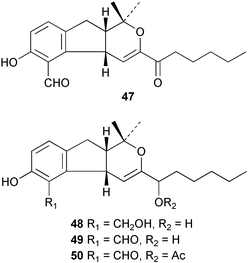
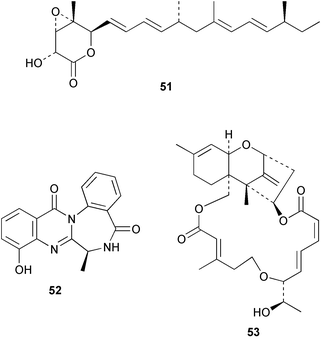
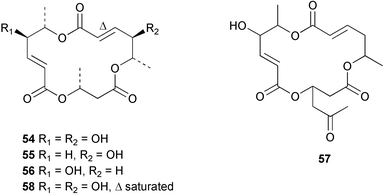
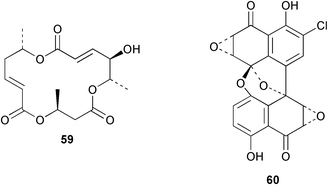
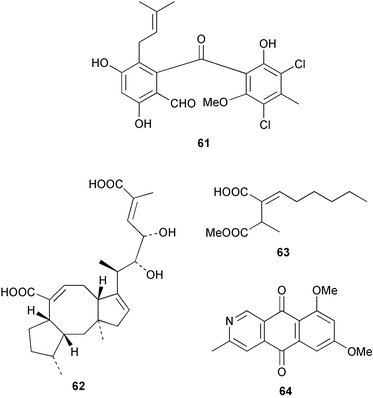
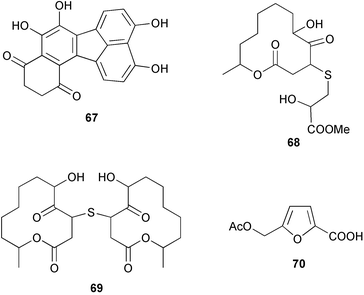
There continues to be much interest in cultured marine organisms. An acidic polysaccharide isolated from Pseudoalteromonas distincta that was obtained from a marine sponge, contained two unusual acidic amino sugars; 2-acetamido-2-deoxy-D-galacturonic acid 1 and 5-acetamido-3,5,7,9-tetradeoxy-7-formamido-L-glycero-L-manno-nonulosonic acid 2.40 A Streptomyces sp. isolated from a shallow sea sediment near Livingston Island, Antarctica was the source of 2-amino-9,13-dimethyl heptadecanoic acid 3, a compound with selective antimicrobial activity.41 The culture broth of a Streptomyces sp. isolated from sediment collected in Korean waters, yielded six novel lactone-containing metabolites 4–9.42 A novel glycerol diether, 2,3-di-O-dihydro-14,15-geranylgeranyl-sn-glycerol 10, was isolated from an anaerobic culture of the archaeon Thermococcus sp., collected from a deep-sea hydrothermal vent.43 A cell-free culture of the marine bacterium Vibrio angustum was the source of the ether [1-(2′-methylpropoxy)-2-hydroxy-2-methylpropoxy]butane 11, which induced both the acylated homoserine lactone (AHL) regulatory system in Agrobacterium tumefaciens and bioluminescence in Vibrio harveyi.44 Macrolactins G–M 12–18 were isolated from a culture broth of a Bacillus sp. The strain had been isolated from the red alga Schizymenia dubyi collected from Japanese waters and also contained macrolactins A and F which were previously isolated from an unclassifiable deep-sea bacterium.45 The macrolactins exhibited selective antimicrobial activity.46 A bacterial isolate from the tissues of an unidentified tube worm collected off Papua New Guinea that was tentatively identified as Bacillus laterosporus yielded the cationic peptide bogorol A 19. This compound displayed reasonably potent activity against both methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcal strains (VRE) of bacteria but exhibited no activity against a range of other bacteria and fungi.47 A novel β-methoxyacrylate antibiotic possessing a conjugated tetraene moiety was isolated from the culture broth of the marine myxobacterium Haliangium luteum and named haliangicin 20. Haliangicin was susceptible to oxidation in air and had to be kept in solution at −20 °C due to rapid decomposition at room temperature when evaporated to dryness.48 Haliangicin inhibited growth of a wide range of fungi but was inactive against bacteria.49 Micromonospolides A–C 21–23, macrolides containing a 16-membered lactone ring, were isolated from an undescribed actinomycete Micromonospora sp. These compounds inhibited the gastrulation of starfish (Asterina pectinifera) embryos.50,51 The crude extract of a halophilic actinomycete, the new species Micromonospora lomaivitiensis, that was isolated from the inner core of the ascidian Polysyncraton lithostrotum, exhibited potent DNA-damaging activity in the biochemical induction assay (BIA) and potent cytotoxicity against a panel of cancer cell lines. Two dimeric diazobenzofluorene glycosides, designated as lomaiviticins A 24 and B 25, were isolated from the extract by BIA-guided fractionation. Both were demonstrated to be potent DNA-damaging agents and lomaiviticin A 24 was shown to cleave double stranded DNA under reducing conditions. Lomaiviticin A possessed a unique cytotoxicity profile as compared to known DNA-damaging drugs such as adriamycin and mitomycin C against a number of cancer cell lines. Both lomaiviticins A and B exhibited potent antibacterial activity against S. aureus and Enterococcus faecium.52 A total synthesis of thiocoraline 26, a potent antibiotic that was isolated from Micromonospora sp.,53,54 has been accomplished and the relative and absolute stereochemistry established. Thiocoraline was also shown to bind to DNA by high-affinity bisintercalation, but with no obvious sequence selectivity. Thiocoraline was exceptionally cytotoxic to the L1210 murine leukaemia cell line with an IC50 value of 200 pM.55 The kahakamides A 27 and B 28 are new indole nucleosides that were isolated from the actinomycete Nocardiopsis dassonvillei, obtained from a shallow water sediment sample from Kauai, Hawaii. Kahakamide A 27 exhibited slight inhibition of B. subtilis in a disc-diffusion assay.56 (Z,Z)-4,8-Dihydroxyundeca-2,9-dienedioic acid diamide 29 was obtained from an unidentified marine actinomycete.57Marine-sourced fungi continue to be of interest. A total synthesis of (±)-epoxysorbicillinol 30, a pigment isolated from the fungus Trichoderma longibrachiatum that was separated from a Haliclona sponge,58 has been accomplished in 13 steps from diethyl methylmalonate.59 The sesquiterpene lactone, 8-hydroxy-9-oxo-7(11)-eremophilien-12,8-olide 31 was isolated from the marine fungus Hypoxylon oceanicum from the South China Sea.60 The isocoumarin avicennin A 32 was isolated from a mangrove endophytic fungus from the South China Sea,61 while the mangrove fungus Xylaria sp., also from the South China Sea, yielded xyloketals A–E 33–37. Xyloketal A 33 is a potent inhibitor of acetylcholine esterase.62 The unusual allenic ether, xyloallenolide A 38, was isolated from a Xylaria sp. from the South China Sea along with an aromatic allenic ether 39 which had not been previously reported from a natural source.63 Chlorogentisylquinone 40 was isolated from the culture broth of an unidentified marine fungus, and was shown to inhibit sphingomyelinase activity of rat brain membranes.64 The marine fungus Aspergillus versicolor, isolated from the sponge Xestospongia exigua from Bali, was the source of six chromone derivatives, the aspergiones A–F 41–46,65 and four further compounds, aspergillone 47, aspergillodiol 48, aspergillol 49 and 12-acetylaspergillol 50.66 An inhibitor of anaerobic electron transport, nafuredin 51, was isolated from Aspergillus niger, isolated from a marine sponge collected in Palau. Nafuredin proved to be a highly selective inhibitor of NADH-fumarate reductase (NFRD) from the pig roundworm Ascaris suum. Biosynthetic studies of nafuredin were carried out.67 The alkaloid circumdatin G 52 was isolated from the culture broth of the fungus Aspergillus ochraceus, isolated from sediment collected in the Sea of Japan.68 A new macrocyclic trichothecene, 12,13-deoxyroridin E 53 was isolated from the filamentous fungus Myrothecium roridum that was obtained from woody material collected in Palau. 12,13-Deoxyroridin E was active against human leukaemia HL-60 and murine L1210 cell lines.69 The macrosphelides E–I 54–58 have been isolated from Periconia byssoides, separated from the sea hare Aplysia kurodai. The absolute stereostructures of the macrosphelides were determined on the basis of spectroscopic analyses and chemical transformations. The absolute configuration of the known compound macrosphelide C 59 was established by X-ray analysis and application of the modified Mosher method. Macrosphelides E–H 54–57 inhibited the adhesion of HL-60 cells to human umbilical vein endothelial cells (HUVEC).70 The absolute stereochemistry of spiroxin A 60, a DNA-cleaving bisnaphthospiroketal from a marine-derived fungus,71 has been determined as (2S,3R,4S,2′S,3′R,4′S) by application of the exciton chirality method following derivatisation.72 A Pestalotia species of fungus isolated from the surface of the brown alga Rosenvingea sp. from the Bahamas, produced a chlorinated benzophenone antibiotic pestalone 61 only when a unicellular marine bacterium was co-cultured in the fungal fermentation. It was not detected when either organism was cultured individually. Pestalone 61, whose structure was confirmed by X-ray analysis, exhibited only moderate in vitro cytotoxicity in the National Cancer Institute's (NCI) 60 human tumour cell line panel but showed potent activity against MRSA and VCE.73 A culture broth of the marine fungus Halorosellinia oceanica collected in Thailand was the source of an ophiobolane sesterterpene halorosellinic acid 62 and of 4-methyl-2-hexylidene-3-methylsuccinate 63. The relative stereochemistry of halorosellinic acid was assigned by analysis of NOESY data. Halorosellinic acid 62 exhibited moderate antimalarial activity against Plasmodium falciparum but only weak antimycobacterial activity.74 3-Methyl-6,8-methoxy-2-aza-9,10-anthraquinone (scorpinone) 64 was isolated from the mycelium of a cultured Bispora-like fungus from sediment collected in the Bahamas, and the structure determined by X-ray analysis.75 Investigations of cultures of the mycelium of an unidentified fungus isolated from the red alga Ceradictyon spongiosum in Okinawa resulted in the isolation of two linear dodecapeptides, dictyonamides A 65 and B 66. Dictyonamide A 65 inhibited cyclin-dependent kinase 4 while dictyonamide B 66 was inactive.76 Hortein, 67, was isolated from the fungus Hortaea werneckii obtained from the Mediterranean sponge Aplysina aerophoba. Hortein possesses an acenaphtho[1′,2′:7,8]naphthalene ring system which is unique among natural products.77 Cultures of Cladosporium herbarum isolated from the sponge Callyspongia aerizusa collected from Bali yielded the 12-membered macrolide pandangolide 3 68, the macrolide dimer pandangolide 4 69 and a new acetyl derivative 70 of 5-hydroxymethylfuran-2-carboxylic acid, (also known as Sumiki's acid). Compound 70 inhibited the growth of B. subtilis and S. aureus in an agar plate diffusion assay.78 The penochalasins D–H 71–75 were isolated from a strain of Penicillium sp. originally separated from the green alga Enteromorpha intestinalis. The compounds were all moderately cytotoxic to P388 murine leukaemia cells.79
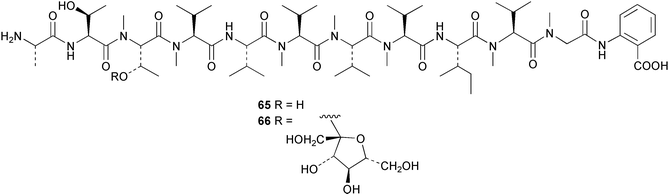
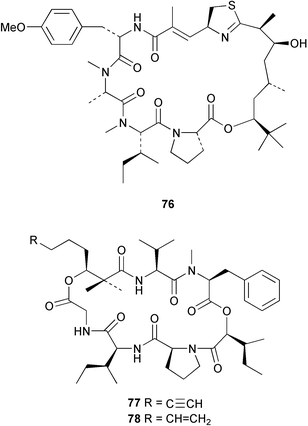
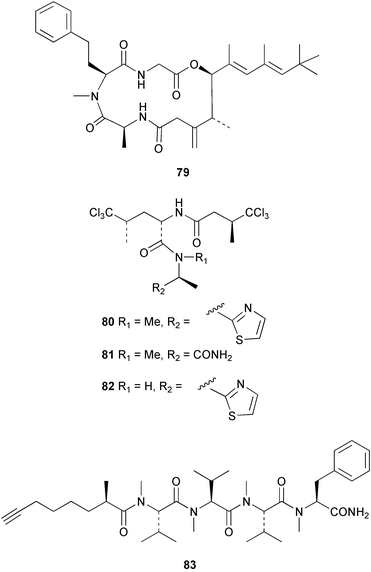
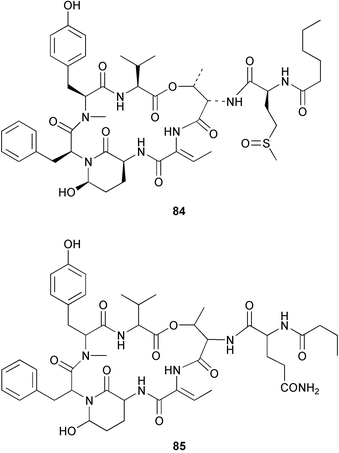
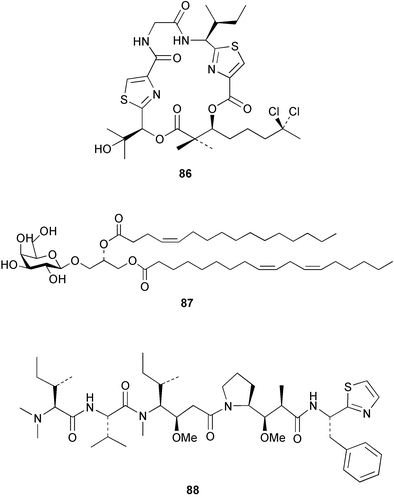
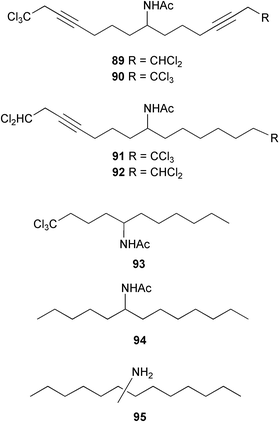
Cyanobacteria continue to yield novel structures. Apratoxin A 76, a cyclic depsipeptide of mixed peptide-polyketide origin, has been isolated from the cyanobacterium Lyngbya majuscula collected from Guam. The absolute configurations of the amino acid-derived units were determined by chiral HPLC analysis of hydrolysis products. The absolute stereochemistry of the new dihydroxylated fatty acid unit, 3,7-dihydroxy-2,5,8,8-tetramethylnonanoic acid was established through analysis by Mosher's method. The solution conformation of apratoxin A 76 was mimicked by molecular modelling. Apratoxin A exhibited potent cytotoxicity in vitro against KB and LoVo cell lines but was toxic in vivo to mice and was poorly tolerated.80 A different population of L. majuscula from Guam was the source of two further cyclic depsipeptides, pitipeptolides A 77 and B 78. Pitipeptolides A and B exhibited weak cytotoxicity against LoVo cells and both compounds were active in the antimycobacterial diffusion susceptibility assay but were less active than a control. Pitipeptolides A and B also stimulated elastase activity.81 Antillatoxin B 79, an N-methyl homophenylalanine analogue of antillatoxin,82 was isolated from samples of L. majuscula from Puerto Rico and the Dry Tortugas. Antillatoxin B was ichthyotoxic to goldfish and activated sodium channels in mouse neuro-2a neuroblastoma cells.83 A Panamanian collection of L. majuscula was the source of four new metabolites, pseudodysidenin 80, dysidenamide 81, nordysidenin 82 and dragonamide 83. The first three of these are closely related to dysidenin84 and isodysidenin,85 isolated from the sponge Dysidea herbacea. This is the first reported instance of the isolation of such compounds from a free-living cyanobacterium, most likely indicating that similar metabolites isolated from sponges are metabolites of associated cyanobacteria. Dragonamide contains a unique C8-alkynoate unit.86 Somamides A 84 and B 85 were isolated from mixed assemblages of the cyanobacteria L. majuscula and Schizothrix sp. from Fiji. The absolute stereochemistries of the amino acid residues for 84 were determined by Marfey's analysis. These depsipeptides are analogous to symplostatin 2, isolated from the cyanobacterium Symploca hydnoides87 and dolastatin 13, originally isolated from the sea hare Dolabella auricularia88 but most likely originating from its cyanobacterial diet.89 The first total synthesis of lyngbyabellin A 86, a peptolide isolated from L. majuscula from Guam,90 has been described. Lyngbyabellin A was synthesised in 58% yield by a convergent strategy.91 A culture of the marine cyanobacterium Oscillatoria sp. yielded a diacylgalactolipid 87.92 The total stereochemistry of symplostatin 1 88, a metabolite of Symploca hydnoides,93 was completed by determination of the stereochemistry of the N,N-dimethylisoleucine unit to be (S,S).94 The same paper reported the isolation of dolastatin 10 from a Symploca species, suggesting that cyanobacteria are the ultimate producers of this metabolite, rather than the sea hare Dolabella auricularia from which it was originally isolated.95 Symplostatin 1, a microtubule inhibitor, was a very potent cytotoxin both in vitro (KB and LoVo cell lines) and in vivo (with mice). It was, however, very toxic, causing lethality on day 1 when injected intravenously at low doses.94 Seven compounds, 89–95, of which five were polychlorinated acetamides, were isolated from Microcoleus lyngbyaceus collected from Chuuk, Micronesia. Compound 95 was tentatively identified as an aminotridecane but the placement of the amino group could not be determined as the compound decomposed before mass spectral studies could be carried out. A positional isomer of compound 93 was synthesised in six steps from δ-decanolactone.96
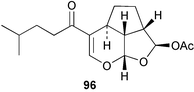
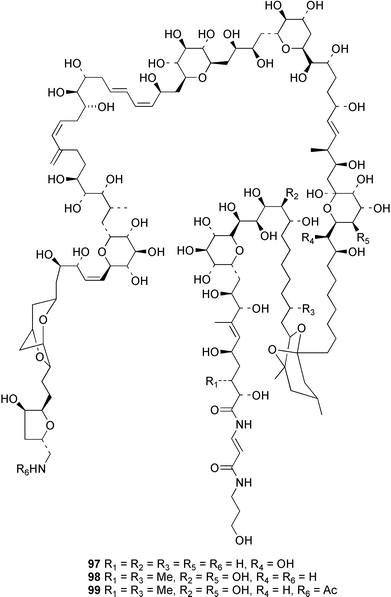
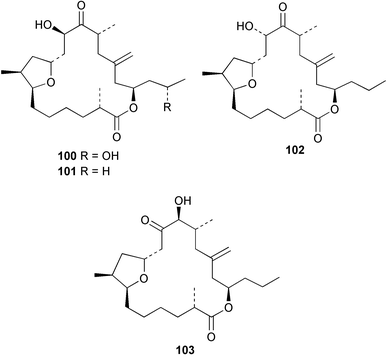
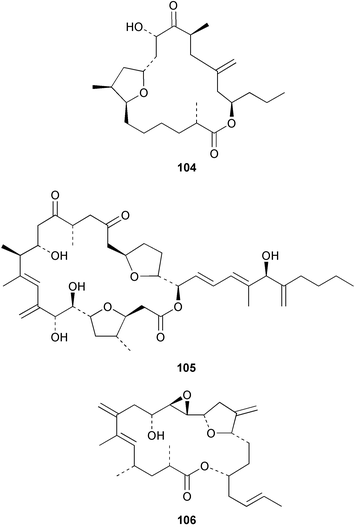
Stereoselective synthesis of an HI/JK ring model of prymnesins 1 and 2, glycosidic toxins isolated from the red tide phytoflagellate Prymnesium parvum,97 was accomplished. Comparison of the NMR data with those of the natural toxins confirmed the original stereochemical assignments.98 The first total synthesis of euplotin A 96, a cytotoxin of the ciliated protist Euplotes crassus99 has been achieved. The synthetic strategy employed retro-cycloaddition reactions of readily available 5-acyl-4-alkyl-4H-1,3-dioxins.100 Ostreocin D 97, a new analogue of palytoxin, was isolated from the dinoflagellate Ostreopsis siamensis. The structure was determined as 42-hydroxy-3,26-didemethyl-19,44-dideoxypalytoxin by 2D NMR analysis of ostreocin D and the ozonolysis products.101 3D Fourier transform and gradient enhanced NMR spectroscopies allowed the full assignment of the 1H and 13C NMR spectra of palytoxin 98 and the N-acetylpalytoxin analogue 99. Notably, the 15N NMR spectrum of N-acetylpalytoxin 99 could be assigned without 13C or 15N enrichment.102 The modestly cytotoxic (L1210 and KB cell lines) amphidinolides T2 100, T3 101 and T4 102, 19-membered macrolides structurally related to amphidinolide T1,103 have been isolated from two strains of the dinoflagellate Amphidinium sp.104 The absolute configurations of 100–102 and of amphidinolide T1 103 were determined by comparison of NMR spectroscopic data with those of synthetic model compounds. The biosynthetic origin of amphidinolide T1 103 was probed using singly and doubly labelled 13C sodium acetate and 13C-labelled sodium bicarbonate. Results suggested that amphidinolide T1 103 was generated through nonsuccessive mixed polyketides and that the main carbon source of amphidinolide T1 was derived from carbon dioxide. Amphidinolide T5 104 was later isolated from the same extract of Amphidinium sp., and the stereostructure of amphidinolide T1 103 was confirmed by a X-ray analysis.105 The absolute stereochemistry of amphidinolide C 105, a 25-membered macrolide from Amphidinium sp.106 was determined by a combination of NMR spectroscopic analyses, degradation experiments and synthesis of the C1–C7 segment.107 The first total synthesis of the related 19-membered lactone amphidinolide K108 has been accomplished.109 The relative and absolute stereochemistry of the synthetic material 106 was confirmed by X-ray analysis, showing that 106 is the antipode of the natural product.
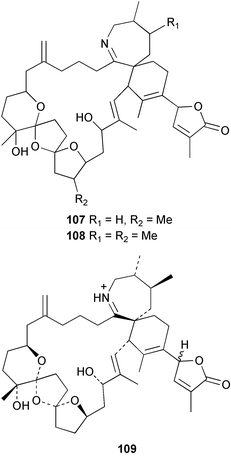
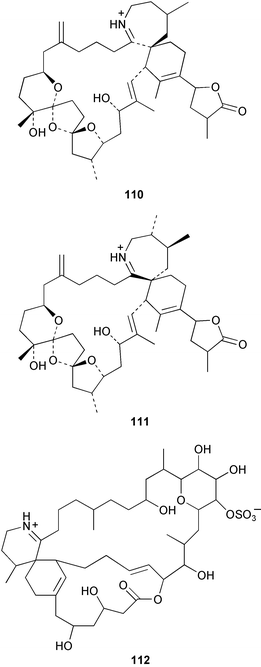
Spirolides A 107, C 108 and 13-desmethylspirolide C 109 were isolated from the annual accumulation of contaminated scallops and phytoplankton obtained from Nova Scotia as well as from batch cultures of the dinoflagellate Alexandrium ostefeldii, isolated from the phytoplankton assemblages. Spirolides B 110 and D 111 were previously isolated from contaminated shellfish in the same area.110 All of the spirolides display “fast-acting” toxicity in the traditional mouse bioassay due to the presence of a cyclic imine moiety. Spirolides containing a vicinal dimethyl group in the seven-membered ring were found to be resistant to oxalic acid hydrolysis.111 The relative stereochemistry of 13-desmethylspirolide C 109, excepting that at one stereogenic centre, was determined from NMR data by application of the ConGen molecular modelling programme. 13-Desmethylspirolide C 109 was found to have the same stereochemistry as pinnatoxins A112,113 and D114 in the regions of common structure. The relative stereochemistries of spirolides B 110 and D 111 were also partially determined by comparison of NMR data with those of 13-desmethylspirolide C 109 and further use of ConGen.115
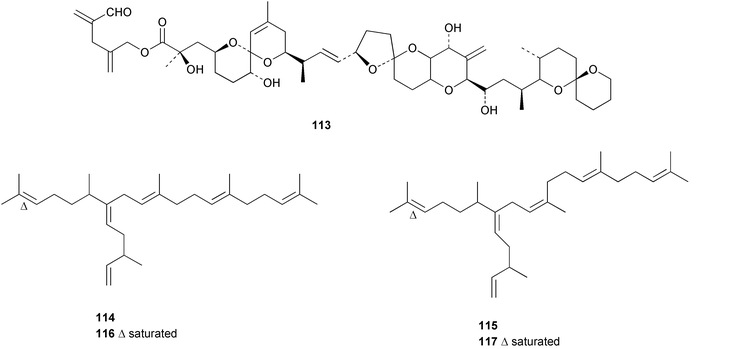
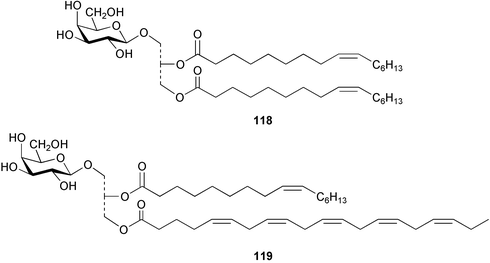
Spiro-prorocentrimine 112, a novel macrocyclic lactone, was isolated from a culture of a benthic Prorocentrum sp. obtained from epiphytes of coral reef seaweeds in Taiwan. This lactone was much less toxic than other known marine cyclic imine toxins in the intraperitoneal mouse bioassay.116 A new ester derivative of okadaic acid, DTX-6 113 was isolated from a culture of a strain of Prorocentrum lima.117 A culture of the marine diatom Rhizosolenia setigera, originally collected in France, was the source of highly branched isoprenoid pentaenes and hexaenes 114–117.118 Two galactopyranosyldiacylglycerols 118 and 119 were isolated from a cultured strain of the marine bacillariophycean microalga Nitzschia sp.119
3 Green algae
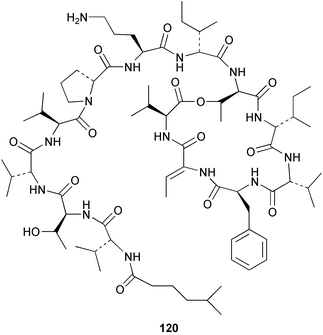
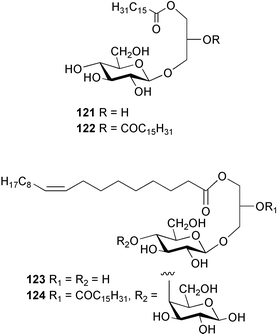
The depsipeptide kahalalide F 120, isolated from the mollusc Elysia rufescens and from Bryopsis sp., the green algal food source,120 has been synthesised.121 HPLC, NMR spectroscopic and biological activity studies indicated that the stereochemistry proposed by Scheuer et al.122 must be that of a less biologically active diastereomer. Four glycerol derivatives, codiosides A–D 121–124 and a new derivative of trans-phytol, codioester 125 were isolated from an Arabian Sea collection of the green alga Codium iyengarii.1234 Brown algae
The brown alga Sargassum crispum collected from the Red Sea was the source of sargassinone 126.124 The weakly cytotoxic (to P388 cells) hedaols A–C 127–129 were isolated from the Japanese brown alga Sargassum sp., and the absolute stereochemistry of hedaol A 127 established by the modified Mosher's method.125 A dolabellane diterpene, hydroclathrol 130, was isolated from the brown alga Hydroclathrus tenuis.126 The first enantiospecific synthesis of (+)-cyclozonarone 131, a sesquiterpene benzoquinone from the brown alga Dictyopteris undulata, has been achieved, utilising polygodial as starting material. The absolute configuration of the naturally occurring (−)-cyclozonarone was established as (5R,10R) by comparison of optical rotation and spectral data with those of (+)-cyclozonarone.127 Four novel acyclic diterpenes 132–135 were isolated from the brown alga Bifurcaria bifurcata collected off the Atlantic coast of Morocco.128 Two new aurones, 4′-chloro-2-hydroxyaurone 136 and 4′-chloroaurone 137 were isolated from Spatoglossum variabile collected off Karachi, Pakistan.129 A synthesis of (all-Z)-henicosa-1,6,9,12,15,18-hexaene 138 starting from (all-Z)-icosa-5,8,11,14,17-pentaenoic acid (EPA) has been completed.130 Compound 138 was originally isolated from the brown alga Fucus vesiculosus.1315 Red algae
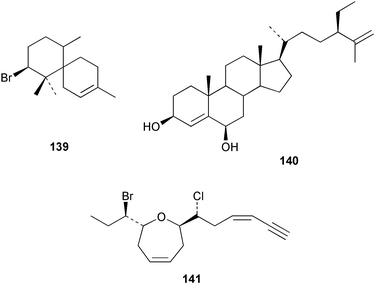
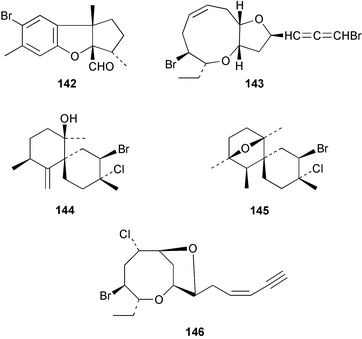
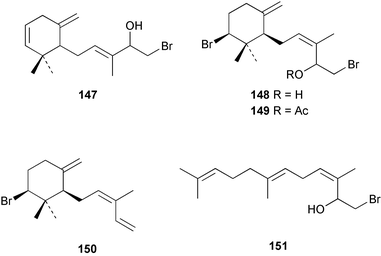
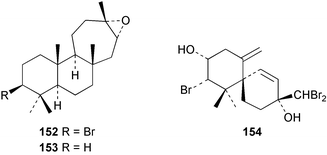
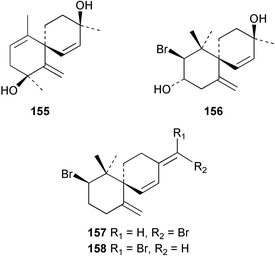
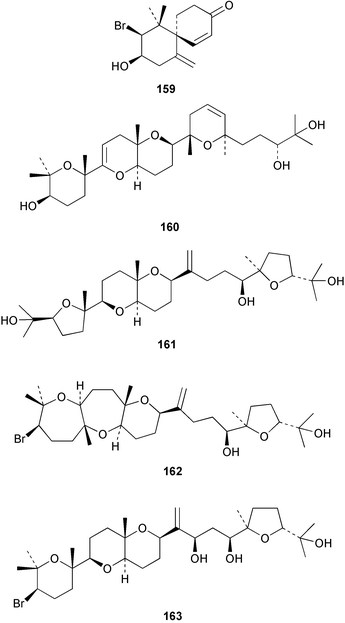
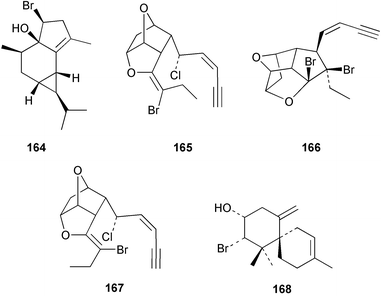
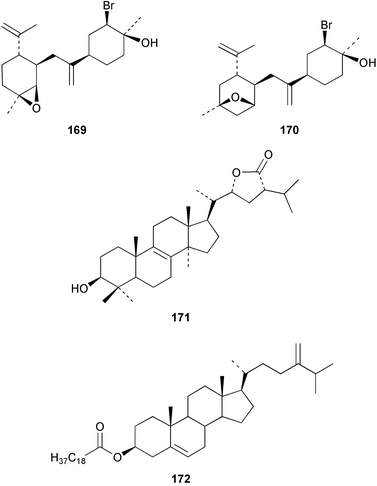
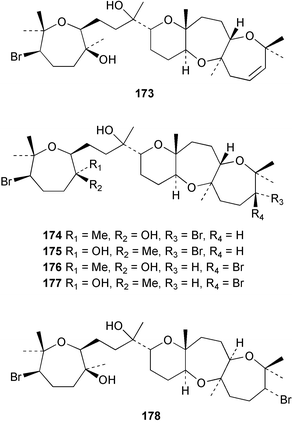
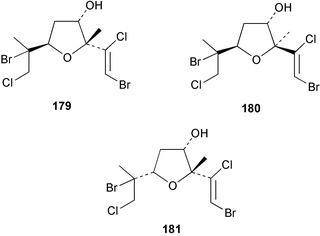
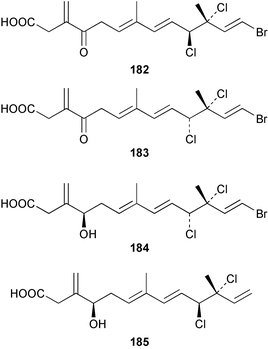
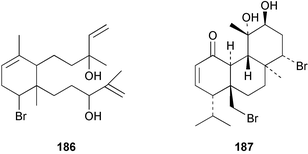
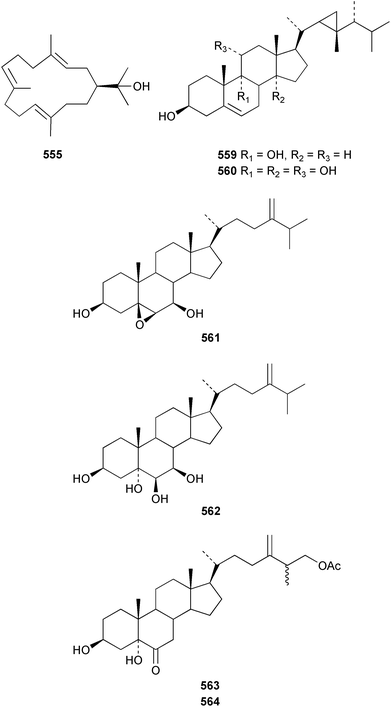
The red alga Laurencia majuscula collected from the South China Sea was the source of two compounds, the sesquiterpene 8-bromochamigren-1-en 139 and a derivative of stigmastadiendiol 140.132 The first total synthesis of the L. microcladia metabolite (+)-rogioloxepane A 141,133 has been accomplished.134 A total synthesis of (±)-aplysinal 142 from Marginisporum aberrans135 has been carried out136 and an asymmetric total synthesis of (−)-isolaurallene 143, originally isolated from L. nipponica yamada has also been achieved.137Laurencia pannosa from Malaysia was the source of three novel antibacterial metabolites: pannosanol 144 and pannosane 145 are halogenated sesquiterpenes with an unusual rearranged chamigrene skeleton while (3Z)-chlorofucin 146 is a halogenated C15 acetogenin.138L. luzonensis from Okinawan waters yielded five bromosesquiterpenes, isopalisol 147, luzonensol 148, luzonensol acetate 149, luzonensin 150 and (3Z,6E)-1-bromo-2-hydroxy-3,7,11-trimethyldodeca-3,6,10-triene 151 and a new bromoditerpene 3-bromobarekoxide 152. X-Ray analysis of 152 determined that the A/B and B/C ring junctions were both trans, in contrast to the trans, cis relative stereochemistry reported for the debrominated compound barekoxide, isolated from the sponge Chelonaplysilla erecta.139 Reductive debromination of 152 and comparison of the NMR and optical data with those reported for barekoxide, confirmed that the absolute configuration of barekoxide is correctly represented as 153.140 Ma'iliohydrin 154, a cytotoxic tribrominated chamigrene was isolated from a Laurencia sp. from the Philippines. Ma'iliohydrin exhibited cytotoxicity in the NCI 60-cell line human tumour screen and displayed especially potent activity against the NCI/ADR-RES breast cancer cell line.141L. scoparia collected in Brazilian waters was the source of four sesquiterpenes 155–158. Scopariol 155 has a rearranged chamigrane skeleton while isorigidol 156, is a β-chamigrene, as are the geometric isomers 157 and 158.142 An X-ray crystal structure of 156 established the absolute stereochemistry as (3R,6S,9S,10S).143 An X-ray analysis of ma'ilione 159, first isolated from L. cartilaginea144 determined the absolute stereochemistry as (6S,9R,10S).142,143 Compound 156 and ma'ilione 159 exhibited moderate in vitro anthelmintic activity against the parasitant stage of Nippostrongylus brasiliensis.142 Four squalene-derived triterpenes, martiriol 160, pseudodehydrothyrsiferol 161, dioxepandehydrothyrsiferol 162 and 16-epihydroxydehydrothyrsiferol 163 were isolated from L. viridis collected off the Canary Islands.145 Calenzanol 164, a sesquiterpene with a previously unreported ring system, was obtained as the major metabolite of L. microcladia off Elba Island in the Mediterranean. Calenzanol was found to be unstable, undergoing rearrangement at 40 °C to a novel indene.146 Two halogenated C15 acetogenins, lembynes A 165 and B 166 were isolated from an unrecorded species of Laurencia collected in Malaysian waters. Lembyne A 165 displayed modest antibacterial activity against a range of marine bacteria.147L. mariannensis from Okinawa contained (12E)-lembyne A 167.148 The halogenated sesquiterpene (6R,9R,10S)-10-bromo-9-hydroxychamigra-2,7(14)-diene 168 was isolated from L. majuscula from Okinawa and is the first report of this compound from a natural source. Both compounds 167 and 168 were active against a range of marine bacteria.148 Prevezols A 169 and B 170 are brominated diterpenes isolated from L. obtusa from Greek waters.149 A lanostanoid lactone, 5α-lanosta-8-en-3β,22ξ-dihydroxy-22(R),24(S)-lactone 171 was isolated from Hypnea cerricornis from the South China Sea.150 The Taiwanese red alga Ceratodictyon spongiosum which contains the symbiotic sponge Sigmadocia symbiotica yielded the novel sterol n-nonadecanoic acid 24-methylenecholesteryl ester 172.151 Armatols A–F 173–178 are bromotriterpene polyethers isolated from an Indian Ocean collection of Chondria armata.152 A Chilean collection of Plocamium cartilagineum yielded three tetrahydrofuran derivatives, furoplocamioids A–C 179–181, which contain a chlorobromo vinyl moiety.153P. cartilagineum from the Mediterranean was the source of four polyhalogenated homosesquiterpenic acids 182–185.154 Two bromoditerpenes, sphaerolabdiene-3,14-diol 186 and bromosphaerone 187, were isolated from a collection of Sphaerococcus coronopifolius from the Atlantic coast of Morocco. Compound 187 exhibited antibacterial activity against S. aureus.1556 Sponges
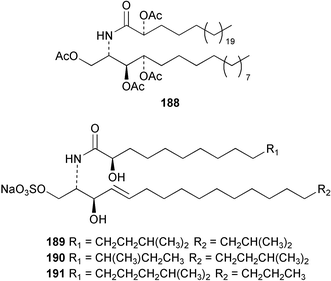
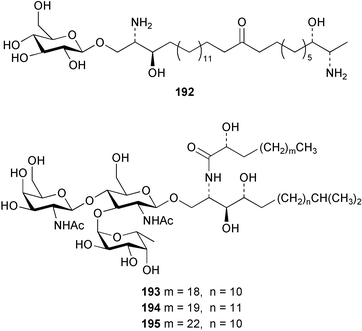
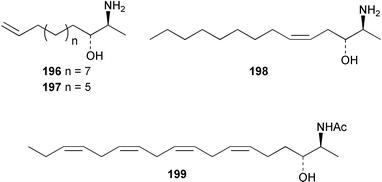
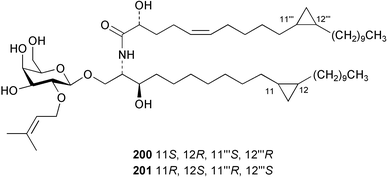
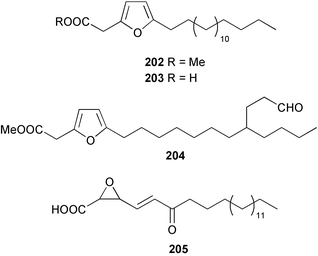
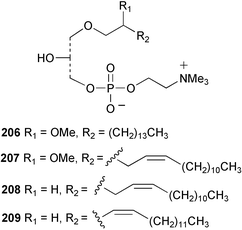
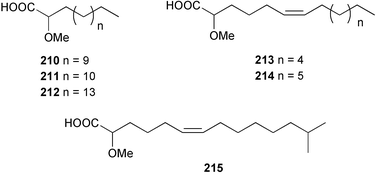
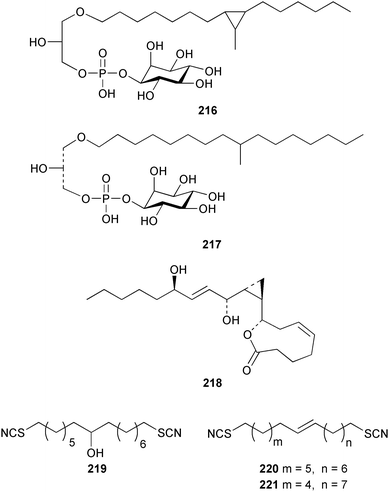
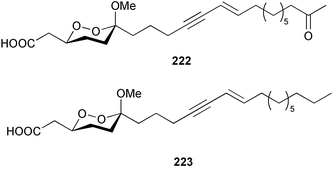
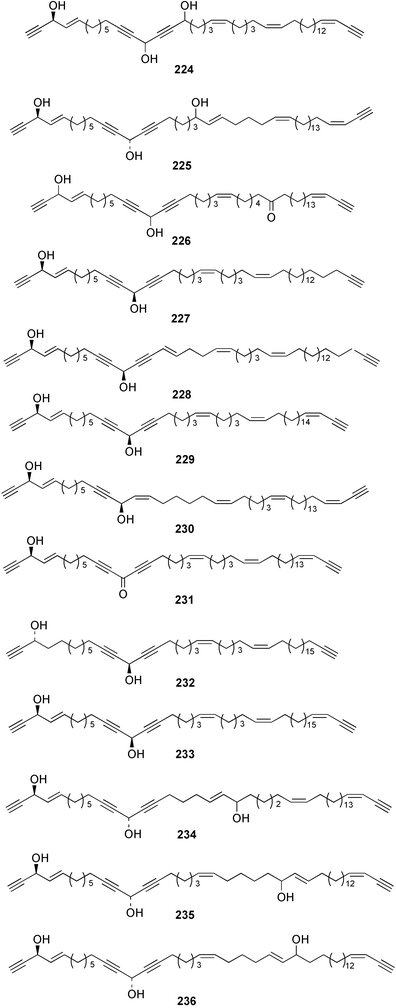
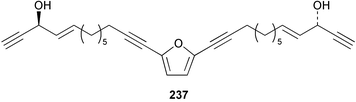
Once again, sponges have provided the greatest number of new marine natural products and have attracted considerable synthetic attention. An acetylated tetrahydroxyceramide 188 was isolated from an acetylated extract of Fasciospongia cavernosa collected on the South East coast of India.156 Three sulfated ceramides, calyceramides A–C 189–191 with neuraminidase inhibition activity were obtained from a Japanese collection of Discodermia calyx.157 A sponge of the genus Calyx, collected in Sulawesi, Indonesia, yielded a ketosphingolipid, calyxoside 192 with DNA-damaging properties.158 The keto substitution of 192 was located by reductive amination of a penta-acetate derivative and analysis of MS fragmentation, while the relative and absolute stereochemistry was proposed from CD analysis of the perbenzoyl aglycone.158 Three glycosphingolipids 193–195 were obtained from Aplysinella rhax collected in New Caledonia.159 A presumably new species of Haliclona from Queensland contained four unsaturated aminoalcohols 196–199 with antifungal properties.160 (2S,3R,11S,12R,2‴R,11‴S,12‴R)-Plakoside A 200 was synthesised161,162 and found to have optical rotation and spectroscopic data identical to plakoside A from Plakortis simplex.163 The spectroscopic data were also identical to the previously synthesised (2S,3R,11R,12S,2‴R,11‴R,12‴S) diastereomer 201; the absolute stereochemistry of the cyclopropyl groups remains unknown.Three furan-containing fatty acid derivatives, plakorsins A–C 202–204, and an epoxide, plakortic acid 205 were isolated from Taiwanese P. simplex specimens.164 The sponge Spirastrella abata collected from Korean waters yielded four phosphatidylcholines 206–209 of which 208 and 209 showed an inhibitory effect on the biosynthesis of cholesterol.165Callyspongia fallax collected in the Caribbean was found to contain the methoxylated acids 210–215.166 Two antimicrobial lysoplasmanylinositols 216–217 were isolated from a Japanese Theonella swinhoei.167 (−)-Halicholactone 218 from Halichondria okadai168 was synthesised stereoselectively using chiral (diene)Fe(CO)3 complexes.169 Three dithiocyanates, thiocyanatins A 219, B 220, and C 221, were isolated from an Oceanapia species collected from South West Australia. These compounds have nematocidal activity and their structures were confirmed by synthesis.170Acarnus bicladotylota collected from the South West coast of India yielded the acetylenic cycloperoxides, peroxyacarnoic acids C 222 and D 223 which were isolated as their methyl esters.171 A further series of cytotoxic polyacetylenic alcohols 224–236, have been isolated from a Korean Petrosia species that has previously yielded similar compounds.172,173 The absolute stereochemistry of (−)-adociacetylene B 237 from Adocia sp.174 was confirmed from a synthesis of both (+) and (−)-isomers employing enzymatic resolution.175
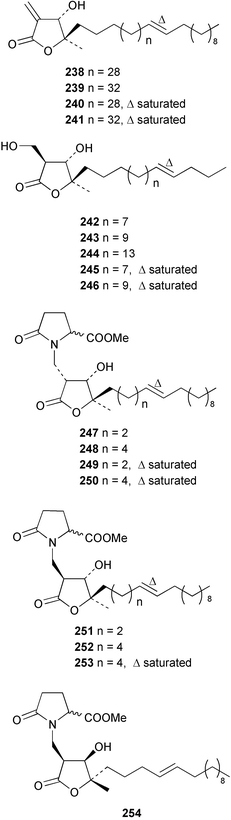
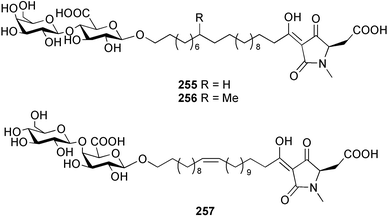
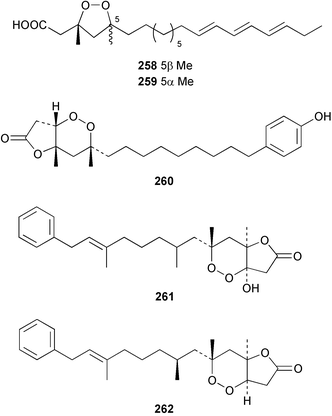
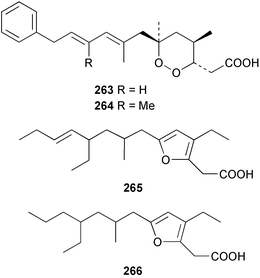
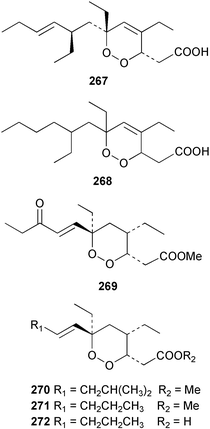
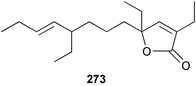
The amphiasterins A1–4 238–241, B1–5 242–246, C1–4 247–250, D1–3 251–253 and E1 254 have been isolated from Plakortis quasiamphiaster collected in Vanuatu.176 The magnesium salt of the previously reported ancorinoside A, isolated from an Ancorina species, has been reported as a new compound.177 Three ancorinosides, B 255, C 256 and D 257, isolated from a Japanese collection of Penares sollasi, were found to be inhibitors of membrane type 1 matrix metalloproteinase.178 The moderately antifungal plakinic acid F 258 and epiplakinic acid F 259 together with plakortolide F 260 were obtained from a Plakinastrella species collected in the Seychelles.179 The name plakortolide F was also given to a different peroxide lactone 261 which was isolated along with plakortolide G 262 from a Jamaican Plakinastrella onkodes collection. The absolute stereochemistry of 262 was proposed from a combination of optical rotation and molecular modelling data.180 The cytotoxic methyl capucinoate A 263 and the related compound 264 were isolated from a Dominican collection of P. onkodes;181 a structure with the same gross connectivity was reported earlier at a conference.182 In the same paper the isolation of glánvillic acid A 265 and B 266 from a Dominican Plakortis halichondrioides was also reported. The compounds were separated and characterised as the methyl esters.181 An Okinawan specimen of P. lita yielded two further cyclic peroxide acids, the haterumadioxins A 267 and B 268 with moderate cytotoxicity.183 Four further plakortides, I–L 269–272 were reported from an undescribed species of Plakortis collected in Jamaica.184 A γ-lactone, plakortone G 273, mildly active against Plasmodium falciparum, was isolated from an apparently different undescribed Jamaican Plakortis species.185
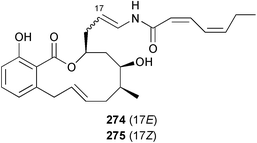
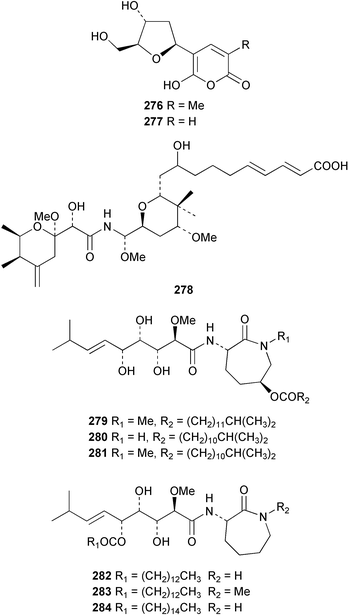
The absolute stereochemistries of the salicylihalamides A 274 and B 275 from Haliclona sp.186 have been revised following a re-interpretation of Mosher ester derivatives187 and enantioselective syntheses of both enantiomers of each.188–190 Two hydroxypyranones tetillapyrone 276 and nortetillapyrone 277 were obtained from the Thai sponge Tetilla japonica.191 Onnamide F 278, isolated from the South Australian sponge Trachycladus laevispirulifer, was found to be a potent nematocide.192 A further six bengamides 279–284 with varying in vitro antitumour activity were obtained from Fijian collections of Japsis cf. coriacea.193
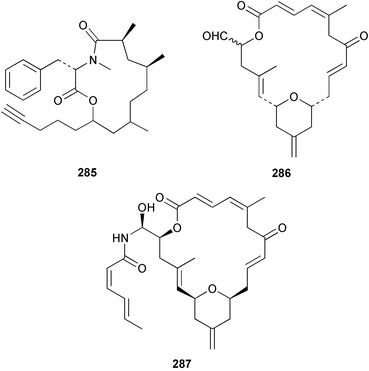
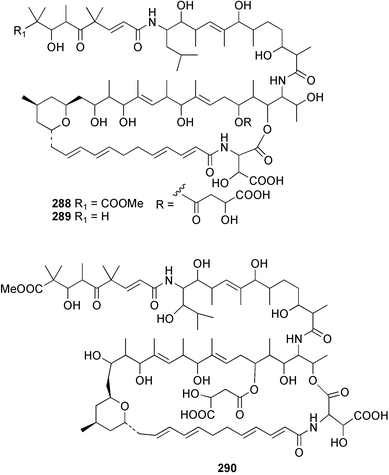
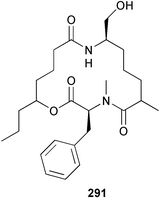
A Vanuatuan specimen of the genus Spongia was found to contain a cytotoxic macrolide spongidepsin 285,194 while the cytotoxic macrolide, dactylolide 286, was found in a Vanuatuan sponge of the genus Dactylospongia.195 The enantioselective synthesis of (+)-zampanolide196 has established the absolute and relative stereochemistry of the (−)-antipode 287 isolated from Fasciospongia rimosa.197 Three further, potently cytotoxic, chondropsin macrolide lactams were reported from three different sponges: 73-deoxychondropsin A 288 was isolated from an Australian Ircinia ramosa, chondropsin C 289 was found in a Philippine Ircinia species,198 while an Australian Chondropsis species yielded chondropsin D 290.199 A Vanuatuan specimen of the genus Haliclona was found to contain the in vitro antitumour macrolide haliclamide 291.200
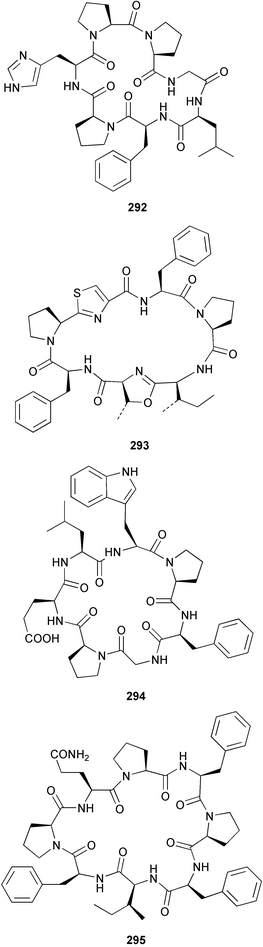
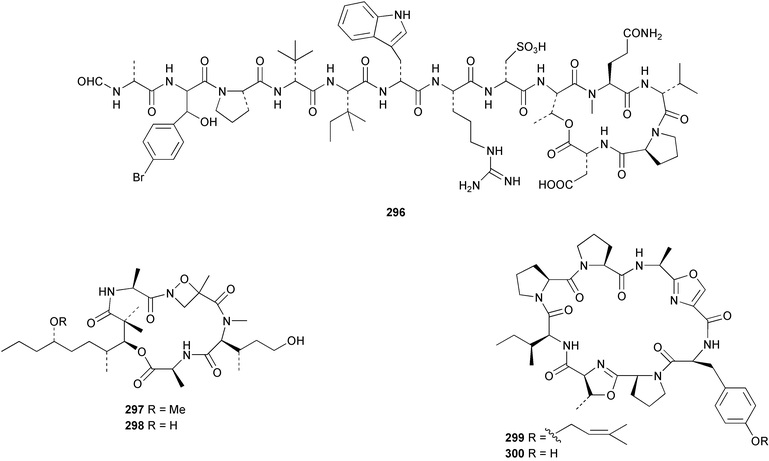
The weakly cytotoxic heptapeptide, wainunuamide 292 was isolated from Stylotella aurantium collected in Fiji.201 The total synthesis of cis,cis-ceratospongamide 293 from the red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica202 has been reported.203 Hymenamide C 294 from Hymeniacidon sp.204 has been synthesised using solid support methodology.205 A total synthesis of phakellistatin 11 295, isolated from Phakellia sp.,206 revealed that the synthetic product is much less cytotoxic than the originally isolated sample.207 A collection of Sidonops microspinosa from Sulawesi, Indonesia was found to contain the HIV-inhibitory depsipeptide microspinosamide 296.208 The structures of two potent anti-inflammatory peptides, halipeptin A 297 and B 298, isolated from a member of the genus Haliclona from Vanuatu, were proposed from an analysis of spectroscopic data.209 Two iron-chelating peptides, haliclonamide A and B, isolated from a Haliclona species collected in Palau were proposed to have structures 299 and 300 respectively on the basis of spectroscopic analysis.210
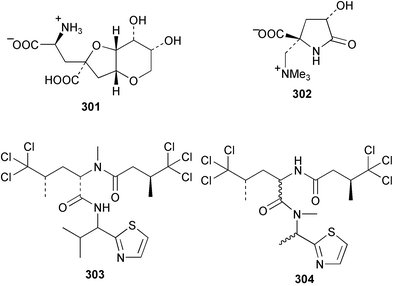
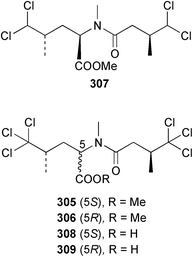
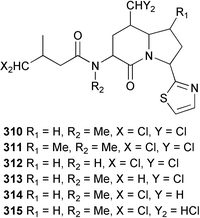
A potent, neurologically-active amino acid, neodysiherbaine A 301 has been reported as a minor metabolite of a Micronesian Dysidea herbacea. The relative and absolute stereochemistries were determined by asymmetric total synthesis.211 The enantioselective synthesis of both (+)- and (−)-dysibetaine 302, isolated from D. herbacea,212 has established the absolute configuration as (S,S).213 Two polychlorinated thiazoles 303 and 304 were isolated from Queensland specimens of D. herbacea.214 From the same collection, several polychlorinated dipeptides 305–309 were reported separately; the absolute stereochemistries were determined by comparison of optical rotation data.215 The methyl esters 305–307 are considered to be artifacts of methanolic extraction. An undescribed species of Dysidea collected in the Philippines yielded the proline-derived dysideaprolines A–F 310–315 together with the enol-ether containing barbaleucamides A 316 and B 317.216
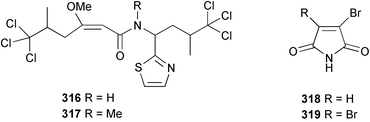
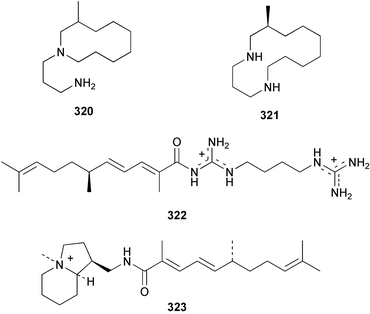
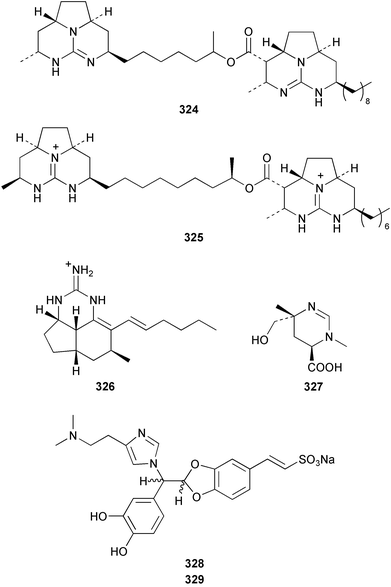
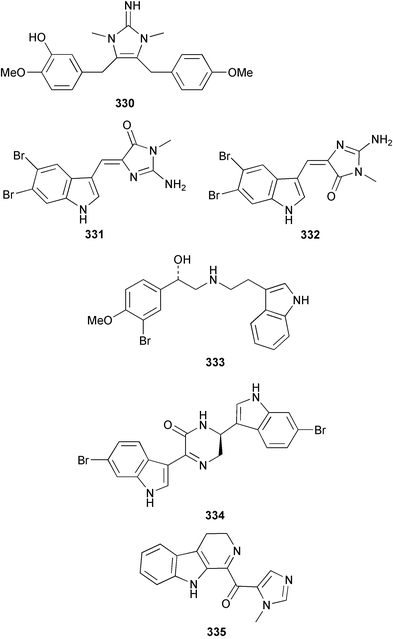
Two antifungal bromopyrroles, 3-bromomaleimide 318 and 3,4-dibromomaleimide 319, were found in Axinella brevistyla collected in Japan.217 (−)-Haliclorensin, isolated from Haliclona tulearensis,218 and assigned the structure 320, was synthesised by two independent groups and found to be spectroscopically non-identical with the natural product.219,220 Subsequently, a re-isolation and re-investigation of the spectroscopic data led to a revised structure 321 that was confirmed by enantioselective synthesis of both enantiomers.221 Both enantiomers of stellettadine A 322 from Stelletta sp.222 were synthesised from (S)- and (R)-citronellal.223,224 The (S) isomer was found to have a negative rotation similar to the natural compound which had previously been assigned as (R).223 (−)-Stellattamide B, originally reported from a Stelletta sp. with a (6″S) configuration,225 has now been established as (1S,4S,8aR,6″R) 323 by total synthesis.226 (+)-Batzelladine F, originally isolated from Batzella sp.,227 recently re-assigned as Monanchora arbuscula,228 was originally assigned structure 324. The structure has been revised to 325 on the basis of the enantioselective synthesis of both the revised and putative structures.229 Mirabilin G 326 was isolated from a South Australian Clathria species.230 The Palauan sponge Protophlitaspongia aga yielded 3,4,5,6-tetrahydro-6-hydroxymethyl-3,6,dimethylpyrimidine-4-carboxylic acid 327 that was found to inhibit the settling of larvae of the barnacle Balanus amphitrite.231 The wondonins A 328 and B 329 were isolated from an association of Poecillastra wondoensis and a Japsis species from Korea.232 Naamine B 330 from Leucetta chagosensis233 has been synthesised.234
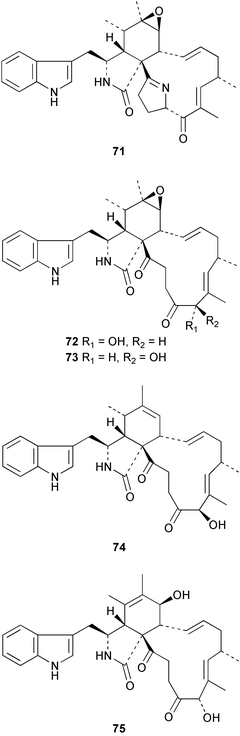
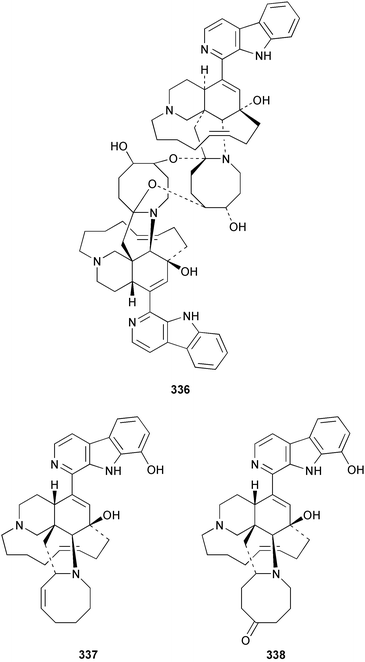
Hyrtios erecta collected in Okinawa yielded two selective inhibitors of neuronal nitric oxide synthase 331 and 332.235 An asymmetric synthesis of (+)-chelonin B 333 from Chelonaplysilla sp.236 employing a Sharpless asymmetric dihydroxylation established the stereogenic centre as (S).237 The synthesis of the (−)-enantiomer of (+)-hamacanthin A 334 isolated from Hamacantha sp.238 established the stereogenic centre as (S).239 Xestomanzamine B 335 from Xestospongia sp.240 was synthesised via a Pictet–Spengler condensation of tryptamine with a vicinal tricarbonyl substituted imidazole.241 A manzamine dimer, neo-kauluamine 336, was isolated from an undescribed genus of the family Petrosiidae collected from Sulawesi, Indonesia along with the antipodes of 8-hydroxymanzamine 337 and manzamine F 338. These antipodes were found to have potent in vivo activity against Plasmodium berghei.242 The manzamines have been isolated from a variety of sponge genera from various orders. The authors speculate that some of these sponges may be members of this new genus of the Petrosiidae family.242
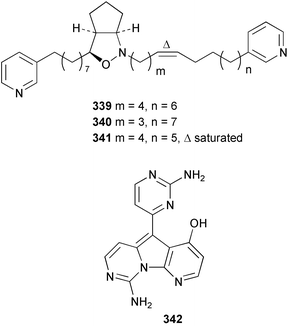
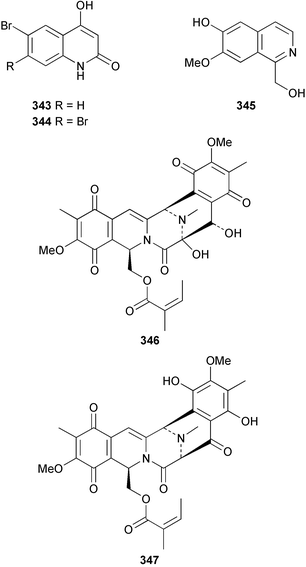
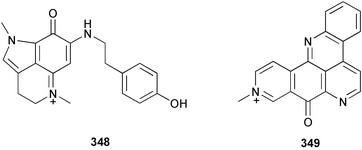
A revised structure 339 for pyrinodemin A 340 from Amphimedon sp.243 has been proposed from spectral comparison of the natural product to the synthesised structures.244 Pyrinodemin B 341 from the same sponge245 was also synthesised.244 Variolin B 342 from Kirkpatrickia varialosa246 was synthesised via a tandem deoxygenation and cyclisation of a triarylmethanol intermediate.247 Two bromoquinolones 343 and 344 were isolated from Okinawan specimens of Hyrtios erecta: 343 is a known synthetic compound.235 A Haliclona species from the Philippines contained 1-hydroxymethyl-7-methoxyisoquinolin-6-ol 345.248 The structure of renieramycin H, previously described from Haliclona cribricutis as 346249 has been re-assigned as 347 on the basis of spectral comparison to synthetic model compounds.250 Subsequently, it was found that this structure was assigned to cribrostatin 4 on the basis of X-ray analysis.251 The NMR spectra of the two compounds were found to be identical, and accordingly, the trivial name renieramycin H must be given to this structure. Makaluvamine P 348, with cytotoxic and antioxidant activity, was isolated from the Vanuatuan sponge Zyzzya cf. fuliginosa.252 Two Xestospongia species collected in Palau and the Philippines contained the DNA-cleaving agent deoxyamphimedine 349.253
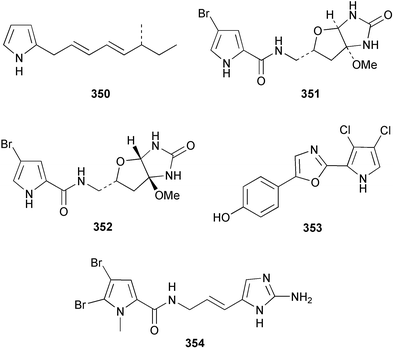
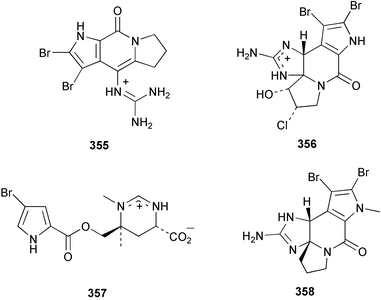
The absolute stereochemistry of (R)-(−)-axinellamine A 350, isolated from Axinella sp.254 was determined by synthesis of the (S)-isomer.255 The enantiomers of (+)-slagenin B 351 and (−)-slagenin C 352 from Agelas nakamurai256 were synthesised stereospecifically, establishing the absolute stereochemistry as (9R,11R,15R) and (9R,11S,15S) respectively.257 A total synthesis of phorbazole C 353 from Phorbas aff. clathrata258 was achieved with the central oxazole ring formed by cyclodehydration of an acylaminoketone.259 The N-methyloroidin derivative, sventrin 354, reported from the Bahaman sponge Agelas sventres, was found to be a feeding deterrent to the reef fish Thalassoma bifasciatum.260Axinella carteri from the Philippines afforded ugibohlin 355, a dibromopyrrole derivative.261A. brevistyla from Japan was found to contain 12-chloro-11-hydroxyldibromoisophakellin 356 and N-methylmanzacidin C 357. Both displayed antifungal and cytotoxic activity.217N-Methyldibromoisophakellin 358, isolated from the Bahaman sponge Stylissa caribica, was found to inhibit the feeding of the reef fish T. bifasciatum.262
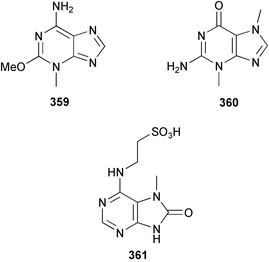
The weakly cytotoxic methoxypurine, mucronatine 359, was isolated from the French Mediterranean sponge Stryphnus mucronatus.263Zyzzya fuliginosa from the Philippines afforded 3,7-dimethylguanine 360.264 A weak inhibitor of CDC2 kinase, microxine 361, a taurine-bearing purine derivative, was isolated from an Australian Microxina species.265
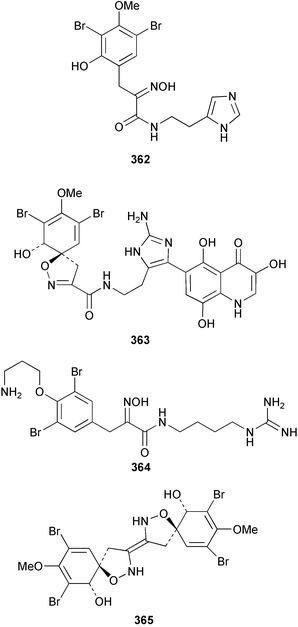
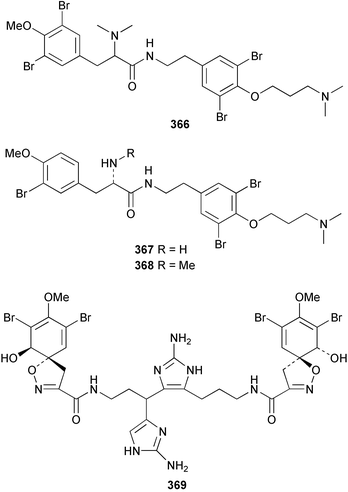
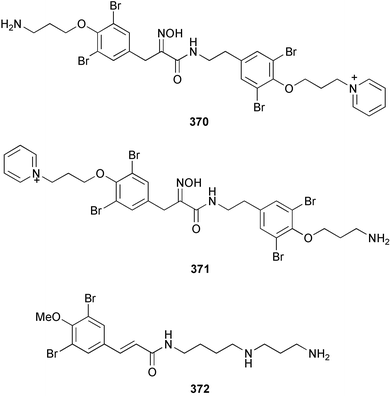
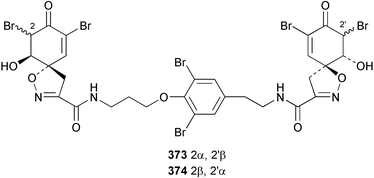
Purealidin N 362 from Psammaplysilla purea266 was synthesised with the oxime formed from addition of hydroxylamine chloride to a silyl-enol ether.267 Inhibitors of the novel mycobacterial enzyme mycothiol S-conjugate amidase, the bromotyrosine-derived alkaloids 363 and 364, were isolated from an Australian Oceanapia species; the absolute stereochemistry of 363 was determined by comparison of the optical rotation to similar compounds.268Pseudoceratina purpurea collected in Okinawa contained zamamistatin 365, a growth inhibitor of the adherent bacterium Rhodospirillum salexigens. The absolute stereochemistry was determined by analysis of the Mosher's acid derivatives.269 Aplyzanzine A 366 was reported from an Aplysina species collected near Zanzibar.270 A Suberea species from Okinawa yielded the cytotoxic suberedamines A 367 and B 368. Chemical degradation to (S)-tyrosine allowed assignment of the absolute configurations.271 Archerine 369, isolated from the Caribbean sponge Aplysina archeri, displayed antihistamine activity in isolated guinea pig ileum.272 The tokaradines A 370, B 371 and C 372, isolated from Pseudoceratina purpurea collected in southern Japan, were found to be toxic to the crab Hemigrapsus sanguineus.273Suberea aff. praetensa, collected in Thailand, yielded 11,17-dideoxyagelorin A 373 and B 374.274
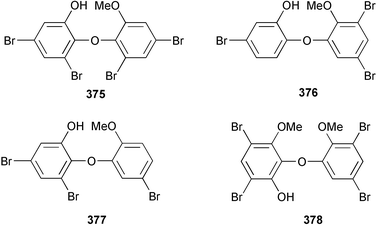
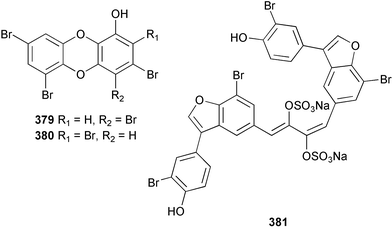
Four polybrominated phenols 375–378 were isolated from Phyllospongia dendyi collected in Palau. These compounds were found to be toxic to a variety of micro- and macro-algae.275Dysidea dendyi collected from the North West coast of Australia yielded two tetrabrominated dibenzo-p-dioxins, spongiadioxins A 379 and B 380.276 Iantheran B 381, isolated from an Ianthella species collected from the Great Barrier Reef, Australia was found to be a Na/K-ATPase inhibitor.277
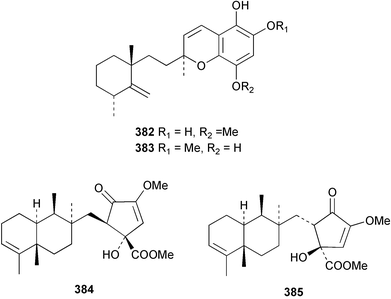
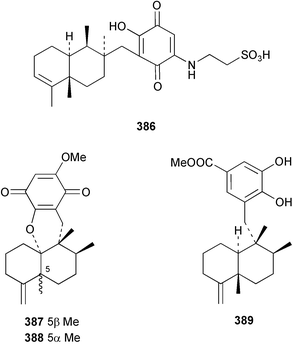
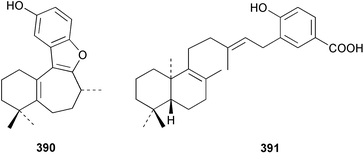
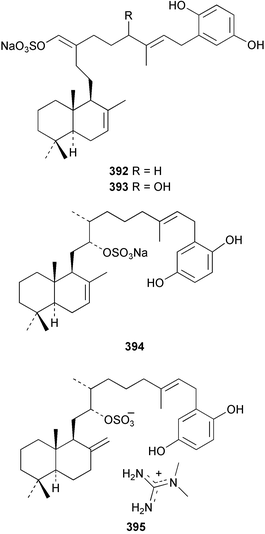
The merosesquiterpenoids hippochromin A 382 and B 383 were isolated as acetates from a Taiwanese specimen of Hippospongia metachromia.278 Two rearranged merosesquiterpenoids dysidenone A 384 and B 385 and the related selective PLA2 inhibitor, dysidine 386 were isolated from a sponge of the genus Dysidea collected in Vanuatu.279 Dactyloquinones A 387 and B 388 were reported from a Dactylospongia elegans specimen collected in Okinawa.280 Smenospondiol 389 from a Smenospongia sp.,281 also known as dictyoceratin A from Hippospongia sp.282 was synthesised by titanium-mediated tandem radical cyclisation as a racemic mixture.283 Two racemic and one asymmetric syntheses were reported for (+)-frondosin B 390, isolated from Dysidea frondosa,284 establishing the configuration of the stereogenic centre as (R).285 A diterpene-4-hydroxybenzoic acid derivative, subersic acid 391, with human lipoxygenase inhibitory activity was isolated from a Papua New Guinean Suberea species together with a diterpene described below.286 The hipposulfates A 392 and B 393, isolated from an Okinawan Hippospongia cf. metachromia, were found to have moderate cytotoxicity.287 The New Caledonian sponge Coscinoderma mathewsi afforded the CDC25 phosphatase inhibitor (+)-coscinosulfate 394 along with an unnamed congener 395.288 The relative and absolute stereochemistry of 394 was confirmed by total asymmetric synthesis.289 Interestingly, halisulfate 1, previously isolated from a Halichondria sp.,290 was reported with the same relative stereochemistry. Halisulfate 1 has a reported optical rotation of −27.3° while that of coscinosulfate is +5°; differences in 1H–1H coupling constants near the sulfate groups were noted,288 but unfortunately the NMR solvent used was different in the two studies.
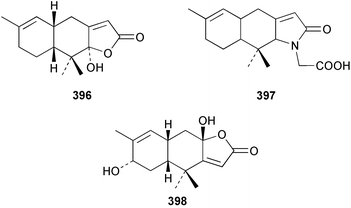
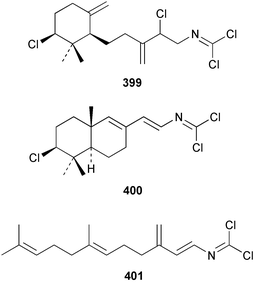
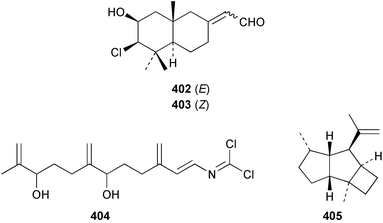
The sesquiterpenoid furodysin lactone 396 and a related amino acid derivative, pyrodysinoic acid 397, were obtained from a blue/grey encrusting member of the genus Dysidea collected from the Philippines.291 A specimen of Dysidea herbacea collected from Lizard Island, Australia was found to contain 6-hydroxyfurodysinin 398.214 Three carbonimidic dichlorides, 399–401, and two related α,β-unsaturated aldehydes 402 and 403, were isolated from Stylotella aurantium collected from Okinawan waters.292 The North Queensland sponge Ulosa spongia also contained 401 along with the related diol 404.293 Two enantiomeric syntheses of (+)-kelsoene 405 from Cymbastela hooperi294 have established the absolute stereochemistry.295,296
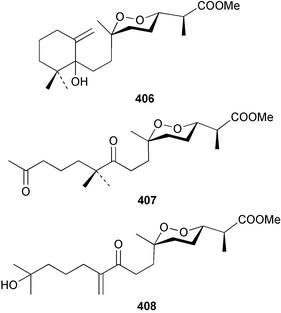
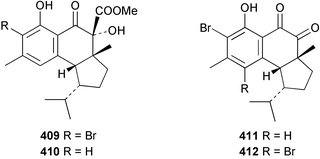
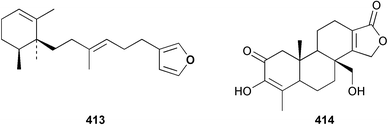
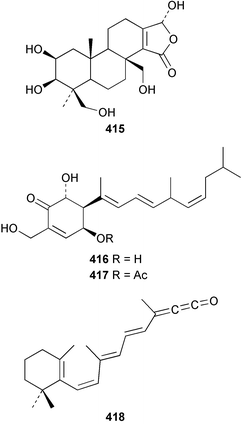
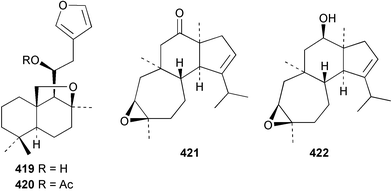
Three weakly cytotoxic C19 norditerpene peroxides, aikupikoxides B 406, C 407, and D 408 were isolated from Diacarnus erythraenus collected from the Red Sea along with a related norsesterterpenoid discussed below.297 The hamigerans 409–412, isolated from Hamigera tarangaensis298 were synthesised via an intramolecular Diels–Alder trapping of a photochemically generated hydroxy-o-quinodimethane.299 (+)-Subersin 413 was isolated from a Papua New Guinean Suberea species.286 The absolute stereochemistry was proposed by comparison of its optical rotation to that of a similar structure. The structure of cacospongin A, isolated from a Philippine sponge of the genus Cacospongia, has been re-assigned to the same diterpene skeleton with no stereochemistry specified.300 The 13C and 1H NMR data are identical to that of 413 but the sign of the optical rotation is opposite, suggesting an enantiomeric relationship. Spongia zimocca subspecies irregularia from China yielded the norditerpenoid zimoclactone B 414 and the diterpenoid zimoclactone C 415.301 A South Australian species of Phorbas yielded phorbasin B 416 and its acetate, phorbasin C 417.302 A quite remarkable cumulated ketene containing compound, irciniketene 418 with moderate cytotoxicity, has been reported from Ircinia selaginea collected in Guangxi Province, China.303 A Cacospongia species collected in Okinawa contained (−)-cacofuran A 419 and the acetate, cacofuran B 420. The absolute stereochemistry was determined from an analysis of the MPTA esters of 419 and by X-ray analysis.304 It was noted that both compounds inhibited the development of fertilised sea urchin eggs. The tricarbocyclic epipolone 421 and epipolol 422 were obtained from Epipolasis reiswigi collected in Puerto Rico.305
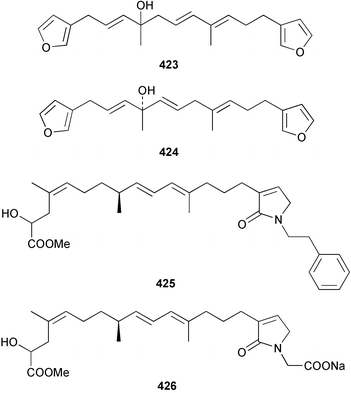
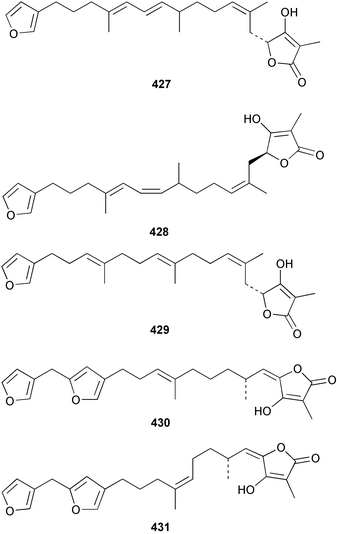
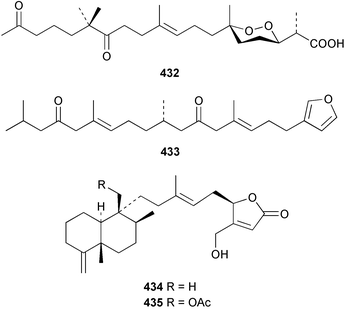
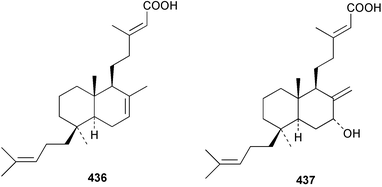
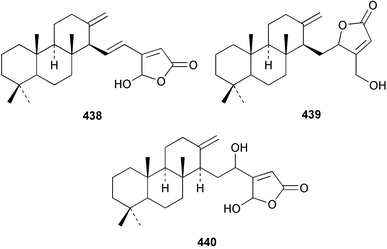
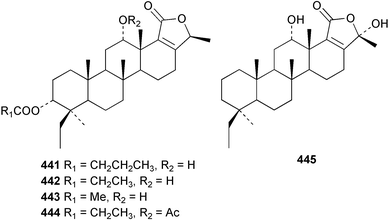
The C21 norsesterterpenoid originally reported with conjugated double bonds 423306 has been revised to 424 on the basis of more complete spectroscopic data obtained from a sample isolated from an Australian specimen of Spirastrella papilosa.307 The absolute stereochemistry was determined by degradation and the name (−)-isotetrahydrofurospongin-1 is proposed for this bisfuranoterpene. Two unusual trisnorsesterterpenoid lactams, the sarcotragins A 425 and B 426 were isolated from a Sarcotragus species from Jaeju Island in Korean waters.308 Five cytotoxic furanosesterterpenoids, the sacotins A–E, 427–431 were reported from a different specimen of Sarcotragus sp. collected at Cheju Island, Korea.309 The absolute stereochemistries of 429–431 were determined by comparison of CD spectra. The Red Sea sponge Diacarnus erythraenus was found to contain the antiviral and cytotoxic C24 norsesterterpenoid muqubilone (aikupikoxide) 432 by two independent groups.310,297 (−)-Idiadione 433 from Spongia idia311 was synthesised from (−)-citronellal, establishing the stereogenic centre as (S).312 A Palauan species of Thorectandra yielded the cytotoxic thorectandrols A 434 and B 435.313 The cytotoxic kohamaic acids A 436 and B 437 were isolated from an Ircinia species from Okinawa.314 Three tricarbocyclic sesterterpenoids 438–440 of the cheilanthane class isolated from a Queensland Ircinia sp. were found to be inhibitors of MSK1 and MAPKAPK-2 protein kinases.315 Five inhibitors of in vitro HIV-1 envelope-mediated fusion, the phyllolactones A–E 441–445, were obtained from specimens of Phyllospongia lamellosa.316
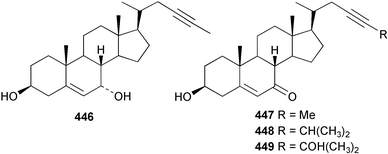
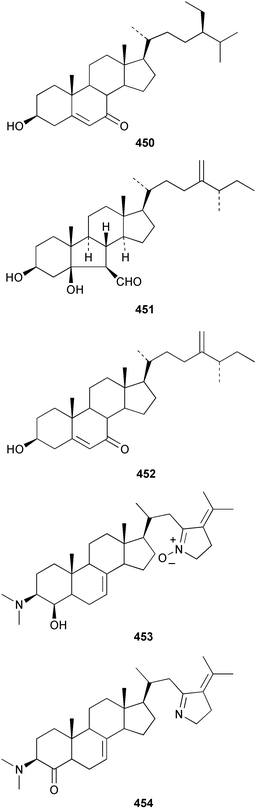
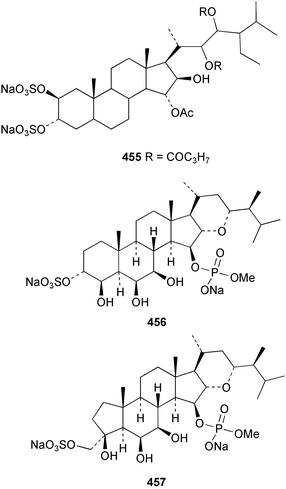
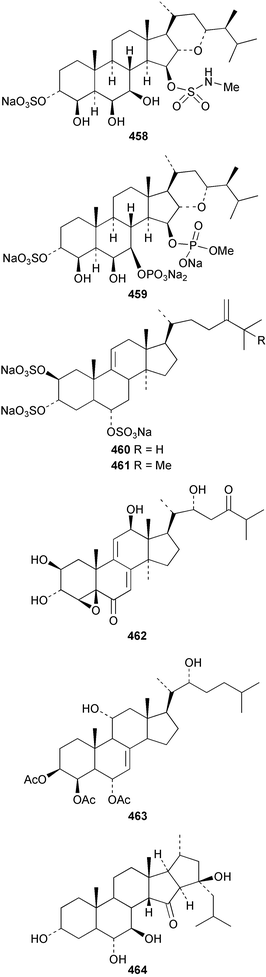
An undescribed species of Gellius from the Caribbean coast of Panama yielded four acetylenic sterols, gelliusterol A–D 446–449; gelliusterols A, B and C were found to be moderately cytotoxic.317 Stigmast-5-en-7-on-3β-ol 450 was obtained from Polymastia sobustia from the South China Sea.318 A cytotoxic and apoptosis-inducing rearranged sterol, orostanal 451 was obtained from Stelletta hiwasaensis collected in Japan.319 The 6–5–6–5 fused ring system has only been found previously in the terrestrial plant Taiwania cryptomeriodes;320 the absolute stereochemistry of 451 was established from analysis of the CD spectrum. A South China Sea specimen of Geodia japonica yielded 26-methylergosta-5,24(28)-dien-3β-ol 452 along with several nortriterpenoids discussed below.321 Two steroidal alkaloids, plakinamine E 453 and F 454 exhibiting moderate cytotoxicity, antifungal activity and nucleic acid-cleaving properties, were isolated from an undescribed species of Corticium collected at Guam.322 A sulfated sterol ester, clathsterol 455 with anti-HIV-1 reverse transcriptase activity was obtained from an Eritrean sponge of the genus Clathria.323 Two phosphorylated sterol sulfates isolated from a Japanese Cribrochalina species are inhibitors of membrane-type matrix metalloproteinase.324 The structures were assigned as 456 and 457. However, 456 was found to be identical to haplosamate A 458 previously isolated from two haplosclerid sponges from the Phillipines,325 requiring a structural revision of 458 to 456 and for assignment of haplosamate B as 459. The absolute stereochemistry of 456 was determined by Mosher's method. A Philippine sponge of the genus Xestospongia contained two sulfated sterols, ibisterol B 460 and C 461 and an epoxysteroid 462 that were found to be inhibitors of HIV-1 integrase.326 (+)-Agosterol A 463 from Spongia sp.327 was synthesised from ergosterol.328 The unusual 15-keto steroid, xestobergsterol 464 from Xestospongia bergquisti329 has been synthesised from stigmasterol.330
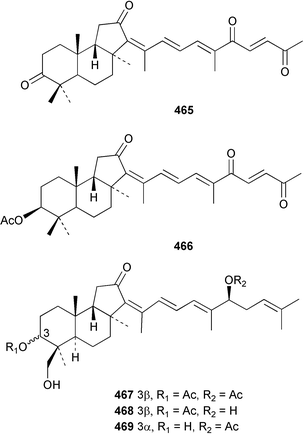
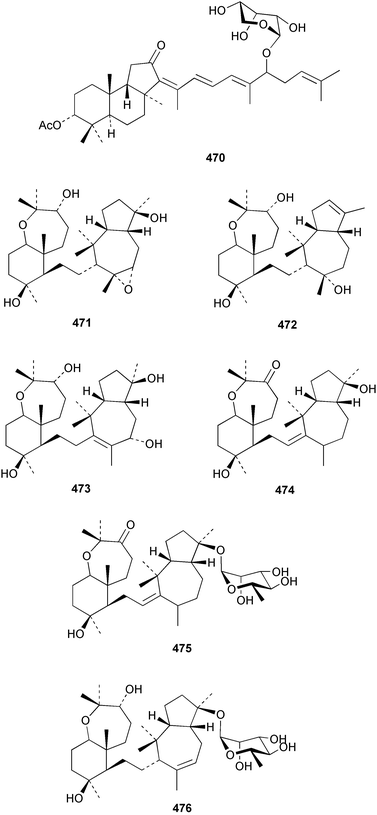
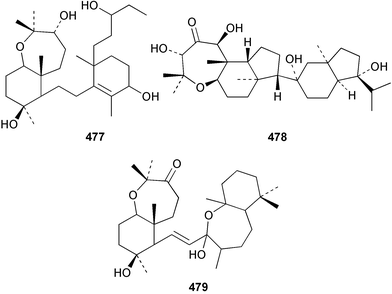
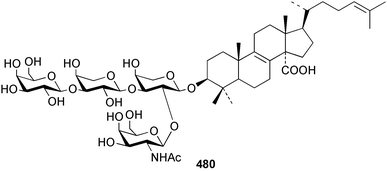
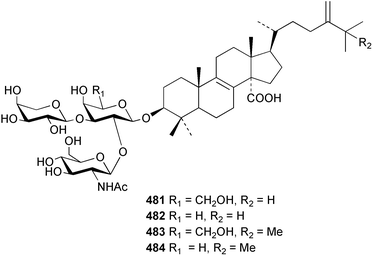
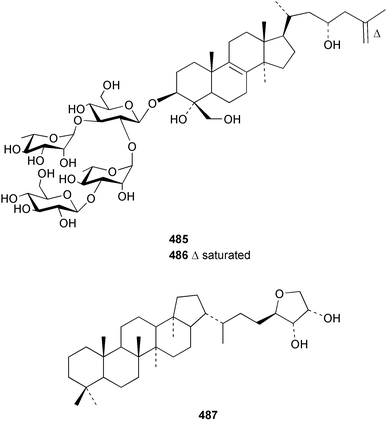
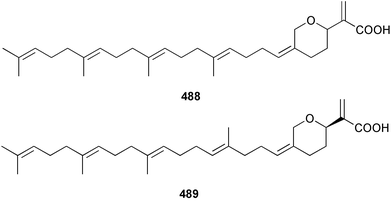
Two nortriterpenes of the isomalabaricane class, geoditins A 465 and B 466 were isolated from Geodia japonica from the South China Sea.321 A Japsis species collected near Tonga yielded three cytotoxic isomalabaricanes, 29-hydroxystelliferin E 467, 29-hydroxystelliferin A 468 and stelliferin G 469.331 The glycosylated triterpenoid, stelliferin riboside 470, was obtained from a Fijian collection of G. globostellifera.332 In an extensive re-investigation of two populations of the Red Sea Sponge Siphonochalina siphonella, sipholenols G 471, F 472 and H 473, sipholenone D 474, sipholenoside A 475 and B 476, siphonellinol B 477, neviotine B 478 and dahabinone A 479 were isolated.333 The relative concentrations of these and previously described triterpenoids were compared between the two populations. An N-acetyl aminoglycoside, formoside B 480, isolated from the Caribbean sponge Erylus formosus was found to be an anti-feedant against the ecologically relevant reef fish Thalassoma bifasciatum.334 The moderately cytotoxic N-acetyl aminoglycosides, erylosides G–J 481–484 were obtained from Korean specimens of Erylus nobilis.335 A Caribbean sponge, Ectyoplasia ferox, afforded the norlanostane glycosides feroxosides A 485 and B 486.336 A bacteriohopanoid, (32R,33S,34S)-32,35-anhydrobacteriohopanetetrol 487 was isolated in significant quantities (0.2% dry weight) from the Bahaman sponge Plakortis simplex.337 A compound having the structure proposed for hippospongic acid A 488, isolated from Hippospongia sp.,338 was synthesised by two independent groups.339,340 It was noted, however, that the spectral details of the synthetic product did not match the natural compound, and a revised structure for (+)-hippospongic acid A 489 was proposed and confirmed by enantioselective total synthesis.339
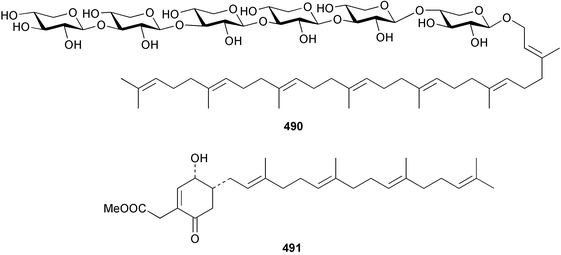
A Caribbean collection of P. simplex contained the heptaisoprenylhexasaccharide, plaxyloside 490. Isolation and structural elucidation were performed on the peracetate.341
7 Coelenterates
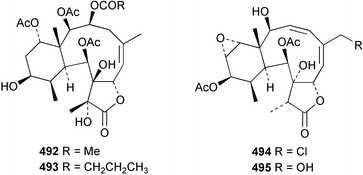
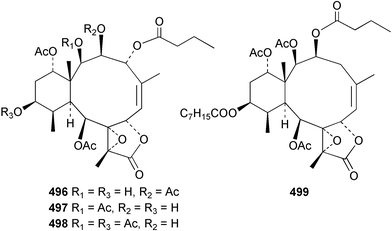
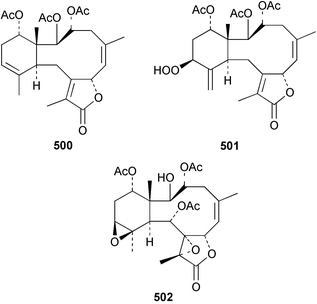
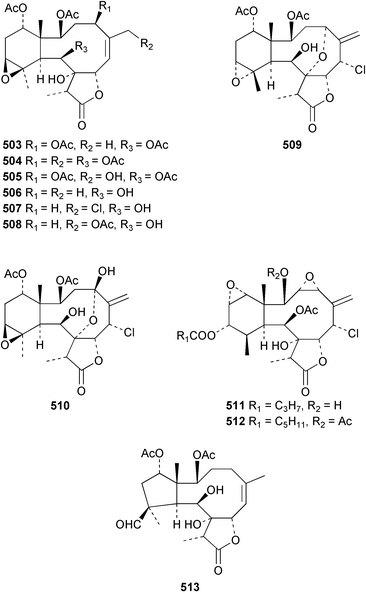
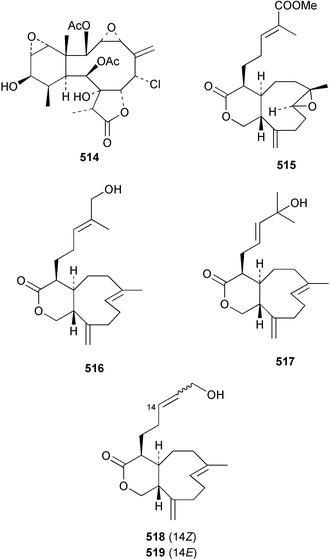
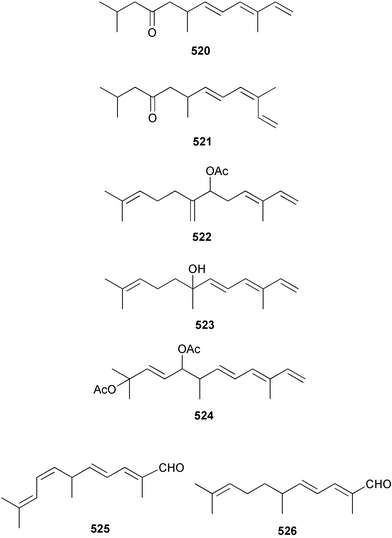
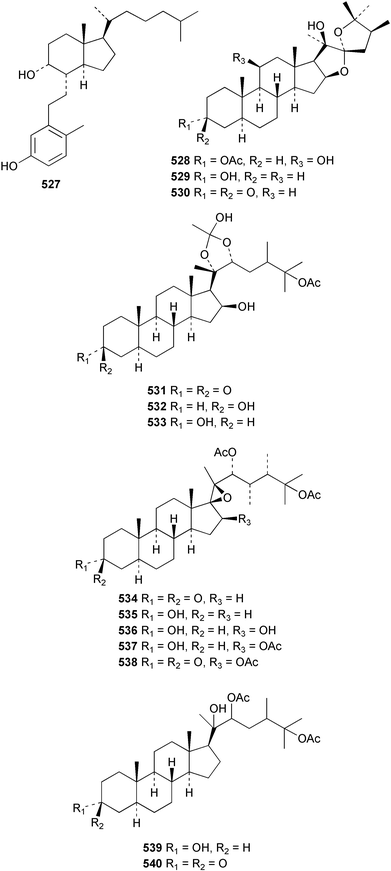
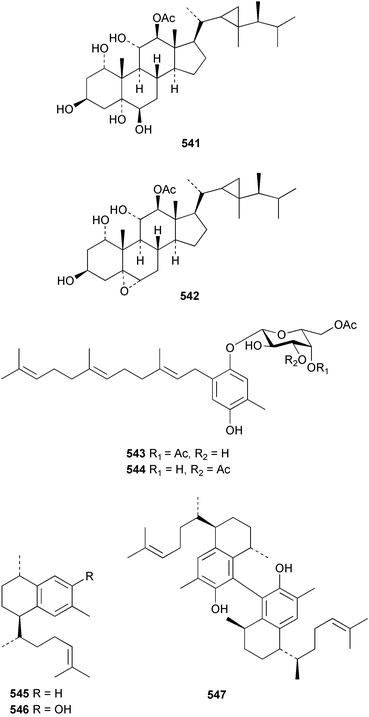
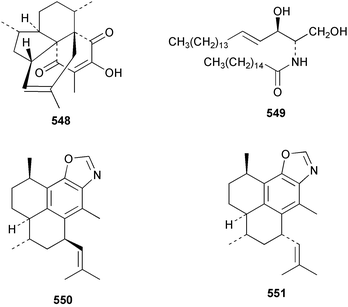
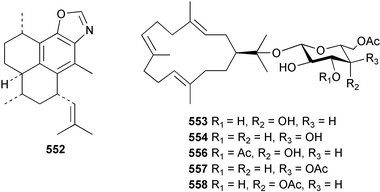
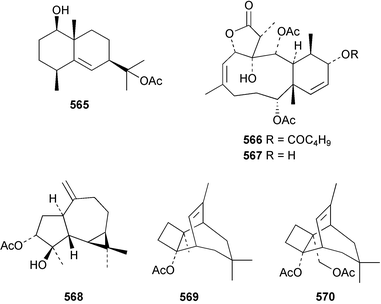
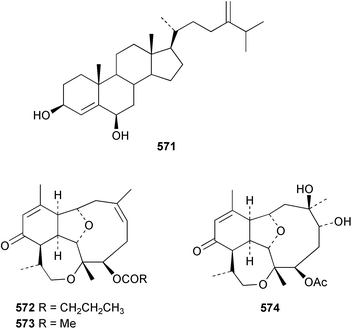
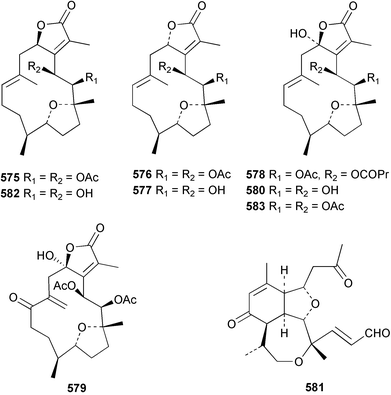
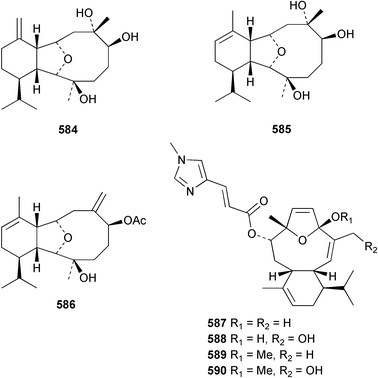
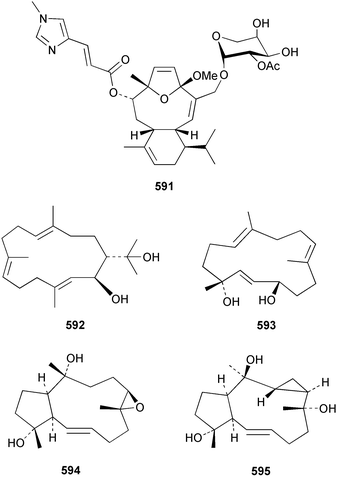
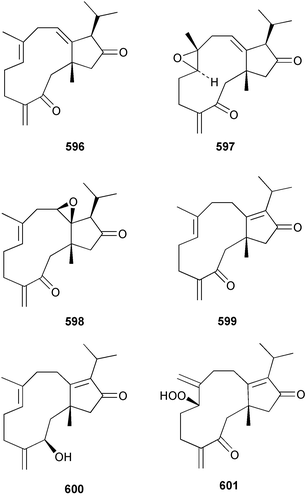
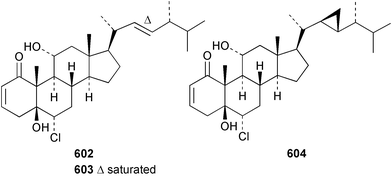
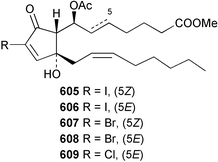
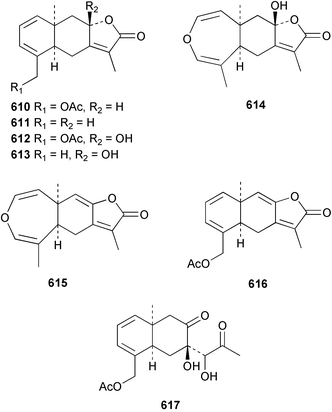
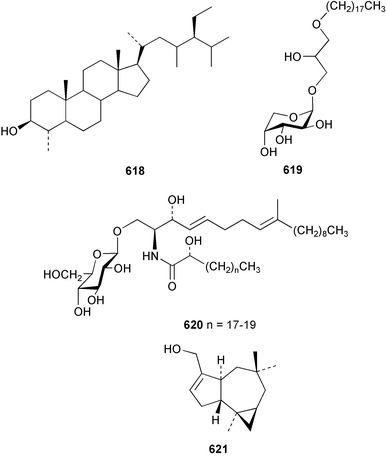
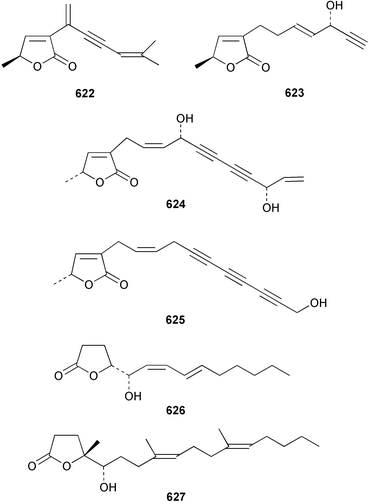
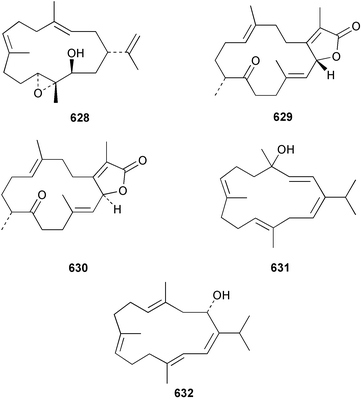
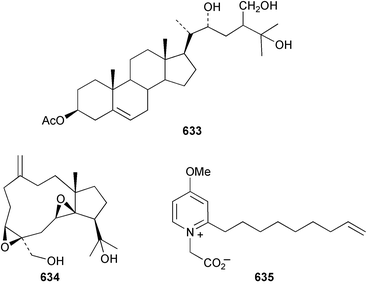
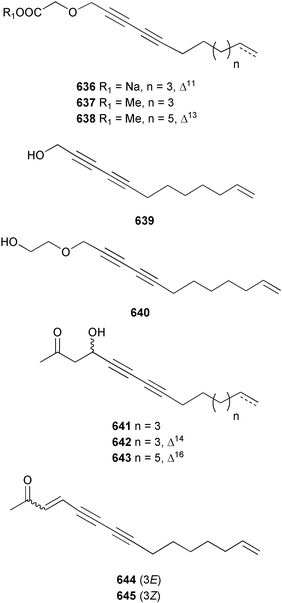
The chemistry of the coelenterates continues to be predominantly terpenoid or steroid in nature. The structure and absolute stereochemistry of eunicenone A 491, a tetraprenylated cyclohexenone metabolite isolated from the gorgonian Eunicea sp.,342 has been confirmed by total synthesis.343 In two separate accounts, eight briarane skeleton diterpenoids briaexcavatolides K–N 492–495344 and O–R 496–499345 were isolated from the gorgonian Briareum excavatum. Relative stereochemistry was secured by X-ray analysis for 492, 496 and 497. In a separate study, the same organism contained the diterpenes, briantheins A–C 500–502.346 The relative stereochemistry of 500 was established by NOESY NMR experiments and the preparation of MTPA esters helped establish the absolute stereochemistry. Brianthein A 500 reversed MDR in a human carcinoma cell line. A Western Pacific collection of the gorgonian B. stechei contained eleven briarane diterpenes, the milolides 503–513 and several known metabolites.347 A consequence of the NMR assignments of 503–513 was the revision of the relative stereochemistry of solenolide C 514,348 also isolated from the organism. New examples of xenicane diterpenes acalycixeniolides H–L 515–519, were isolated as cytotoxic components of the gorgonian Acalycigorgia inermis.349 The mildly cytotoxic acyclic sesquiterpenes and norsesquiterpenes 520–526 were isolated from the Caribbean gorgonian Plexaurella grisea.350 The structure and absolute stereochemistry of (−)-astrogorgiadiol 527, isolated from the gorgonian Astrogorgia sp.,351 has been secured by total synthesis.352 Thirteen polyoxygenated sterols 528–540 were isolated from an Indonesian collection of the gorgonian Isis hippuris.353 Of the compounds tested, spiroketal-containing sterol 528 exhibited the most potent cytotoxicity. A Taiwanese collection of I. hippuris contained two polyhydroxylated sterols isihippurols A 541 and B 542, as well as nine known sterols.354 The gorgonian Euplexaura anastomosans contained two farnesylhydroquinone derivatives euplexides F 543 and G 544.355 Both metabolites exhibited mild cytotoxicity and inhibited PLA2. Two antimycobacterial diterpenes and a bisditerpenoid 545–547 were isolated from the sea whip Pseudopterogorgia elisabethae.356 A racemic synthesis of colombiasin A 548, also isolated from P. elisabethae,357 has been reported.358 An Indian Ocean collection of the gorgonian Pseudopterogorgia sp. contained the antibacterial ceramide derivative 549.359 The structure reported for the antimycobacterial diterpene pseudopteroxazole 550360 and its C-1 diastereomer 551 have been synthesised but neither have the same spectroscopic data as the natural product.361 The revised structure of pseudopteroxazole was proposed to be 552. Examination of the Caribbean gorgonian Eunicea sp.362 afforded two cembrane glycosides, calyculaglycosides D 553 and E 554, and the (+)-antipode of the known cembrane nephthenol 555.363 Chemical conversions and closer comparison with the spectroscopic data observed for 553 and 554 required revision of the previously published structures of calyculaglycosides A–C364 to 556–558. The Caribbean gorgonian Plexaurella grisea contained six sterols 559–564 and several known compounds.365 Many of the sterols exhibited in vitro antitumour activity towards the HT-29 cell-line. A mildly cytotoxic sesquiterpene, junceol A 565, was isolated from the sea pen Virgularia juncea.366 Briarane skeleton terpenoids, cavernulin A 566 and B 567 were isolated from a Cavernularia sp.367 A metabolite containing an aromadendrane-type skeleton, 3-acetoxyspathulenol 568, was isolated from the soft coral Parerythropodium fulvum.368 Paesslerins A 569 and B 570, the first examples of metabolites containing the 2,8,8,10-tetramethyltricyclo[4.3.2.02,5]undecane (paesslerane) skeleton, were isolated from a South Georgia Island collection of the pink soft coral Alcyonium paessleri.369 The structure of the cytotoxic sterol 571, isolated from A. patagonicum,370 has been confirmed by total synthesis.371 The diterpenes pachyclavulariaenones A–C 572–574 were isolated from the soft coral Pachyclavularia violacea.372 The structure and relative stereochemistry of 574 was confirmed by X-ray analysis. Seven diterpenes, pachyclavulariolides G–L 575–580, and secopachyclavulariaenone A 581, and the two known analogues pachyclavulariolide 582373 and pachyclavulariolide E 583374 were also reported from P. violacea.375 The structures of 575, 576, 582 and 583 were secured by X-ray analysis. Several accounts of the total synthesis of the revised structure of sclerophytin A 584,376,377 cladiell-11-ene-3,6,7-triol 585378 and 6-acetoxycladiell-7(16),11-dien-3-ol 586379 have been reported.380–382 Two diterpenes caribaeorane 587 and 15-hydroxycaribaeorane 588 were isolated from the soft coral Erythropodium caribaeorum as their C-4 methylketals 589 and 590.383 The observation of facile ketal formation suggests that the C-4 methylketal contained in the antimitotic drug lead eleutherobin 591384 is an artifact of isolation. In addition to three known diterpenes that included 2-hydroxynephthenol 592,385 three norditerpenes, chabrolols A–C 593–595 were isolated from the soft coral Nephthea chabroli.386 X-Ray analysis of 592–595 secured the respective relative stereochemistries. Six dolabellane diterpenes 596–601 have been isolated as the cytotoxic components of the Formosan soft coral Clavularia inflata.387 An Okinawan collection of C. viridis contained three chlorinated steroids, yonarasterols G, H, and I 602–604.388 The absolute stereochemistry of 603 was equated with the stereochemically-defined analogue stoloniferone-c389 by chemical conversion. In a separate study, five halogenated prostanoids 605–609 were obtained from C. viridis.390 Absolute stereochemistries were assigned based upon chemical conversion and the modified Mosher's method for 605 and by subsequent comparison of CD data for 606–609. Eight sesquiterpenes, tubipolides A–G 610–616 and tubiporone 617 were isolated as mildly cytotoxic components of the Formosan stolonifer Tubipora musica.391 Specimens of the soft coral Cladiella sp. collected from Andaman and Nicobar Islands contained the sterol 618 and glycolipid 619,392 while an Andaman Island collection of the same genus contained a mixture of cerebroside homologues 620.393 The soft coral Sinularia dissecta yielded the sesquiterpene 621.394 Absolute stereochemistry of 621 was deduced from interpretation of CD data and by preparation of MTPA esters. The soft corals Sarcophyton trocheliophorum and Lithophyton arboreum, collected in the Red Sea, contained six novel fatty acid derivatives 622–627.395 The structure and absolute stereochemistry of (−)-13-hydroxy-11,12-epoxyneocembrene 628, also isolated from S. trocheliophorum,396 has been secured by total synthesis.397 The soft coral S. molle contained the known diterpene lactone sarcophinone 629398 and the new diastereomer isosarcophinone 630.399 Both compounds exhibited in vitro antitumour activity. Two cembrene alcohols, one new, acutanol 631 and one known, sarcophytol A 632,400 have been reported from the soft coral S. acutangulum.401 The absolute configuration of 632 was confirmed by the preparation of the NMA ester. A polyhydroxylated sterol sardisterol 633 was isolated from the soft coral S. digitatum.402 The structure and absolute configuration of stolonidiol 634, isolated from the soft coral Clavularia sp.,403 was established by total synthesis.404 Pyridinium alkaloids are predominantly the preserve of sponges. However, a new pyridinium alkaloid, montipyridine 635, has been reported from the stony coral Montipora sp.405 Also isolated from the same genus were a range of cytotoxic diacetylenes 636–645.406 Absence of optical activity for 641–643 and the formation of diastereomeric mixtures of MTPA esters suggested that 641–643 were isolated as racemic mixtures. A crystal structure to 1.9 Å resolution was reported for the pore-forming toxin equinatoxin II (EqtII) purified from the sea anemone Actinia equina.407 Two polypeptide toxins, RSAP I and II, of molecular masses 5008 and 4992 Da respectively, have been purified from the sea anemone Actinia ceri.4088 Bryozoans
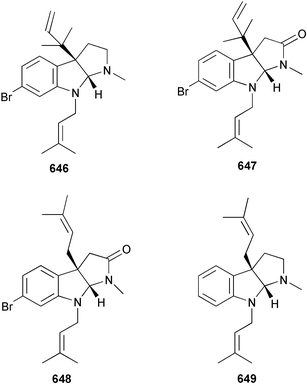
Despite their history of yielding novel natural products, there continues to be very little new work reported on bryozoan metabolites, although some syntheses have been carried out. Total syntheses of the Flustra foliacea metabolites flustramine A 646409 and flustramides A 647410 and B 648411 have been accomplished412 and the first synthesis of (−)-debromoflustramine B 649, also from F. foliacea,413 has been achieved.4149 Molluscs
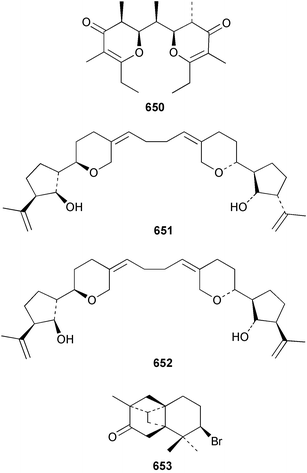
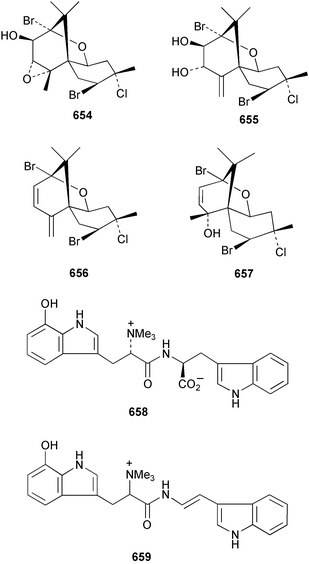
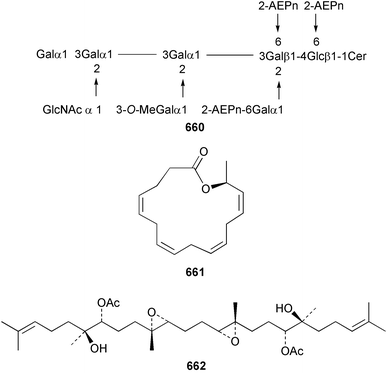
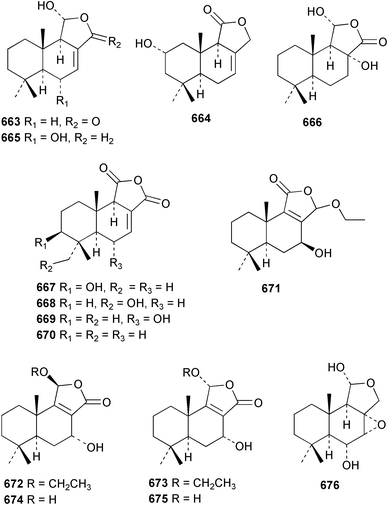
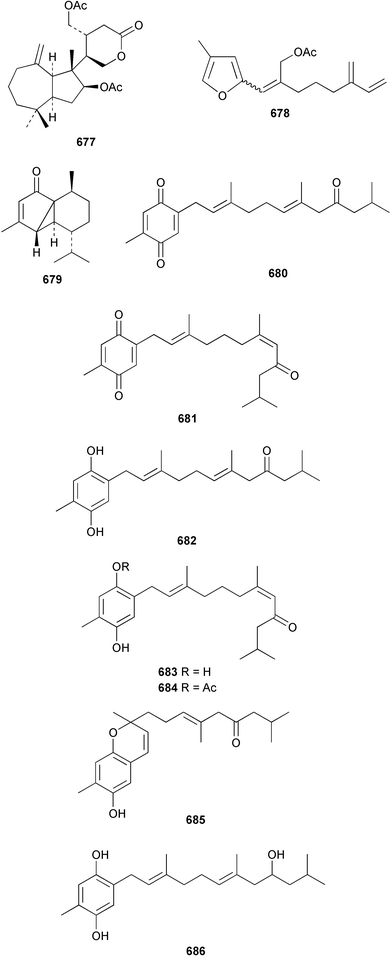
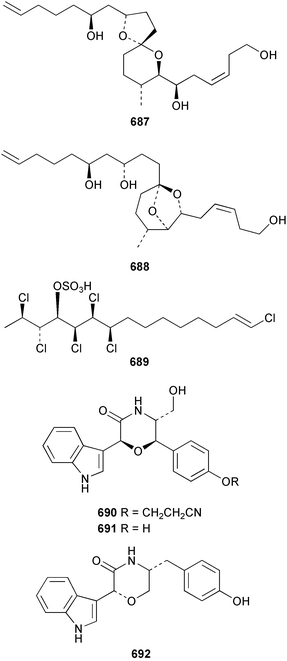
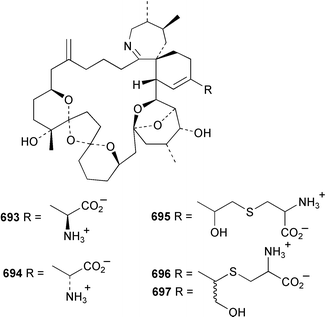
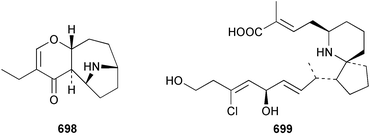
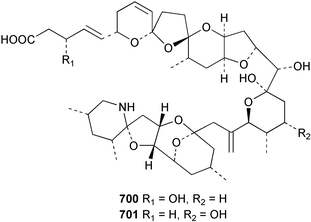
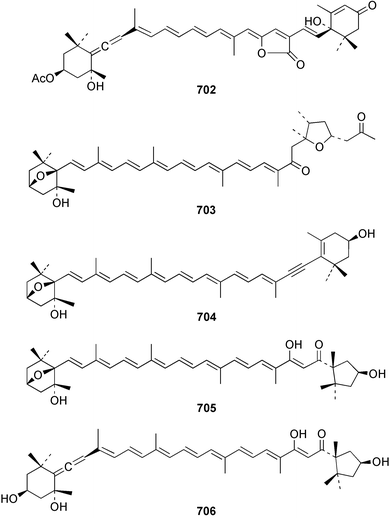
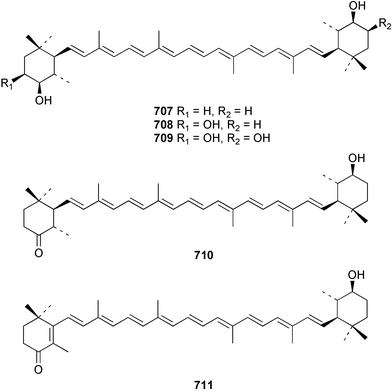
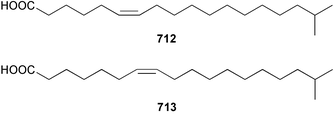
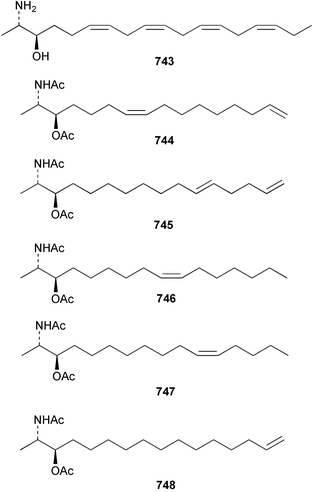
The relative and absolute stereochemistry of (−)-membrenone C 650, isolated from Pleurobranchus membranaceus,415 has been secured by synthesis.416 Several accounts of stereoselective syntheses of (+)-testudinariols A 651 and B 652, ichthyotoxic metabolites from P. testudinarius,417 have been reported.418–420 Aplydactone 653, a structurally unprecedented sesquiterpene was isolated from the sea hare Aplysia dactylomela.421 The absolute stereochemistry of the metabolite was established by X-ray analysis. The complete 1H and 13C NMR assignments of the A. dactylomela metabolites johnstonol 654, pacifenediol 655, pacifidiene 656, and pacifenol 657 have been reported.422 Two tryptophan-derived dipeptides 658 and 659 were isolated from a New Zealand collection of the sea hare A. dactylomela.423 The absolute stereochemistry of 658 was determined by synthesis of deoxo-diastereomers and comparison of CD spectra. A novel glycosphingolipid, EGL-II 660 has been isolated from eggs of the sea hare A. kurodai.424 The stereochemistry of (−)-aplyolide A 661, an ichthyotoxic metabolite from A. depilans,425 has been confirmed by synthesis.426 A Japanese collection of the sea hare Dolabella auricularia contained auriculol 662, a novel cytotoxic squalene metabolite.427 The structure and absolute stereochemistry was confirmed by synthesis. Fourteen drimane sesquiterpenes, dendocarbins A–N 663–676 were isolated from the nudibranch Dendrodoris carbunculosa.428 The ethoxy groups present in 671, 672 and 673 suggest that these compounds are probably artifacts of the use of ethanol to extract the organism. Compound 672 exhibited cytotoxicity towards MDR tumour cell lines. The relative and absolute stereochemistry of (+)-shahamin K 677, which was isolated from Chromodoris gleniei,429 has been confirmed by synthesis.430 Nine metabolites 678–686 were identified in chemical studies of the South African nudibranch Leminda millecra.431 GC-MS analysis of potential octacoral prey species identified two likely dietary sources for some of the metabolites, including 679. Attenols A 687 and B 688, cytotoxic spiro-acetals isolated from the Chinese bivalve Pinna attenuata,432 have been synthesised enantioselectively.433 A novel shellfish-derived chlorosulfolipid toxin 689 was isolated from Mytilus galloprovincialis.434 The absolute stereochemistry of 689 was determined by a combination of molecular modelling and Mosher's methodology. From the digestive glands of the same species were isolated three bioactive alkaloids, oxazinins 1–3 690–692.435 The absolute stereochemistry of oxazinin-1 690 was subsequently secured by derivatisation and NMR analysis.436 Pinnatoxins B 693 and C 694, isolated from the Okinawan bivalve Pinna muricata, are the most active members of the pinnatoxin family of marine toxins.437 The LD99 of the isolated 1 : 1 mixture of 693 and 694 was 22 µg kg−1. By chemical transformations, the absolute stereochemistries of 693 and 694 were equated to the synthetically defined absolute stereochemistry of pinnatoxin A.438 The structurally related pteriatoxins A–C 695–697 were isolated from the bivalve Pteria penguin.439 Based upon spectroscopic similarities, the absolute stereochemistry of the polyether macrocyclic core of the toxins was proposed to be the same as that observed for the pinnatoxins. The absolute stereochemistry of pinnamine 698, a toxin isolated from Pinna muricata,440 has been confirmed by synthesis.441 The absolute stereochemistry of pinnaic acid 699, a toxin also isolated from P. muricata,442 has been secured by a combination of synthetic and degradative studies.443 Two new members of the AZP (azaspiracid poisoning) inducing class of toxins 700 and 701 were reported from a collection of the mussel Mytilus edulis.444 The absolute configuration at C-3 was secured by degradation of 701 and comparison with synthesised fragments. Five carotenoids 702–706 have been reported from the oyster Crassostrea gigas445 and carotenoids 707–711 were also reported from the spindle shell Fusinus perplexus.446 Two novel fatty acids 712 and 713 were isolated from the siphonarid limpet Siphonaria denticulata. The structures were confirmed by synthesis.44710 Tunicates (ascidians)
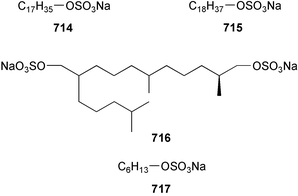
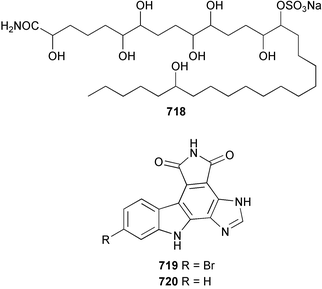
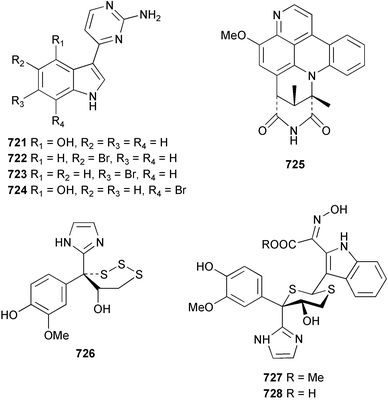
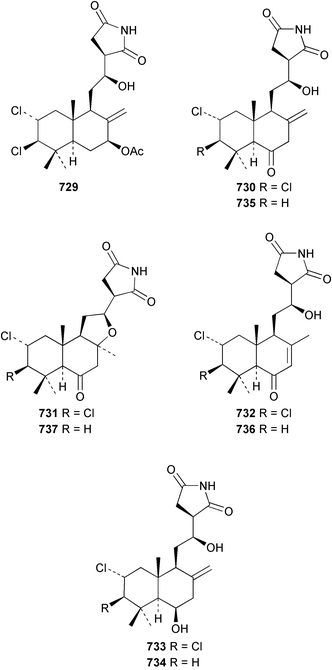
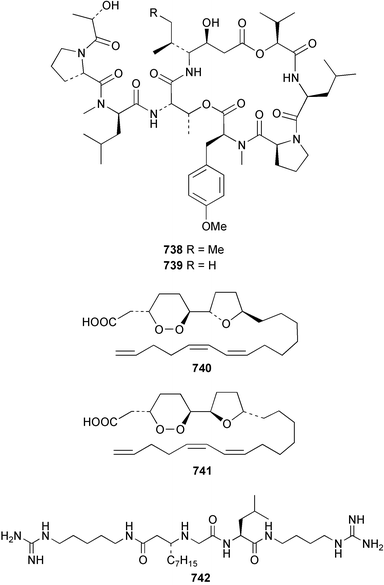
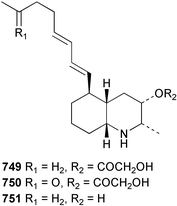
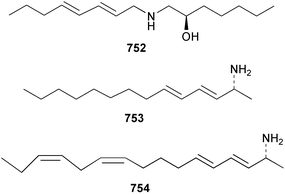
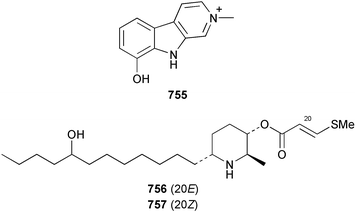
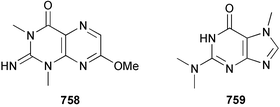
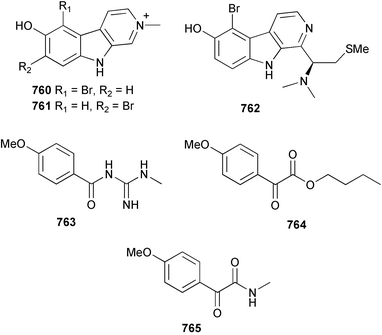
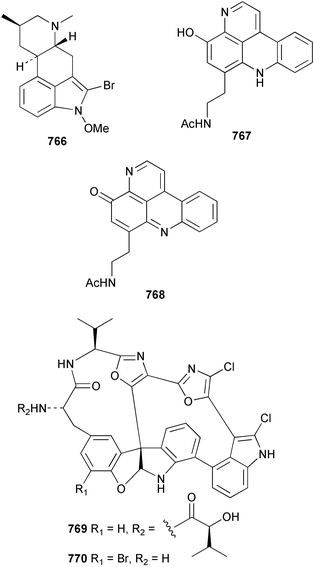
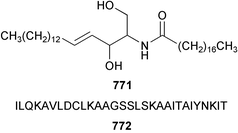
The chemistry of ascidians continues to be dominated by amino acid-derived metabolites, although there are a growing number of examples based upon terpene and alkyl biosynthesis. A collection of the Mediterranean tunicate Sidnyum turbinatum afforded a range of antiproliferative alkyl sulfates 714–717448 while subsequent further examination of the same species afforded turbinamide 718, a novel cytotoxic sulfated polyhydroxylated amide.449 Continued investigation of the Brazilian ascidian Didemnum granulatum has led to the isolation of the novel alkaloid 6-bromogranulatimide 719, and the known analogue granulatimide 720, confirming the latter as a natural product.450 Meridianins A 721 and C–E 722–724, originally isolated from Aplidium meridianum,451 have been synthesised in good yield from N-tosylacetylindoles.452 Segoline C 725, the enantiomer of previously reported segoline B,453 has been reported from an Indian Ocean collection of Eudistoma bituminis.454 Differences observed in the 13C NMR data for segolines C and B suggests some discrepancies with the earlier assignments of segoline B resonances. The (−)-enantiomer of 1,2,3-trithiane 726, previously isolated as the (+)-enantiomer from Aplidium sp.455 has been reported from the New Zealand ascidian Hypsistozoa fasmeriana.456 The structurally related alkaloids fasmerianamine A 727 and B 728 were also reported from the ascidian. In two separate accounts, nine mono- and di-chlorinated diterpenoids, the haterumaimides A–I 729–737 were isolated as cytotoxic components from Okinawan collections of Lissoclinum sp.457,458 The absolute stereochemistries of 729–732 and 734, 736 and 737 were determined by chemical modification and application of the modified Mosher's method, while relative stereochemistries were determined for 733 and 735 only. A full account of the synthesis of tamandarin A 738 and B 739, cytotoxic cyclic depsipeptides isolated from a Brazilian collection of an unidentified didemnid ascidian,459 and related compounds, has been reported.460 The methyl ester derivatives of previously reported endoperoxide stolonoxides A 740 and C 741, isolated from Stolonica socialis461,462 have been identified as potent inhibitors of the mitochondrial respiratory chain.463 The absolute stereochemistry of minalemine A 742, originally isolated from Didemnum rodriguesi,464 has been defined by stereospecific synthesis.465 The ascidian Pseudodistoma obscurum collected in Cadiz, Spain, has afforded the unsaturated amino alcohols 743–748 of which 744–748 were characterised as the diacetate derivatives.466 The absolute configuration of 743 was determined as (2S,3R) by Mosher's method. The first syntheses of (−)-lepadins A 749 and C 750, and a new synthesis of (−)-lepadin B 751 have been reported,467 confirming the relative and establishing the absolute configuration of the alkaloids first reported from Clavelina lepadiformis.468,469 A South African collection of Pseudodistoma sp. yielded four alkaloids, comprising three aliphatic amines 752–754 and a β-carboline alkaloid 755.470 Mosher's methodology was used to assign the absolute configuration of 752. Two monocyclic piperidine alkaloids, uoamines A 756 and B 757 have been reported from the ascidian Aplidium uouo.471 The structure of a modified pterin 758 isolated from a Fijian Eudistoma species of ascidian was secured using 1H–15N NMR spectroscopic techniques,472 while the trimethylguanine derivative 759 was reported from the New Zealand ascidian Lissoclinum notti.473 Three members of the eudistomin-family of alkaloids 760–762 were reported from the ascidian Eudistoma gilboverde collected in Palau.474 An Australian collection of Polycarpa aurata contained three simple p-methoxybenzoyl derivatives 763–765.475 The first example of a marine alkaloid bearing the rare N-O-methylindole functionality, pibocin B 766 was isolated from a Eudistoma sp. ascidian collected in the Sea of Japan.476 A simple biomimetic synthesis of the pyridoacridine alkaloid styelsamine B 767, isolated from an Indonesian collection of Eusynstyela latericius,477 has been reported.478 Mild oxidation of styelsamine B yielded cystodytin J 768.479 In two elegant accounts, total synthesis has dictated that the structures originally proposed for (−)-diazonamide A and B480 be revised to 769481 and 770482 respectively. A ceramide derivative 771 was isolated from a Dayawan Bay collection of Styela canopus.483 An antimicrobial 6.2 kDa peptide, dicynthaurin 772 which is comprised of two 30-residue monomers, has been reported from hemocytes of the solitary ascidian Halocynthia aurantium.484 Investigation of the hemocytes of S. clava has afforded the clavanins and styelins, high molecular weight α-helical antimicrobial peptides.48511 Echinoderms
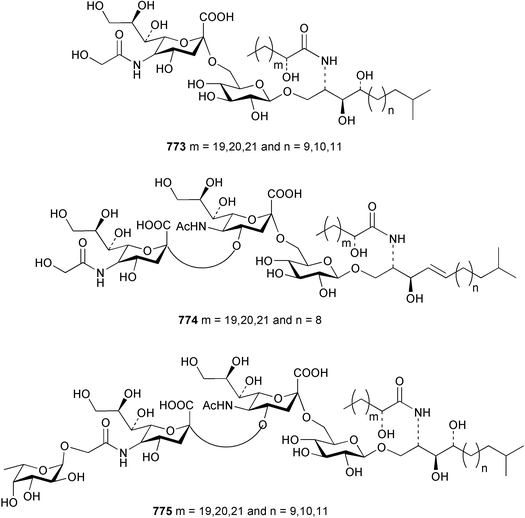
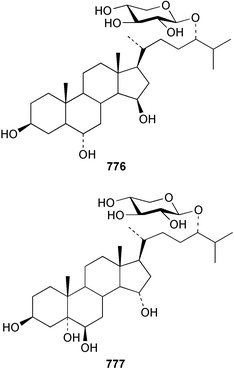
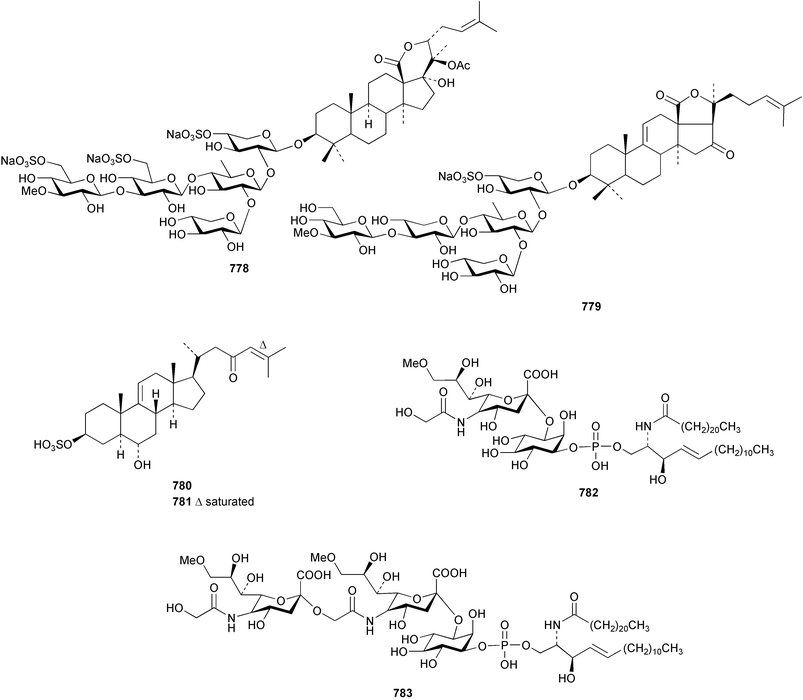
The sea cucumber Holothuria leucospilota has afforded three ganglioside molecular species 773–775. The structures of the ceramide portion of all three species are heterogeneous mixtures of alkyl homologues.486 Studies of the star fish Asterias rathbuni collected in the Bering Sea resulted in the isolation of two novel steroidal glycosides 776 and 777.487 Two saponins, frondoside F 778 and E2779 were isolated from a collection of the sea cucumber Cucumaria frondosa.488 Two new steroids 780 and 781 have been reported from the Pacific starfish Lysastrosoma anthosticta.489 Novel gangliosides CJP2 782 and CJP3 783 were isolated from the feather star Comanthus japonica.490 The starfish Aphelasterias japonica yielded four sulfated steroids, including the new example 784.491 Two sulfated triterpene glycosides with anti-HSV (Herpes simplex virus) activity 785 and 786 were reported from the Antarctic sea cucumber Staurocucumis liouvillei.492 Four novel nonmethylene-interrupted polyunsaturated fatty acid derivatives 787–790 were identified in extracts of the brittle star Ophiura sarsi.493 Hedathiosulfonic acids A 791 and B 792 were isolated as acute toxicity-exhibiting constituents of the deep-sea urchin Echinocardium cordatum.494 A triterpene glycoside patagonicoside A 793, bearing a novel aglycon moiety, was reported from the sea cucumber Psolus patagonicus.495 The glycoside exhibited potent antifungal activity towards the pathogen Cladosporium cucumerinum.12 Conclusions
Over the previous 30 years there has been a marked increase in the number of marine natural products reported annually. During the period 1996–2000 there was, however, a decrease in the number of new compounds reported and the numbers for 2001 are following the same trend (Fig. 1). These numbers suggest we are observing a lessening in the rate of discovery of new compounds from the marine environment.496,497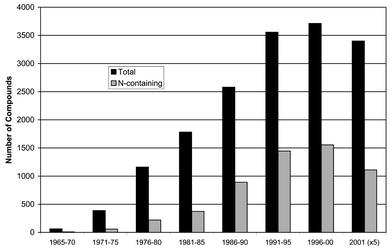 | ||
| Fig. 1 Marine Natural Products, 1965 onwards. | ||
Also depicted graphically in Fig. 1 is the rate of discovery of N-containing compounds over the same period.496 The relative dearth of N-containing compounds reported earlier has been well compensated for in recent years, which is perhaps a reflection of the greater emphasis that is now placed on finding bioactive compounds. Of course, bioactivity could also be correlated with other possible structural indicators such as polyethers.
The breakdown of discoveries by phylum for 2001 is shown graphically in Fig. 2. Sponges continue to dominate as a source of new compounds followed by coelenterates and the grouping of microorganisms and phytoplankton.
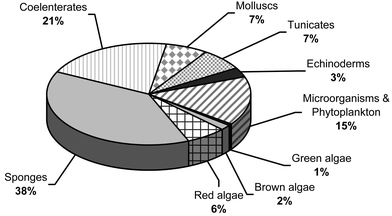 | ||
| Fig. 2 Distribution of Marine Natural Products by Phylum, 2001. | ||
Acknowledgements
We thank Eleanor Becker, Liesl Marsh, Kathryn Stillwell and Ekkehard Unger for assistance in collecting data for the preparation of this review.References
- D. J. Faulkner, Nat. Prod. Rep., 1984, 1, 251 RSC.
- D. J. Faulkner, Tetrahedron, 1977, 33, 1421 CrossRef CAS.
- A. B. Da Rocha, R. M. Lopes and G. Schwartsmann, Curr. Opin. Pharmacol., 2001, 1, 364 CrossRef CAS.
- R. X. Tan and W. X. Zou, Nat. Prod. Rep., 2001, 18, 448 RSC.
- S. Hibino and T. Choshi, Nat. Prod. Rep., 2001, 18, 66 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2001, 18, 88 RSC.
- B. R. O'Keefe, J. Nat. Prod., 2001, 64, 1373 CrossRef CAS.
- S. S. Yang, G. M. Cragg, D. J. Newman and J. P. Bader, J. Nat. Prod., 2001, 64, 265 CrossRef CAS.
- A. M. Burja, B. Banaigs, E. Abou-Mansour, J. Grant Burgess and P. C. Wright, Tetrahedron, 2001, 57, 9347 CrossRef CAS.
- J. Patocka, Acta Med., 2001, 44, 69 Search PubMed.
- W. H. Gerwick, L. T. Tan and N. Sitachitta, Alkaloids Chem. Biol. Perspect., 2001, 57, 75 Search PubMed.
- Y. Geng, X. Zhang and X. Zhao, Tianran Chanwu Yanjiu Yu Kaifa, 2001, 13, 73 Search PubMed.
- T. K. Chakraborty and S. Das, Curr. Med. Chem.: Anti-Cancer Agents, 2001, 1, 131 Search PubMed.
- K. Miyazawa and T. Noguchi, J. Toxicol. Toxin Rev., 2001, 20, 11 Search PubMed.
- L. Mos, Environ. Toxicol. Pharmacol., 2001, 9, 79 CrossRef CAS.
- V. Burgess and G. Shaw, Environ. Int., 2001, 27, 275 CrossRef CAS.
- Y. Wang, S. Yan, J. Su, L. Zeng and H. Li, Youji Huaxue, 2001, 21, 16 Search PubMed.
- T. Matsuno, Fish. Sci., 2001, 67, 771 Search PubMed.
- U. Pindur and T. Lemster, Curr. Med. Chem., 2001, 8, 1681 Search PubMed.
- C. Jiménez, Stud. Nat. Prod. Chem., 2001, 25, 811 Search PubMed.
- C. D. Amsler, K. B. Iken, J. B. McClintock and B. J. Baker, Mar. Chem. Ecol., 2001, 267 Search PubMed.
- S. H. Sennett, Mar. Chem. Ecol., 2001, 523 Search PubMed.
- G. Cimino and M. T. Ghiselin, Mar. Chem. Ecol., 2001, 115 Search PubMed.
- G. Blunden, Phytother. Res., 2001, 15, 89 CrossRef CAS.
- R. J. Capon, Eur. J. Org. Chem., 2001, 4, 633 CrossRef.
- A. M. S. Mayer and V. K. B. Lehmann, Anticancer Res., 2001, 21, 2489 Search PubMed.
- G. Schwartsmann, A. Brondani da Rocha, R. G. S. Berlinck and J. Jimeno, Lancet Oncol., 2001, 2, 221 Search PubMed.
- B. Austin, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 1 Search PubMed.
- M. Kuniyoshi and T. Higa, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 21 Search PubMed.
- F. M. Y. Faith, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 85 Search PubMed.
- Y. Venkateswarlu, N. Srinivasa Reddy and U. Venkatesham, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 101 Search PubMed.
- G. Anderluh and G. Menestrina, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 131 Search PubMed.
- R. G. Kerr, A. C. Kohl and J. M. Boehnlein, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 149 Search PubMed.
- M. R. Prinsep, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 165 Search PubMed.
- W. R. Kem, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 187 Search PubMed.
- C. de Albuquerque, A. Muhlia-Almazán, P. Hernández-Cortés and F. L. García-Carreño, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 209 Search PubMed.
- D. Rittschof and K. K. Parker, in Recent Advances in Marine Biotechnology: Volume 6 Bio-organic Compounds: Chemistry and Biomedical Applications, ed. M. Fingerman and R. Nagabhushanam, Science Publishers, Inc., New Hampshire, USA, 2001, 239 Search PubMed.
- J. Pei, J. Lei, T. Peng and J. Zhou, Jisuanji Yu Yingyong Huaxue, 2001, 18, 353 Search PubMed.
- MarinLit database, Department of Chemistry, University of Canterbury, http://www.chem.canterbury.ac.nz/research/marinlit.htm.
- J. Muldoon, A. S. Shashkov, S. N. Senchenkova, S. V. Tomshich, N. A. Komandrova, L. A. Romanenko, Y. A. Knirel and A. V. Savage, Carbohydr. Res., 2001, 330, 231 CrossRef CAS.
- V. Ivanova, M. Oriol, M.-J. Montes, A. García and J. Guinea, Z. Naturforsch. C Biosci., 2001, 56, 1 Search PubMed.
- K. W. Cho, H.-S. Lee, J.-R. Rho, T. S. Kim, S. J. Mo and J. Shin, J. Nat. Prod., 2001, 64, 664 CrossRef CAS.
- I. Gonthier, M.-N. Rager, P. Metzger, J. Guezennec and C. Largeau, Tetrahedron Lett., 2001, 42, 2795 CrossRef CAS.
- R. de Nys, N. Kumar, K. A. Sharara, S. Srinivasan, G. Ball and S. Kjelleberg, J. Nat. Prod., 2001, 64, 531 CrossRef CAS.
- K. Gustafson, M. Roman and W. Fenical, J. Am. Chem. Soc., 1989, 111, 7519 CrossRef CAS.
- T. Nagao, K. Adachi, M. Sakai, M. Nishijima and H. Sano, J. Antibiot., 2001, 54, 333 CAS.
- T. Barsby, M. T. Kelly, S. M. Gagné and R. J. Andersen, Org. Lett., 2001, 3, 437 CrossRef CAS.
- R. Fudou, T. Iizuka, S. Sato, T. Ando, N. Shimba and S. Yamanaka, J. Antibiot., 2001, 54, 153 CAS.
- R. Fudou, T. Iizuka and S. Yamanaka, J. Antibiot., 2001, 54, 149 CAS.
- E. Ohta, S. Ohta, N. K. Kubota, M. Suzuki, T. Ogawa, A. Yamasaki and S. Ikegami, Tetrahedron Lett., 2001, 42, 4179 CrossRef CAS.
- E. Ohta, N. K. Kubota, S. Ohta, M. Suzuki, T. Ogawa, A. Yamasaki and S. Ikegami, Tetrahedron, 2001, 57, 8463 CrossRef CAS.
- H. He, W.-D. Ding, V. S. Bernan, A. D. Richardson, C. M. Ireland, M. Greenstein, G. A. Ellestad and G. T. Carter, J. Am. Chem. Soc., 2001, 123, 5362 CrossRef CAS.
- F. Romero, F. Espliego, J. Perez Baz, T. Garcia de Quesada, D. Gravalos, F. de la Calle and J. L. Fernandez-Puentes, J. Antibiot., 1997, 50, 734 CAS.
- J. Perez Baz, L. M. Canedo, J. L. Fernandez Puentes and M. V. Silva Elipe, J. Antibiot., 1997, 50, 738.
- D. L. Boger, S. Ichikawa, W. C. Tse, M. P. Hedrick and Q. Jin, J. Am. Chem. Soc., 2001, 123, 561 CrossRef CAS.
- R. W. Schumacher, B. L. Harrigan and B. S. Davidson, Tetrahedron Lett., 2001, 42, 5133 CrossRef CAS.
- A. A. Smelcerovic, S. M. Dordevic and R. M. Palic, Hem. Ind., 2001, 55, 399 Search PubMed.
- S. Sperry, G. J. Samuels and P. Crews, J. Org. Chem., 1998, 63, 10011 CrossRef CAS.
- J. L. Wood, B. D. Thompson, N. Yusuff, D. A. Pflum and M. S. P. Matthäus, J. Am. Chem. Soc., 2001, 123, 2097 CrossRef CAS.
- H. Li, Y. Lin, L. Wang, S. Zhou and L. L. P. Vrijmoed, Zhongshan Daxue Xuebao, Ziran Kexueban, 2001, 40, 70 Search PubMed.
- J. Wan, Y. Lin, X. Wu, S. Zhou and L. L. P. Vrijmoed, Zhongshan Daxue Xuebao, Ziran Kexueban, 2001, 40, 127 Search PubMed.
- Y. Lin, X. Wu, S. Feng, G. Jiang, J. Luo, S. Zhou, L. L. P. Vrijmoed, E. B. G. Jones, K. Krohn, K. Steingröver and F. Zsila, J. Org. Chem., 2001, 66, 6252 CrossRef CAS.
- Y. Lin, X. Wu, S. Feng, G. Jiang, S. Zhou, L. L. P. Vrijmoed and E. B. Gareth Jones, Tetrahedron Lett., 2001, 42, 449 CrossRef CAS.
- R. Uchida, H. Tomoda, M. Arai and S. Omura, J. Antibiot., 2001, 54, 882 CAS.
- W. H. Lin, H. Z. Fu, J. Li and P. Proksch, Chin. Chem. Lett., 2001, 12, 235 CAS.
- W. H. Lin, J. Li, H. Z. Fu and P. Proksch, Chin. Chem. Lett., 2001, 12, 435 CAS.
- H. Ui, K. Shiomi, Y. Yamaguchi, R. Masuma, T. Nagamitsu, D. Takano, T. Sunazuka, M. Namikoshi and S. Omura, J. Antibiot., 2001, 54, 234 CAS.
- J.-R. Dai, B. K. Carte, P. J. Sidebottom, A. L. S. Yew, S.-B. Ng, Y. Huang and M. S. Butler, J. Nat. Prod., 2001, 64, 125 CrossRef CAS.
- M. Namikoshi, K. Akano, S. Meguro, I. Kasuga, Y. Mine, T. Takahashi and H. Kobayashi, J. Nat. Prod., 2001, 64, 396 CrossRef CAS.
- T. Yamada, M. Iritani, M. Doi, K. Minoura, T. Ito and A. Numata, J. Chem. Soc., Perkin Trans. 1, 2001, 3046 RSC.
- L. A. McDonald, D. R. Abbanat, L. R. Barbieri, V. S. Bernan, C. M. Discafani, M. Greenstein, K. Janota, J. D. Korshalla, P. Lassota, M. Tischler and G. T. Carter, Tetrahedron Lett., 1999, 40, 2489 CrossRef CAS.
- T. Wang, O. Shirota, K. Nakanishi, N. Berova, L. A. McDonald, L. R. Barbieri and G. T. Carter, Can. J. Chem., 2001, 79, 1786 CrossRef CAS.
- M. Cueto, P. R. Jensen, C. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Nat. Prod., 2001, 64, 1444 CrossRef CAS.
- M. Chinworrungsee, P. Kittakoop, M. Isaka, A. Rungrod, M. Tanticharoen and Y. Thebtaranonth, Bioorg. Med. Chem. Lett., 2001, 11, 1965 CrossRef CAS.
- A. Miljkovic, P. G. Mantle, D. J. Williams and B. Rassing, J. Nat. Prod., 2001, 64, 1251 CrossRef CAS.
- K. Komatsu, H. Shigemori and J. Kobayashi, J. Org. Chem., 2001, 66, 6189 CrossRef CAS.
- G. Brauers, R. Ebel, R. Edrada, V. Wray, A. Berg, U. Gräfe and P. Proksch, J. Nat. Prod., 2001, 64, 651 CrossRef CAS.
- R. Jadulco, P. Proksch, V. Wray, Sudarsono, A. Berg and U. Gräfe, J. Nat. Prod., 2001, 64, 527 CrossRef CAS.
- C. Iwamoto, T. Yamada, Y. Ito, K. Minoura and A. Numata, Tetrahedron, 2001, 57, 2997 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and T. H. Corbett, J. Am. Chem. Soc., 2001, 123, 5418 CrossRef CAS.
- H. Luesch, R. Pangilinan, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2001, 64, 304 CrossRef CAS.
- J. Orjala, D. G. Nagle, V. Hsu and W. H. Gerwick, J. Am. Chem. Soc., 1995, 117, 8281 CrossRef CAS.
- L. M. Nogle, T. Okino and W. H. Gerwick, J. Nat. Prod., 2001, 64, 983 CrossRef CAS.
- R. Kazlauskas, R. O. Lidgard and R. J. Wells, Tetrahedron Lett., 1977, 18, 3183 CrossRef.
- C. Charles, J. C. Braekman, D. Daloze, B. Tursch and R. Karlsson, Tetrahedron Lett., 1978, 1519 CrossRef CAS.
- J. I. Jiménez and P. J. Scheuer, J. Nat. Prod., 2001, 64, 200 CrossRef CAS.
- G. G. Harrigan, H. Luesch, W. Y. Yoshida, R. E. Moore, D. G. Nagle and V. J. Paul, J. Nat. Prod., 1999, 62, 655 CrossRef CAS.
- G. R. Pettit, Y. Kamano, C. L. Herald, C. Dufresne, R. L. Cerny, D. L. Herald, J. M. Schmidt and H. Kizu, J. Am. Chem. Soc., 1989, 111, 5015 CrossRef CAS.
- L. M. Nogle, R. T. Williamson and W. H. Gerwick, J. Nat. Prod., 2001, 64, 716 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and S. L. Mooberry, J. Nat. Prod., 2000, 63, 611 CrossRef CAS.
- F. Yokokawa, H. Sameshima and T. Shioiri, Tetrahedron Lett., 2001, 42, 4171 CrossRef CAS.
- B. W. Son, J. C. Kim and H. D. Choi, Lipids, 2001, 36, 427 Search PubMed.
- G. G. Harrigan, H. Luesch, W. Y. Yoshida, R. E. Moore, D. G. Nagle, V. J. Paul, S. L. Mooberry, T. H. Corbett and F. A. Valeriote, J. Nat. Prod., 1998, 61, 1075 CrossRef CAS.
- H. Luesch, R. E. Moore, V. J. Paul, S. L. Mooberry and T. H. Corbett, J. Nat. Prod., 2001, 64, 907 CrossRef CAS.
- G. R. Pettit, Y. Kamano, C. L. Herald, Y. Fujii, H. Kizu, M. R. Boyd, F. E. Boettner, D. L. Doubek, J. M. Schmidt, J.-C. Chapuis and C. Michel, Tetrahedron, 1993, 49, 9151 CrossRef CAS.
- M. A. Orsini, L. K. Pannell and K. L. Erickson, J. Nat. Prod., 2001, 64, 572 CrossRef CAS.
- T. Igarashi, M. Satake and T. Yasumoto, J. Am. Chem. Soc., 1996, 118, 479 CrossRef CAS.
- M. Sasaki, T. Shida and K. Tachibana, Tetrahedron Lett., 2001, 42, 5725 CrossRef CAS.
- F. Dini, G. Guella, P. Giubbilini, I. Mancini and F. Pietra, Naturwissenschaften, 1993, 80, 84 CAS.
- R. A. Aungst and R. L. Funk, J. Am. Chem. Soc., 2001, 123, 9455 CrossRef CAS.
- T. Ukena, M. Satake, M. Usami, Y. Oshima, H. Naoki, T. Fujita, Y. Kan and T. Yasumoto, Biosci. Biotechnol. Biochem., 2001, 65, 2585 CrossRef CAS.
- Y. Kan, D. Uemura, Y. Hirata, M. Ishiguro and T. Iwashita, Tetrahedron Lett., 2001, 42, 3197 CrossRef CAS.
- M. Tsuda, T. Endo and J. Kobayashi, J. Org. Chem., 2000, 65, 1349 CrossRef CAS.
- J. Kobayashi, T. Kubota, T. Endo and M. Tsuda, J. Org. Chem., 2001, 66, 134 CrossRef CAS.
- T. Kubota, T. Endo, M. Tsuda, M. Shiro and J. Kobayashi, Tetrahedron, 2001, 57, 6175 CrossRef CAS.
- J. Kobayashi, M. Ishibashi, M. R. Wälchli, H. Nakamura, Y. Hirata, T. Sasaki and Y. Ohizumi, J. Am. Chem. Soc., 1988, 110, 490 CrossRef CAS.
- T. Kubota, M. Tsuda and J. Kobayashi, Org. Lett., 2001, 3, 1363 CrossRef CAS.
- M. Ishibashi, M. Sato and J. Kobayashi, J. Org. Chem., 1993, 58, 6928 CrossRef CAS.
- D. R. Williams and K. G. Meyer, J. Am. Chem. Soc., 2001, 123, 765 CrossRef CAS.
- T. Hu, J. M. Curtis, J. A. Walter, Y. Oshima, M. A. Quilliam and J. L. C. Wright, J. Chem. Soc., Chem. Commun., 1995, 2159 RSC.
- T. Hu, I. W. Burton, A. D. Cembella, J. M. Curtis, M. A. Quilliam, J. A. Walter and J. L. C. Wright, J. Nat. Prod., 2001, 64, 308 CrossRef CAS.
- D. Uemura, T. Chou, T. Haino, A. Nagatsu, S. Fukuzawa, S. Zheng and H. Chen, J. Am. Chem. Soc., 1995, 117, 1155 CrossRef CAS.
- T. Chou, O. Kamo and D. Uemura, Tetrahedron Lett., 1996, 37, 4023 CrossRef CAS.
- T. Chou, T. Haino, M. Kuramoto and D. Uemura, Tetrahedron Lett., 1996, 37, 4027 CrossRef CAS.
- M. Falk, I. W. Burton, T. Hu, J. A. Walter and J. L. C. Wright, Tetrahedron, 2001, 57, 8659 CrossRef CAS.
- C.-K. Lu, G.-H. Lee, R. Huang and H.-N. Chou, Tetrahedron Lett., 2001, 42, 1713 CrossRef CAS.
- B. Suárez-Gómez, M. L. Souto, M. Norte and J. J. Fernández, J. Nat. Prod., 2001, 64, 1363 CrossRef CAS.
- S. T. Belt, W. G. Allard, G. Massé, J.-M. Robert and S. J. Rowland, Tetrahedron Lett., 2001, 42, 5583 CrossRef CAS.
- B. W. Son, Y. J. Cho, J. S. Choi, W. K. Lee, D.-S. Kim, H. D. Choi, J. H. Jung, K. S. Im and W. C. Choi, Nat. Prod. Lett., 2001, 15, 299 Search PubMed.
- M. T. Hamann and P. J. Scheuer, J. Am. Chem. Soc., 1993, 115, 5825 CrossRef CAS.
- A. López-Macià, J. C. Jiménez, M. Royo, E. Giralt and F. Albericio, J. Am. Chem. Soc., 2001, 123, 11398 CrossRef CAS.
- G. Goetz, W. Y. Yoshida and P. J. Scheuer, Tetrahedron, 1999, 55, 7739 CrossRef CAS.
- M. S. Ali, M. Saleem, V. U. Ahmad and S. Shameel, Z. Naturforsch. B Chem. Sci., 2001, 56, 837 CAS.
- S.-E. N. Ayyad, M. O. Slama, A. H. MoKhtar and A. F. Anter, Boll. Chim. Farm., 2001, 140, 155 Search PubMed.
- N. Takada, R. Watanabe, K. Suenaga, K. Yamada and D. Uemura, J. Nat. Prod., 2001, 64, 653 CrossRef CAS.
- X. H. Xu, X. Chen, J. H. Lu, G. M. Yao and Y. M. Li, Chin. Chem. Lett., 2001, 12, 709 CAS.
- M. Cortés, J. A. Valderrama, M. Cuellar, V. Armstrong and M. Preite, J. Nat. Prod., 2001, 64, 348 CrossRef CAS.
- G. Culioli, M. Daoudi, A. Ortalo-Magné, R. Valls and L. Piovetti, Phytochemistry, 2001, 57, 529 CrossRef CAS.
- Atta-ur-Rahman, M. I. Choudhary, S. Hayat, A. M. Khan and A. Ahmed, Chem. Pharm. Bull., 2001, 49, 105 CrossRef.
- A. K. Holmeide, l. Skattebol and M. Sydnes, J. Chem. Soc., Perkin Trans. 1, 2001, 1942 RSC.
- T. G. Halsall and I. R. Hills, J. Chem. Soc., Chem. Commun., 1971, 448 RSC.
- X. Xu, J. Lu, G. Yao, Y. Li, J. Su and L. Zeng, Tianran Chanwu Yanjiu Yu Kaifa, 2001, 13, 5 Search PubMed.
- G. Guella, I. Mancini, G. Chiasera and F. Pietra, Helv. Chim. Acta, 1992, 75, 310 CrossRef CAS.
- R. Matsumura, T. Suzuki, H. Hagiwara, T. Hoshi and M. Ando, Tetrahedron Lett., 2001, 42, 1543 CrossRef CAS.
- K. Ohta and M. Takagi, Phytochemistry, 1977, 16, 1062–3 CrossRef CAS.
- D. C. Harrowven, M. C. Lucas and P. D. Howes, Tetrahedron, 2001, 57, 791 CrossRef CAS.
- M. T. Crimmins and K. A. Emmitte, J. Am. Chem. Soc., 2001, 123, 1533 CrossRef CAS.
- M. Suzuki, M. Daitoh, C. S. Vairappan, T. Abe and M. Masuda, J. Nat. Prod., 2001, 64, 597 CrossRef CAS.
- A. Rudi and Y. Kashman, J. Nat. Prod., 1992, 55, 1408 CrossRef CAS.
- M. Kuniyoshi, M. S. Marma, T. Higa, G. Bernardinelli and C. W. Jefford, J. Nat. Prod., 2001, 64, 696 CrossRef CAS.
- M. E. Y. Francisco and K. L. Erickson, J. Nat. Prod., 2001, 64, 790 CrossRef CAS.
- D. Davyt, R. Fernandez, L. Suescun, A. W. Mombrú, J. Saldaña, L. Domínguez, J. Coll, M. T. Fujii and E. Manta, J. Nat. Prod., 2001, 64, 1552 CrossRef CAS.
- L. Suescun, A. W. Mombrú, R. A. Mariezcurrena, D. Davyt, R. Fernández and E. Manta, Acta Crystallogr. Sect. C. Cryst. Struct. Commun., 2001, 57, 286 CrossRef CAS.
- E. G. Juagdan, R. Kalidindi and P. J. Scheuer, Tetrahedron, 1997, 53, 521 CrossRef CAS.
- C. P. Manríquez, M. L. Souto, J. A. Gavín, M. Norte and J. J. Fernández, Tetrahedron, 2001, 57, 3117 CrossRef CAS.
- G. Guella, D. Skropeta, S. Breuils, I. Mancini and F. Pietra, Tetrahedron Lett., 2001, 42, 723 CrossRef CAS.
- C. S. Vairappan, M. Daitoh, M. Suzuki, T. Abe and M. Masuda, Phytochemistry, 2001, 58, 291 CrossRef CAS.
- C. S. Vairappan, M. Suzuki, T. Abe and M. Masuda, Phytochemistry, 2001, 58, 517 CrossRef CAS.
- N. Mihopoulos, C. Vagias, E. Mikros, M. Scoullos and V. Roussis, Tetrahedron Lett., 2001, 42, 3749 CrossRef CAS.
- X.-H. Xu, X. Chen, J.-H. Lu, G.-M. Yao, Y.-M. Li and L.-M. Zheng, Chin. J. Chem., 2001, 19, 702 Search PubMed.
- J.-M. Lo, W.-L. Wang, Y.-M. Chiang and C.-M. Chen, J. Chin. Chem. Soc., 2001, 48, 821 CAS.
- M. L. Ciavatta, S. Wahidulla, L. D'Souza, G. Scognamiglio and G. Cimino, Tetrahedron, 2001, 57, 617 CrossRef CAS.
- J. Darias, J. Rovirosa, A. San-Martin, A.-R. Díaz, E. Dorta and M. Cueto, J. Nat. Prod., 2001, 64, 1383 CrossRef CAS.
- T. Rezanka and V. M. Dembitsky, Phytochemistry, 2001, 57, 607 CrossRef CAS.
- S. Etahiri, V. Bultel-Poncé, C. Caux and M. Guyot, J. Nat. Prod., 2001, 64, 1024 CrossRef CAS.
- P. Ramesh, V. Ravikanth, V. L. N. Reddy, T. V. Goud and Y. Venkateswarlu, J. Chem. Res. Synop., 2001, 232 Search PubMed.
- Y. Nakao, K. Takada, S. Matsunaga and N. Fusetani, Tetrahedron, 2001, 57, 3013 CrossRef CAS.
- B. N. Zhou, M. P. Mattern, R. K. Johnson and D. G. I. Kingston, Tetrahedron, 2001, 57, 9549 CrossRef CAS.
- N. Borbone, S. De Marino, M. Iorizzi, F. Zollo, C. Debitus, A. Ianaro and B. Pisano, Eur. J. Org. Chem., 2001, 4651 CrossRef CAS.
- R. J. Clark, M. J. Garson and J. N. A. Hooper, J. Nat. Prod., 2001, 64, 1568 CrossRef CAS.
- M. Seki, A. Kayo and K. Mori, Tetrahedron Lett., 2001, 42, 2357 CrossRef CAS.
- M. Seki and K. Mori, Eur. J. Org. Chem., 2001, 3797 CrossRef CAS.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa and A. Ianaro, J. Am. Chem. Soc., 1997, 119, 12465 CrossRef CAS.
- Y. C. Shen, C. V. S. Prakash and Y. H. Kuo, J. Nat. Prod., 2001, 64, 324 CrossRef CAS.
- N. Alam, B. H. Bae, J. Hong, C. O. Lee, B. A. Shin, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 533 CrossRef CAS.
- N. M. Carballeira and M. Pagan, J. Nat. Prod., 2001, 64, 620 CrossRef CAS.
- S. Matsunaga, S. Nishimura and N. Fusetani, J. Nat. Prod., 2001, 64, 816 CrossRef CAS.
- H. Niwa, K. Wakamatsu and K. Yamada, Tetrahedron Lett., 1989, 30, 4543 CrossRef CAS.
- Y. Baba, G. Saha, S. Nakao, C. Iwata, T. Tanaka, T. Ibuka, H. Ohishi and Y. Takemoto, J. Org. Chem., 2001, 66, 81 CrossRef CAS.
- R. J. Capon, C. Skene, E. H. Liu, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, J. Org. Chem., 2001, 66, 7765 CrossRef CAS.
- A. Fontana, G. d'Ippolito, L. D'Souza, E. Mollo, P. S. Parameswaram and G. Cimino, J. Nat. Prod., 2001, 64, 131 CrossRef CAS.
- Y. J. Lim, H. S. Park, K. S. Im, C. Lee, J. Hong, M. Lee, D. Kim and J. H. Jung, J. Nat. Prod., 2001, 64, 46 CrossRef CAS.
- Y. J. Lim, C. Lee, J. Hong, D. Kim, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 1565 CrossRef CAS.
- M. Kobayashi, T. Mahmud, H. Tajima, W. Wang, S. Aoki, S. Nakagawa, T. Mayumi and I. Kitagawa, Chem. Pharm. Bull., 1996, 44, 720 CAS.
- B. W. Gung, H. Dickson and S. Shockley, Tetrahedron Lett., 2001, 42, 4761 CrossRef CAS.
- A. Zampella, C. Giannini, C. Debitus and M. V. D'Auria, Tetrahedron, 2001, 57, 257 CrossRef CAS.
- E. Ohta, S. Ohta and S. Ikegami, Tetrahedron, 2001, 57, 4699 CrossRef CAS.
- M. Fujita, Y. Nakao, S. Matsunaga, M. Seiki, Y. Itoh, R. W. M. van Soest and N. Fusetani, Tetrahedron, 2001, 57, 1229 CrossRef CAS.
- Y. Chen, K. B. Killday, P. J. McCarthy, R. Schimoler, K. Chilson, C. Selitrennikoff, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 2001, 64, 262 CrossRef CAS.
- T. L. Perry, A. Dickerson, A. A. Khan, R. K. Kondru, D. N. Beratan, P. Wipf, M. Kelly and M. T. Hamann, Tetrahedron, 2001, 57, 1483 CrossRef CAS.
- D. E. Williams, T. M. Allen, R. B. van Soest, W. Behrish and R. J. Andersen, J. Nat. Prod., 2001, 64, 281 CrossRef CAS.
- D. J. Faulkner, R. W. Armstrong, P. Djura, M. D. Higgs, B. N. Ravi, D. B. Stierle and S. J. Wratten, Colloq. Int. CNRS, 1979, 291, 401 Search PubMed.
- N. Takada, M. Watanabe, A. Yamada, K. Suenaga, K. Yamada, K. Ueda and D. Uemura, J. Nat. Prod., 2001, 64, 356 CrossRef CAS.
- J. F. Hu, H. F. Gao, M. Kelly and M. T. Hamann, Tetrahedron, 2001, 57, 9379 CrossRef CAS.
- D. J. Gochfeld and M. T. Hamann, J. Nat. Prod., 2001, 64, 1477 CrossRef CAS.
- K. L. Erickson, J. A. Beutler, J. H. Cardellina II and M. R. Boyd, J. Org. Chem., 1997, 62, 8188 CrossRef CAS.
- K. L. Erickson, J. A. Beutler, J. H. Cardellina II and M. R. Boyd, J. Org. Chem., 2001, 66, 1532 CrossRef CAS.
- D. Labrecque, S. Charron, R. Rej, C. Blais and S. Lamothe, Tetrahedron Lett., 2001, 42, 2645 CrossRef CAS.
- B. B. Snider and F. Song, Org. Lett., 2001, 3, 1817 CrossRef CAS.
- A. Fürstner, D. Thorsten, O. R. Thiel and G. Blanda, Chem. Eur. J., 2001, 7, 5286 CrossRef CAS.
- R. Watanadilok, P. Sonchaeng, A. Kijjoa, A. M. Damas, L. Gales, A. M. S. Silva and W. Herz, J. Nat. Prod., 2001, 64, 1056 CrossRef CAS.
- D. Vuong, R. J. Capon, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, J. Nat. Prod., 2001, 64, 640 CrossRef CAS.
- Z. Thale, F. R. Kinder, K. W. Bair, J. Bontempo, A. M. Czuchta, R. W. Versace, P. E. Phillips, M. L. Sanders, S. Wattanasin and P. Crews, J. Org. Chem., 2001, 66, 1733 CrossRef CAS.
- A. Grassia, I. Bruno, C. Debitus, S. Marzocco, A. Pinto, L. Gomez-Paloma and R. Riccio, Tetrahedron, 2001, 57, 6257 CrossRef CAS.
- A. Cutignano, I. Bruno, G. Bifulco, A. Casapullo, C. Debitus, L. Gomez-Paloma and R. Riccio, Eur. J. Org. Chem., 2001, 4, 775 CrossRef.
- A. B. Smith III, I. G. Safonov and R. M. Corbett, J. Am. Chem. Soc., 2001, 123, 12426 CrossRef.
- J. Tanaka and T. Higa, Tetrahedron Lett., 1996, 37, 5535 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, Tetrahedron Lett., 2001, 42, 1623 CrossRef CAS.
- M. A. Rashid, C. L. Cantrell, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1341 CrossRef CAS.
- A. Randazzo, C. Debitus and L. Gomez-Paloma, Tetrahedron, 2001, 57, 4443 CrossRef CAS.
- J. Tabudravu, L. A. Morris, J. J. Kettenes-van den Bosch and M. Jaspars, Tetrahedron Lett., 2001, 42, 9273 CrossRef CAS.
- L. T. Tan, R. T. Williamson and W. H. Gerwick, J. Org. Chem., 2000, 65, 419 CrossRef CAS.
- F. Yokokawa, H. Sameshima and T. Shioiri, Synlett, 2001, 986 CrossRef CAS.
- M. Tsuda, H. Shigemori, Y. Mikami and J. Kobayashi, Tetrahedron, 1993, 49, 6785 CrossRef CAS.
- A. Napolitano, I. Bruno, P. Rovero, R. Lucas, M. P. Peris, L. Gomez-Paloma and R. Riccio, Tetrahedron, 2001, 57, 6249 CrossRef CAS.
- G. R. Pettit, R. Tan, Y. Ichihara, M. D. Williams, D. L. Doubek, L. P. Tackett and J. M. Schmidt, J. Nat. Prod., 1995, 58, 961 CrossRef CAS.
- G. R. Pettit, J. W. Lippert III, S. R. Taylor, R. Tan and M. D. Williams, J. Nat. Prod., 2001, 64, 883 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson, L. K. Cartner, N. Shigematsu, L. K. Pannell and M. R. Boyd, J. Nat. Prod., 2001, 64, 117 CrossRef CAS.
- A. Randazzo, G. Bifulco, C. Giannini, M. Bucci, C. Debitus, G. Cirino and L. Gomez-Paloma, J. Am. Chem. Soc., 2001, 123, 10870 CrossRef CAS.
- L. L. Guan, Y. Sera, K. Adachi, F. Nishida and Y. Shizuri, Biochem. Biophys. Res. Commun., 2001, 283, 976 CrossRef CAS.
- R. Sakai, T. Koike, M. Sasaki, K. Shimamoto, C. Oiwa, A. Yano, K. Suzuki, K. Tachibana and H. Kamiya, Org. Lett., 2001, 3, 1479 CrossRef CAS.
- R. Sakai, C. Oiwa, K. Takaishi, H. Kamiya and M. Tagawa, Tetrahedron Lett., 1999, 40, 6941 CrossRef CAS.
- B. B. Snider and Y. Gu, Org. Lett., 2001, 3, 1761 CrossRef CAS.
- N. Dumrongchai, C. Ponglimanont, B. L. Stapleton and M. J. Garson, ACGC Chem. Res. Commun., 2001, 13, 17 Search PubMed.
- B. L. Stapleton, G. M. Cameron and M. J. Garson, Tetrahedron, 2001, 57, 4603 CrossRef CAS.
- G. G. Harrigan, G. H. Goetz, H. Luesch, S. Yang and J. Likos, J. Nat. Prod., 2001, 64, 1133 CrossRef CAS.
- S. Tsukamoto, K. Tane, T. Ohta, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2001, 64, 1576 CrossRef CAS.
- G. Koren-Goldshlager, Y. Kashman and M. Schleyer, J. Nat. Prod., 1998, 61, 282 CrossRef CAS.
- M. R. Heinrich and W. Steglich, Tetrahedron Lett., 2001, 42, 3287 CrossRef CAS.
- M. G. Banwell, A. M. Bray, A. J. Edwards and D. J. Wong, New J. Chem., 2001, 25, 1347 RSC.
- M. R. Heinrich, Y. Kashman, P. Spiteller and W. Steglich, Tetrahedron, 2001, 57, 9973 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron Lett., 1996, 37, 5555 CrossRef.
- D. Nozawa, H. Takikawa and K. Mori, Bioorg. Med. Chem. Lett., 2001, 11, 1481 CrossRef CAS.
- H. Takikawa, D. Nozawa and K. Mori, J. Chem. Soc., Perkin Trans. 1, 2001, 657 RSC.
- J. Shin, Y. Seo, K. W. Cho, J. Rho and C. J. Sim, J. Nat. Prod., 1997, 60, 611 CrossRef CAS.
- N. Yamazaki, W. Dokoshi and C. Kibayashi, Org. Lett., 2001, 3, 193 CrossRef CAS.
- A. D. Patil, A. J. Freyer, P. B. Taylor, B. Carté, G. Zuber, R. K. Johnson and D. J. Faulkner, J. Org. Chem., 1997, 62, 1814 CrossRef CAS.
- J. C. Braekman, D. Daloze, R. Tavares, E. Hajdu and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 193 CrossRef CAS.
- F. Cohen and L. E. Overman, J. Am. Chem. Soc., 2001, 123, 10782 CrossRef CAS.
- R. J. Capon, M. Miller and F. Rooney, J. Nat. Prod., 2001, 64, 643 CrossRef CAS.
- T. Hattori, S. Matsuo, K. Adachi and Y. Shizuri, Fish. Sci., 2001, 67, 690 Search PubMed.
- J. Shin, J. R. Rho, Y. Seo, H. S. Lee, K. W. Cho, H. J. Kwon and C. J. Sim, Tetrahedron Lett., 2001, 42, 1965 CrossRef CAS.
- S. Carmely, M. Ilan and Y. Kashman, Tetrahedron, 1989, 45, 2193 CrossRef CAS.
- I. Kawasaki, S. Nakamura, S. Yanagitani, A. Kakumo, M. Yamashita and S. Ohta, J. Chem. Soc., Perkin Trans. 1, 2001, 3095 RSC.
- S. Aoki, Y. Ye, K. Higuchi, A. Takashima, Y. Tanaka, I. Kitagawa and M. Kobayashi, Chem. Pharm. Bull., 2001, 49, 1372 CrossRef CAS.
- S. C. Bobzin and D. J. Faulkner, J. Org. Chem., 1991, 56, 4403 CrossRef CAS.
- N. J. Lawrence and S. M. Bushell, Tetrahedron Lett., 2001, 42, 7671 CrossRef CAS.
- S. P. Gunasekera, P. J. McCarthy and M. Kelly-Borges, J. Nat. Prod., 1994, 57, 1437 CrossRef CAS.
- B. Jiang, C. G. Yang and J. Wang, J. Org. Chem., 2001, 66, 4865 CrossRef CAS.
- M. Kobayashi, Y. J. Chen, S. Aoki, Y. In, T. Ishida and I. Kitagawa, Tetrahedron, 1995, 51, 3727 CrossRef CAS.
- B. E. A. Burm, P. Blokker, E. Jongmans, E. van Kampen, M. J. Wanner and G. J. Koomen, Heterocycles, 2001, 55, 495 CAS.
- K. A. El Sayed, M. Kelly, U. A. K. Kara, K. K. H. Ang, I. Katsuyama, D. C. Dunbar, A. A. Khan and M. T. Hamman, J. Am. Chem. Soc., 2001, 123, 1804 CrossRef CAS.
- M. Tsuda, K. Hirano, T. Kubota and J. Kobayashi, Tetrahedron Lett., 1999, 40, 4819 CrossRef CAS.
- B. B. Snider and B. Shi, Tetrahedron Lett., 2001, 42, 1639 CrossRef CAS.
- K. Hirano, T. Kubota, M. Tsuda, Y. Mikami and J. Kobayashi, Chem. Pharm. Bull., 2000, 48, 974 CAS.
- N. B. Perry, L. Ettouati, M. Litaudon, J. W. Blunt and M. H. G. Munro, Tetrahedron, 1994, 50, 3987 CrossRef CAS.
- R. J. Anderson and J. C. Morris, Tetrahedron Lett., 2001, 42, 8697 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1249 CrossRef CAS.
- P. S. Parameswaran, C. G. Naik, S. Y. Kamat and B. N. Pramanik, Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem., 1998, 37, 1258.
- N. Saito, H. Sakai, K. Suwanborirux, S. Pummangura and A. Kubo, Heterocycles, 2001, 55, 21 CAS.
- G. R. Pettit, J. C. Knight, J. C. Collins, D. L. Herald, R. K. Pettit, M. R. Boyd and V. G. Young, J. Nat. Prod., 2000, 63, 793 CrossRef CAS.
- A. Casapullo, A. Cutignano, I. Bruno, G. Bifulco, C. Debitus, L. Gomez-Paloma and R. Riccio, J. Nat. Prod., 2001, 64, 1354 CrossRef CAS.
- D. Tasdemir, K. M. Marshall, G. C. Mangalindan, G. P. Concepcion, L. R. Barrows, M. K. Harper and C. M. Ireland, J. Org. Chem., 2001, 66, 3246 CrossRef CAS.
- K. C. Bascombe, S. R. Peter, W. F. Tinto, S. M. Bissada, S. McLean and W. F. Reynolds, Heterocycles, 1998, 48, 1461 CAS.
- M. Seki and K. Mori, Eur. J. Org. Chem., 2001, 3, 503 CrossRef.
- M. Tsuda, H. Uemoto and J. Kobayashi, Tetrahedron Lett., 1999, 40, 5709 CrossRef CAS.
- B. Jiang, J. F. Liu and S. Y. Zhao, Org. Lett., 2001, 3, 4011 CrossRef CAS.
- A. Rudi, Z. Stein, S. Green, I. Goldberg and Y. Kashman, Tetrahedron Lett., 1994, 35, 2589 CrossRef CAS.
- A. Radspieler and J. Liebscher, Tetrahedron, 2001, 57, 4867 CrossRef CAS.
- M. Assmann, S. Zea and M. Köck, J. Nat. Prod., 2001, 64, 1593 CrossRef CAS.
- G. H. Goetz, G. G. Harrigan and J. Likos, J. Nat. Prod., 2001, 64, 1581 CrossRef CAS.
- M. Assmann, R. W. M. van Soest and M. Köck, J. Nat. Prod., 2001, 64, 1345 CrossRef CAS.
- M. Bourguet-Kondracki, M. Martin, J. Vacelet and M. Guyot, Tetrahedron Lett., 2001, 42, 7257 CrossRef CAS.
- D. Tasdemir, G. C. Mangalidan, G. P. Concepcion, M. K. Harper and C. M. Ireland, Chem. Pharm. Bull., 2001, 49, 1628 CrossRef CAS.
- K. B. Killday, D. Yarwood, M. A. Sills, P. T. Murphy, J. N. A. Hooper and A. E. Wright, J. Nat. Prod., 2001, 64, 525 CrossRef CAS.
- J. Kobayashi, K. Honma, T. Sasaki and M. Tsuda, Chem. Pharm. Bull., 1995, 43, 403 CAS.
- T. R. Boehlow, J. J. Harburn and C. D. Spilling, J. Org. Chem., 2001, 66, 3111 CrossRef CAS.
- G. M. Nicholas, G. L. Newton, R. C. Fahey and C. A. Bewley, Org. Lett., 2001, 3, 1543 CrossRef CAS.
- N. Takada, R. Watanabe, K. Suenaga, K. Yamada, K. Ueda, M. Kita and D. Uemura, Tetrahedron Lett., 2001, 42, 5265 CrossRef CAS.
- T. Evan, A. Rudi, M. Ilan and Y. Kashman, J. Nat. Prod., 2001, 64, 226 CrossRef CAS.
- M. Tsuda, Y. Sakuma and J. Kobayashi, J. Nat. Prod., 2001, 64, 980 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso and S. Magno, Eur. J. Org. Chem., 2001, 1, 55 CrossRef.
- N. Fusetani, Y. Masuda, Y. Nakao, S. Matsunaga and R. W. M. van Soest, Tetrahedron, 2001, 57, 7507 CrossRef CAS.
- A. Kijjoa, R. Watanadilok, P. Sonchaeng, A. M. S. Silva, G. Eaton and W. Herz, Z. Naturforsch., C: Biosci., 2001, 56, 1116 Search PubMed.
- T. Hattori, A. Konno, K. Adachi and Y. Shizuri, Fish. Sci., 2001, 67, 899 Search PubMed.
- N. K. Utkina, V. A. Denisenko, O. V. Scholokova, M. V. Virovaya, A. V. Gerasimenko, D. Y. Popov, V. B. Krasokhin and A. M. Popov, J. Nat. Prod., 2001, 64, 151 CrossRef CAS.
- Y. Okamoto, M. Ojika, S. Suzuki, M. Murakami and Y. Sakagami, Bioorg. Med. Chem., 2001, 9, 179 Search PubMed.
- Y. Shen, C. Chen and Y. Kuo, J. Nat. Prod., 2001, 64, 801 CrossRef CAS.
- C. Giannini, C. Debitus, R. Lucas, A. Ubeda, M. Paya, J. N. A. Hooper and M. V. D'Auria, J. Nat. Prod., 2001, 64, 612 CrossRef CAS.
- H. Mitome, T. Nagasawa, H. Miyaoka, Y. Yamada and R. W. M. van Soest, J. Nat. Prod., 2001, 64, 1506 CrossRef CAS.
- M. L. Kondracki, D. Davoust and M. Guyot, J. Chem. Res. Synop., 1989, 3, 74 Search PubMed.
- H. Nakamura, S. Deng, J. Kobayashi, Y. Ohizumi and Y. Hirata, Tetrahedron, 1986, 42, 4197 CrossRef CAS.
- Y. Haruo, T. Hasegawa, H. Tanaka and T. Takahashi, Synlett, 2001, 1935 CrossRef CAS.
- A. D. Patil, A. J. Freyer, L. Killmer, P. Offen, B. Carte, A. J. Jurewicz and R. K. Johnson, Tetrahedron, 1997, 53, 5047 CrossRef CAS.
- M. Inoue, M. W. Carson, A. J. Frontier and S. J. Danishefsky, J. Am. Chem. Soc., 2001, 123, 1878 CrossRef CAS.
- J. Carroll, E. N. Jonsson, R. Ebel, M. S. Hartman, T. R. Holman and P. Crews, J. Org. Chem., 2001, 66, 6847 CrossRef CAS.
- M. Musman, I. I. Ohtani, D. Nagaoka, J. Tanaka and T. Higa, J. Nat. Prod., 2001, 64, 350 CrossRef CAS.
- A. Loukaci, I. Le Saout, M. Samadi, S. Leclerc, E. Damiens, L. Meijer, C. Debitus and M. Guyot, Bioorg. Med. Chem., 2001, 9, 3049 Search PubMed.
- S. Poigny, S. Nouri, A. Chiaroni, M. Guyot and M. Samadi, J. Org. Chem., 2001, 66, 7263 CrossRef CAS.
- M. R. Kernan and D. J. Faulkner, J. Org. Chem., 1988, 53, 4574 CrossRef CAS.
- G. H. Goetz, G. G. Harrigan and J. Likos, J. Nat. Prod., 2001, 64, 1486 CrossRef CAS.
- M. Musman, J. Tanaka and T. Higa, J. Nat. Prod., 2001, 64, 111 CrossRef CAS.
- S. Kehraus, G. M. König and A. D. Wright, J. Nat. Prod., 2001, 64, 939 CrossRef CAS.
- G. M. König and A. D. Wright, J. Org. Chem., 1997, 62, 3837 CrossRef.
- G. Mehta and K. Srinivas, Tetrahedron Lett., 2001, 42, 2855 CrossRef CAS.
- S. Fietz-Razavian, S. Schulz, I. Dix and P. G. Jones, Chem. Commun., 2001, 2154 RSC.
- D. T. A. Youssef, W. Y. Yoshida, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2001, 64, 1332 CrossRef CAS.
- K. D. Wellington, R. C. Cambie, P. R. Rutledge and P. R. Bergquist, J. Nat. Prod., 2000, 63, 79 CrossRef CAS.
- K. C. Nicolaou, D. Gray and J. Tae, Angew. Chem., Int. Ed., 2001, 40, 3679 CrossRef CAS.
- D. Tasdemir, G. P. Concepcion, G. C. Mangalindan, M. K. Harper, E. Hajdu and C. M. Ireland, Tetrahedron, 2001, 57, 5681 CrossRef CAS.
- L. M. Zeng, Z. Guan, J. Y. Su, X. L. Feng and J. W. Cai, Acta Chim. Sin. (Engl. Ed.), 2001, 59, 1675 Search PubMed.
- M. McNally and R. J. Capon, J. Nat. Prod., 2001, 64, 645 CrossRef CAS.
- S. Yan, G. Zhang, J. Su and L. Zeng, Gaodeng Xuexiao Huaxue Xuebao, 2001, 22, 949 Search PubMed.
- J. Tanaka, G. Marriott, T. Higa and T. Higa, J. Nat. Prod., 2001, 64, 1468 CrossRef CAS.
- A. D. Rodriguez and B. Vera, J. Org. Chem., 2001, 66, 6364 CrossRef CAS.
- R. J. Capon, E. L. Ghisalberti and P. R. Jefferies, Experientia, 1982, 38, 1444 Search PubMed.
- R. J. Capon, A. Jenkins, F. Rooney and E. L. Ghisalberti, J. Nat. Prod., 2001, 64, 638 CrossRef CAS.
- J. Shin, J. R. Rho, Y. Seo, H. S. Lee, K. W. Cho and C. J. Sim, Tetrahedron Lett., 2001, 42, 3005 CrossRef CAS.
- Y. Liu, B. H. Bae, N. Alam, J. Hong, C. J. Sim, C. Lee, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 1301 CrossRef CAS.
- K. A. El Sayed, M. T. Hamann, N. E. Hashish, W. T. Shier, M. Kelly and A. A. Khan, J. Nat. Prod., 2001, 64, 522 CrossRef CAS.
- R. P. Walker, J. E. Thompson and D. J. Faulkner, J. Org. Chem., 1980, 45, 4976 CrossRef CAS.
- Y. Noda, H. Hashimoto and T. Norizuki, Heterocycles, 2001, 55, 1839 CAS.
- R. D. Charan, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2001, 64, 661 CrossRef CAS.
- S. Kokubo, K. Yogi, M. J. Uddin, T. Inuzuka, K. Suenaga, K. Ueda and D. Uemura, Chem. Lett., 2001, 176 CrossRef CAS.
- M. S. Buchanan, A. Edser, G. King, J. Whitmore and R. J. Quinn, J. Nat. Prod., 2001, 64, 300 CrossRef CAS.
- L. C. Chang, S. Otero-Quintero, G. M. Nicholas and C. A. Bewley, Tetrahedron, 2001, 57, 5731 CrossRef CAS.
- W. A. Gallimore, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2001, 64, 741 CrossRef CAS.
- S. H. Xu and L. M. Zeng, Youji Huaxue, 2001, 21, 45 Search PubMed.
- T. Miyamoto, K. Kodama, Y. Aramaki, R. Higuchi and R. W. M. van Soest, Tetrahedron Lett., 2001, 42, 6349 CrossRef CAS.
- W. H. Lin, J. M. Fang and Y. S. Cheng, Phytochemistry, 1998, 48, 1391 CrossRef CAS.
- W. Zhang and C. Che, J. Nat. Prod., 2001, 64, 1489 CrossRef CAS.
- H. S. Lee, Y. Seo, J. R. Rho, J. Shin and V. J. Paul, J. Nat. Prod., 2001, 64, 1474 CrossRef CAS.
- A. Rudi, T. Yosief, S. Loya, A. Hizi, M. Schleyer and Y. Kashman, J. Nat. Prod., 2001, 64, 1451 CrossRef CAS.
- M. Fujita, Y. Nakao, S. Matsunaga, M. Seiki, Y. Itoh, R. W. M. van Soest, M. Heubes, D. J. Faulkner and N. Fusetani, Tetrahedron, 2001, 57, 3885 CrossRef CAS.
- A. Qureshi and D. J. Faulkner, Tetrahedron, 1999, 55, 8323 CrossRef CAS.
- M. L. Lerch and D. J. Faulkner, Tetrahedron, 2001, 7, 4091 CrossRef.
- S. Aoki, Y. Yoshioka, Y. Miyamoto, K. Higuchi, A. Setiawan, N. Murakami, Z. S. Chen, T. Sumizawa, S. Akiyama and M. Kobayashi, Tetrahedron Lett., 1998, 39, 6303 CrossRef CAS.
- N. Murakami, M. Sugimoto, M. Morita and M. Kobayashi, Chem. Eur. J., 2001, 7, 2663 CrossRef CAS.
- N. Shoji, A. Umeyama, K. Shin, K. Takeda, S. Arihara, J. Kobayashi and M. Takei, J. Org. Chem., 1992, 57, 2996 CrossRef CAS.
- M. E. Jung and T. W. Johnson, Tetrahedron, 2001, 57, 1449 CrossRef CAS.
- K. M. Meragelman, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2001, 64, 389 CrossRef CAS.
- J. N. Tabudravu and M. Jaspars, J. Nat. Prod., 2001, 64, 813 CrossRef CAS.
- Y. Kashman, T. Yosief and S. Carmeli, J. Nat. Prod., 2001, 64, 175 CrossRef CAS.
- J. Kubanek, W. Fenical and J. R. Pawlik, Nat. Prod. Lett., 2001, 15, 275 Search PubMed.
- J. Shin, H. S. Lee, L. Woo, J. R. Rho, Y. Seo, K. W. Cho and C. J. Sim, J. Nat. Prod., 2001, 64, 767 CrossRef CAS.
- C. Campagnuolo, E. Fattorusso and O. Taglialatela-Scafati, Tetrahedron, 2001, 57, 4049 CrossRef CAS.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, Tetrahedron, 2001, 57, 4045 CrossRef CAS.
- S. Ohta, M. Uno, M. Tokumasu, Y. Hiraga and S. Ikegami, Tetrahedron Lett., 1996, 37, 7765 CrossRef CAS.
- H. Hioki, H. Ooi, M. Hamano, Y. Mimura, S. Yoshio, M. Kodama, S. Ohta, M. Yanai and S. Ikegami, Tetrahedron, 2001, 57, 1235 CrossRef CAS.
- M. Ichihashi, H. Takikawa and K. Mori, Biosci. Biotechnol. Biochem., 2001, 65, 2569 CrossRef CAS.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, Eur. J. Org. Chem., 2001, 4457 CrossRef CAS.
- J. Shin and W. Fenical, Tetrahedron, 1993, 49, 9277 CrossRef CAS.
- T. W. Lee and E. J. Corey, J. Am. Chem. Soc., 2001, 123, 1872 CrossRef CAS.
- P. J. Sung, J. H. Su, C. Y. Duh, M. Y. Chiang and J. H. Sheu, J. Nat. Prod., 2001, 64, 318 CrossRef CAS.
- S. L. Wu, P. J. Sung, M. Y. Chiang, J. Y. Wu and J. H. Sheu, J. Nat. Prod., 2001, 64, 1415 CrossRef CAS.
- S. Aoki, M. Okano, K. Matsui, T. Itoh, R. Satari, S. Akiyama and M. Kobayashi, Tetrahedron, 2001, 57, 8951 CrossRef CAS.
- J. H. Kwak, F. J. Schmitz and G. C. Williams, J. Nat. Prod., 2001, 64, 754 CrossRef CAS.
- A. Groweiss, S. A. Look and W. Fenical, J. Org. Chem., 1988, 53, 2401 CrossRef CAS.
- J. R. Rho, M. S. Oh, K. H. Jang, K. W. Cho and J. Shin, J. Nat. Prod., 2001, 64, 540 CrossRef CAS.
- A. Rueda, E. Zubia, M. J. Ortega and J. Salva, J. Nat. Prod., 2001, 64, 401 CrossRef CAS.
- N. Fusetani, H. Nagata, H. Hirota and T. Tsuyuki, Tetrahedron Lett., 1989, 30, 7079 CrossRef CAS.
- D. F. Taber and S. C. Malcolm, J. Org. Chem., 2001, 66, 944 CrossRef CAS.
- N. Gonzalez, M. A. Barral, J. Rodriguez and C. Jimenez, Tetrahedron, 2001, 57, 3487 CrossRef CAS.
- Y. C. Shen, C. V. S. Prakash and Y. T. Chang, Steroids, 2001, 66, 721 CrossRef CAS.
- Y. Seo, J. R. Rho, K. W. Cho and J. Shin, Nat. Prod. Lett., 2001, 15, 81 Search PubMed.
- A. D. Rodriguez and C. Ramirez, J. Nat. Prod., 2001, 64, 100 CrossRef CAS.
- A. D. Rodriguez and C. Ramirez, Org. Lett., 2000, 2, 507 CrossRef CAS.
- K. C. Nicolaou, G. Vassilikogiannakis, W. Mägerlein and R. Kranich, Angew. Chem., Int. Ed., 2001, 40, 2482 CrossRef CAS.
- M. Vanisree, G. V. Subbaraju and C. B. Rao, J. Asian Nat. Prod. Res., 2001, 3, 23 Search PubMed.
- A. D. Rodriguez, C. Ramirez, I. I. Rodriguez and E. Gonzalez, Org. Lett., 1999, 1, 527 CrossRef CAS.
- T. W. Johnson and E. J. Corey, J. Am. Chem. Soc., 2001, 123, 4475 CrossRef CAS.
- Y. P. Shi, A. D. Rodriguez and O. L. Padilla, J. Nat. Prod., 2001, 64, 1439 CrossRef CAS.
- F. J. Schmitz, D. J. Vanderah and L. S. Ciereszko, J. Chem. Soc., Chem. Commun., 1974, 407 RSC.
- O. M. Cobar, A. D. Rodriguez, O. L. Padilla and J. A. Sanchez, J. Org. Chem., 1997, 62, 7183 CrossRef CAS.
- A. Rueda, E. Zubia, M. J. Ortega and J. Salva, Steroids, 2001, 66, 897 CrossRef CAS.
- S. P. Chen, P. J. Sung, C. Y. Duh, C. F. Dai and J. H. Sheu, J. Nat. Prod., 2001, 64, 1241 CrossRef CAS.
- A. Patra, A. Majumdar, K. K. Mandal, A. Ghosh, D. Banerjee and B. P. Haldar, J. Indian Chem. Soc., 2001, 78, 619 Search PubMed.
- M. Wessels, G. M. König and A. D. Wright, J. Nat. Prod., 2001, 64, 370 CrossRef.
- M. F. Rodriguez Brasco, A. M. Seldes and J. A. Palermo, Org. Lett., 2001, 3, 1415 CrossRef CAS.
- L. M. Zeng, X. Q. Li, J. Y. Su, X. Fu and F. J. Schmitz, J. Nat. Prod., 1995, 58, 296 CrossRef CAS.
- J. G. Cui, L. M. Zeng, J. Y. Su and W. G. Lu, Steroids, 2001, 66, 33 CrossRef CAS.
- G. H. Wang, J. H. Sheu, M. Y. Chiang and T. J. Lee, Tetrahedron Lett., 2001, 42, 2333 CrossRef CAS.
- W. Inman and P. Crews, J. Org. Chem., 1989, 54, 2526 CrossRef CAS.
- L. Xu, B. O. Patrick, M. Roberge, T. Allen, L. van Ofwegen and R. J. Andersen, Tetrahedron, 2000, 56, 9031 CrossRef.
- J. H. Sheu, G. H. Wang, P. J. Sung, C. Y. Duh and M. Y. Chiang, Tetrahedron, 2001, 57, 7639 CrossRef CAS.
- P. Sharma and M. Alam, J. Chem. Soc., Perkin Trans. 1, 1988, 2537 RSC.
- D. Friedrich, R. W. Doskotch and L. A. Paquette, Org. Lett., 2000, 2, 1879 CrossRef CAS.
- Y. Uchio, M. Nakatani, T. Hase, M. Kodama, S. Usui and Y. Fukazawa, Tetrahedron Lett., 1989, 30, 3331 CrossRef CAS.
- Y. Uchio, M. Kodama, S. Usui and Y. Fukazawa, Tetrahedron Lett., 1992, 33, 1317 CrossRef CAS.
- F. Gallou, D. W. C. MacMillan, L. E. Overman, L. A. Paquette, L. D. Pennington and J. Yang, Org. Lett., 2001, 3, 135 CrossRef CAS.
- D. W. C. MacMillan, L. E. Overman and L. D. Pennington, J. Am. Chem. Soc., 2001, 123, 9033 CrossRef CAS.
- P. Bernardelli, O. M. Moradei, D. Friedrich, J. Yang, F. Gallou, B. P. Dyck, R. W. Doskotch, T. Lange and L. A. Paquette, J. Am. Chem. Soc., 2001, 123, 9021 CrossRef CAS.
- R. Britton, M. Roberge, H. Berisch and R. J. Andersen, Tetrahedron Lett., 2001, 42, 2953 CrossRef CAS.
- T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carboni and C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744 CrossRef CAS.
- B. Tursch, J. C. Braekman and D. Daloze, Bull. Soc. Chim. Belg., 1975, 84, 767 CAS.
- W. H. Zhang, I. D. Williams and C. T. Che, Tetrahedron Lett., 2001, 42, 4681 CrossRef CAS.
- C. Y. Duh, M. C. Chia, S. K. Wang, H. J. Chen, A. A. H. El-Gamal and C. F. Dai, J. Nat. Prod., 2001, 64, 1028 CrossRef CAS.
- M. Iwashima, K. Nara, Y. Nakamichi and K. Iguchi, Steroids, 2001, 66, 25 CrossRef CAS.
- M. Kobayashi, N. K. Lee, B. W. Son, K. Yanagi, Y. Kyogoku and I. Kitagawa, Tetrahedron Lett., 1984, 25, 5925 CrossRef CAS.
- K. Watanabe, M. Sekine, H. Takahashi and K. Iguchi, J. Nat. Prod., 2001, 64, 1421 CrossRef CAS.
- C. Y. Duh, K. J. Chen, A.A. H. El-Gamal and C. F. Dai, J. Nat. Prod., 2001, 64, 1430 CrossRef CAS.
- V. Anjaneyulu, P. V. Subba Rao, P. Radhika, H. Laatsch and R. N. Asolkar, Indian J. Chem. Sect. B., 2001, 40, 405.
- A. S. Dmitrenok, V. Anjaneyulu, P. V. Subba Rao, P. Radhika, P. S. Dmitrenok, V. M. Boguslavsky and V. A. Stonik, Russ. Chem. Bull., 2001, 50, 1474 CrossRef CAS.
- P. Ramesh, V. Ravikanth and Y. Venkateswarlu, Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem., 2001, 40, 867.
- T. V. Rezanka and V. M. Dembitsky, Tetrahedron, 2001, 57, 8743 CrossRef CAS.
- A. M. Suleimenova, A. I. Kalinovskii, V. A. Raldugin, S. A. Shevtsov, I. Y. Bagryanskaya, Y. V. Gatilov, T. A. Kuznetsova and G. B. Elyalkov, Khim. Prir. Soedin., 1988, 4, 535.
- T. Zhang, Z. Liu and Y. Li, Synthesis, 2001, 3, 393 CrossRef.
- Z. Yan, C. Chen and L. Zeng, Redai Haiyang, 1985, 4, 80 Search PubMed.
- J. Su, S. Yan and L. Zeng, Gaodeng Xuexiao Huaxue Xuebao, 2001, 22, 1515 Search PubMed.
- M. Kobayashi, T. Nakagawa and H. Mitsuhashi, Chem. Pharm. Bull., 1979, 27, 2382 CAS.
- K. Mada, T. Ooi and T. Kusumi, Spectroscopy, 2001, 15, 177 Search PubMed.
- J. Y. Su, R. L. Yang and L. M. Zeng, Chin. J. Chem., 2001, 19, 515 Search PubMed.
- K. Mori, K. Iguchi, N. Yamada, Y. Yamada and Y. Inouye, Tetrahedron Lett., 1987, 28, 5673 CrossRef CAS.
- H. Miyaoka, T. Baba, H. Mitome and Y. Yamada, Tetrahedron Lett., 2001, 42, 9233 CrossRef CAS.
- N. Alam, J. Hong, C. O. Lee, K. S. Im, B. W. Son, J. S. Choi, W. C. Choi and J. H. Jung, J. Nat. Prod., 2001, 64, 956 CrossRef CAS.
- N. Alam, B. H. Bae, J. Hong, C. O. Lee, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 1059 CrossRef CAS.
- A. Athanasiadis, G. Anderluh, P. Macek and D. Turk, Structure, 2001, 9, 341 CrossRef CAS.
- R. Zhuo, H. Fu, C. Zhong, X. Wang, W. Lin and L. Zhang, Shengwu Huaxue Yu Shengwu Wuli Jinzhan, 2001, 28, 514 Search PubMed.
- J. S. Carlé and C. Christophersen, J. Am. Chem. Soc., 1979, 101, 4012 CrossRef CAS.
- P. Wulff, J. S. Carlé and C. Christophersen, Comp. Biochem. Physiol., 1982, 71B, 523 Search PubMed.
- P. Keil, E. G. Nielsen, U. Anthoni and C. Christophersen, Acta Chem. Scand. B, 1986, 40, 555 Search PubMed.
- M. S. Morales-Ríos, O. R. Suárez-Castillo, J. J. Trujillo-Serrato and P. Joseph-Nathan, J. Org. Chem., 2001, 66, 1186 CrossRef CAS.
- P. B. Holst, U. Anthoni, C. Christophersen and P. H. Nielsen, J. Nat. Prod., 1994, 57, 997 CrossRef CAS.
- A. S. Cardoso, N. Srinivasan, A. M. Lobo and S. Prabhakar, Tetrahedron Lett., 2001, 42, 6663 CrossRef CAS.
- M. L. Ciavatta, E. Trivellone, G. Villani and G. Cimino, Tetrahedron Lett., 1993, 34, 6791 CrossRef CAS.
- M. V. Perkins and R. A. Sampson, Org. Lett., 2001, 3, 123 CrossRef CAS.
- A. Spinella, E. Mollo, E. Trivellone and G. Cimino, Tetrahedron, 1997, 53, 16891 CrossRef CAS.
- H. Takikawa, M. Yoshida and K. Mori, Tetrahedron Lett., 2001, 42, 1527 CrossRef CAS.
- M. Yoshida, H. Takikawa and K. Mori, J. Chem. Soc. Perkin Trans. 1, 2001, 1007 RSC.
- H. Hioki, M. Hamano, M. Kubo, T. Uno and M. Kodama, Chem. Lett., 2001, 898 CrossRef CAS.
- S. N. Fedorov, O. S. Radchenko, L. K. Shubina, A. I. Kalinovsky, A. V. Gerasimenko, D. Y. Popov and V. A. Stonik, J. Am. Chem. Soc., 2001, 123, 504 CrossRef.
- C. R. Kaiser, L. F. Pitombo and A. C. Pinto, Magn. Reson. Chem., 2001, 39, 147 CrossRef CAS.
- D. R. Appleton, R. C. Babcock and B. R. Copp, Tetrahedron, 2001, 57, 10181 CrossRef CAS.
- S. Araki, S. Yamada, S. Abe, H. Waki, K. Kon, S. Itonori, M. Sugita and S. Ando, J. Biochem., 2001, 129, 93 CAS.
- A. Spinella, E. Zubia, E. Martinez, J. Ortea and G. Cimino, J. Org. Chem., 1997, 62, 5471 CrossRef CAS.
- T. V. Hansen and Y. Stenstrom, Tetrahedron: Asymmetry, 2001, 12, 1407 CrossRef CAS.
- H. Kigoshi, T. Itoh, T. Ogawa, K. Ochi, M. Okada, K. Suenaga and K. Yamada, Tetrahedron Lett., 2001, 42, 7461 CrossRef CAS.
- Y. Sakio, Y. J. Hirano, M. Hayashi, K. Komiyama and M. Ishibashi, J. Nat. Prod., 2001, 64, 726 CrossRef CAS.
- E. D. de Silva, S. A. Morris, S. C. Miao, E. Dumdei and R. J. Andersen, J. Nat. Prod., 1991, 54, 993.
- A. D. Lebsack, L. E. Overman and R. J. Valentekovich, J. Am. Chem. Soc., 2001, 123, 4851 CrossRef CAS.
- K. L. McPhail, M. T. Davies-Coleman and J. Starmer, J. Nat. Prod., 2001, 64, 1183 CrossRef CAS.
- N. Takada, K. Suenaga, K. Yamada, S. Z. Zheng, H. S. Chen and D. Uemura, Chem. Lett., 1999, 1025 CAS.
- K. Suenaga, K. Araki, T. Sengoku and D. Uemura, Org. Lett., 2001, 3, 527 CrossRef CAS.
- P. Ciminiello, E. Fattorusso, M. Forino, M. Di Rosa, A. Ianaro and R. Poletti, J. Org. Chem., 2001, 66, 578 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, A. Ianaro and M. Di Rosa, Eur. J. Org. Chem., 2001, 1, 49 CrossRef.
- P. Ciminiello, C. Dell'Aversano, C. Fattorusso, E. Fattorusso, M. Forino and S. Magno, Tetrahedron, 2001, 57, 8189 CrossRef CAS.
- N. Takada, N. Umemura, K. Suenaga, T. Chou, A. Nagatsu, T. Haino, K. Yamada and D. Uemura, Tetrahedron Lett., 2001, 42, 3491 CrossRef CAS.
- J. A. McCauley, K. Nagasawa, P. A. Lander, S. G. Mischke, M. A. Semones and Y. Kishi, J. Am. Chem. Soc., 1998, 120, 7647 CrossRef CAS.
- N. Takada, N. Umemura, K. Suenaga and D. Uemura, Tetrahedron Lett., 2001, 42, 3495 CrossRef CAS.
- N. Takada, M. Iwatsuki, K. Suenaga and D. Uemura, Tetrahedron Lett., 2000, 41, 6425 CrossRef CAS.
- H. Kigoshi, N. Hayashi and D. Uemura, Tetrahedron Lett., 2001, 42, 7469 CrossRef CAS.
- T. Chuo, M. Kuramoto, Y. Otani, M. Shikano, K. Yazawa and D. Uemura, Tetrahedron Lett., 1996, 37, 3871 CrossRef.
- M. W. Carson, G. Kim and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4453 CrossRef CAS.
- K. Ofuji, M. Satake, T. McMahon, K. J. James, H. Naoki, Y. Oshima and T. Yasumoto, Biosci. Biotechnol. Biochem., 2001, 65, 740 CrossRef CAS.
- T. Maoka, K. Hashimoto, N. Akimoto and Y. Fujiwara, J. Nat. Prod., 2001, 64, 578 CrossRef CAS.
- M. Tsushima, T. Maoka and T. Matsuno, J. Nat. Prod., 2001, 64, 1139 CrossRef CAS.
- N. M. Carballeira, H. Cruz, C. A. Hill, J. J. de Voss and M. Garson, J. Nat. Prod., 2001, 64, 1426 CrossRef CAS.
- A. Aiello, S. Carbonelli, E. Fattorusso, T. Iuvone and M. Menna, J. Nat. Prod., 2001, 64, 219 CrossRef CAS.
- A. Aiello, S. Carbonelli, G. Esposito, E. Fattorusso, T. Iuvone and M. Menna, Org. Lett., 2001, 3, 2941 CrossRef CAS.
- R. Britton, J. H. H. L. de Oliveira, R. J. Andersen and R. G. S. Berlinck, J. Nat. Prod., 2001, 64, 254 CrossRef CAS.
- L. H. Franco, E. B. de Kier Joffe, L. Puricelli, M. Tatian, A. M. Seldes and J. A. Palermo, J. Nat. Prod., 1998, 61, 1130 CrossRef CAS.
- P. M. Fresneda, P. Molina and J. A. Bleda, Tetrahedron, 2001, 57, 2355 CrossRef CAS.
- A. Rudi and Y. Kashman, J. Org. Chem., 1989, 54, 5331 CrossRef CAS.
- I. Viracaoundin, R. Faure, E. M. Gaydou and M. Aknin, Tetrahedron Lett., 2001, 42, 2669 CrossRef CAS.
- B. R. Copp, J. W. Blunt, M. H. G. Munro and L. K. Pannell, Tetrahedron Lett., 1989, 30, 3703 CrossRef CAS.
- A. N. Pearce, R. C. Babcock, C. N. Battershill, G. Lambert and B. R. Copp, J. Org. Chem., 2001, 66, 8257 CrossRef CAS.
- M. J. Uddin, S. Kokubo, K. Suenaga, K. Ueda and D. Uemura, Heterocycles, 2001, 54, 1039 CAS.
- M. J. Uddin, S. Kokubo, K. Ueda, K. Suenaga and D. Uemura, J. Nat. Prod., 2001, 64, 1169 CrossRef CAS.
- H. Vervoort, W. Fenical and R. de A. Epifanio, J. Org. Chem., 2000, 65, 782 CrossRef CAS.
- B. Liang, D. J. Richard, P. S. Portonovo and M. M. Joullie, J. Am. Chem. Soc., 2001, 123, 4469 CrossRef CAS.
- A. Fontana, M. C. Gonzalez, M. Gavagnin, J. Templado and G. Cimino, Tetrahedron Lett., 2000, 41, 429 CrossRef CAS.
- R. Duran, E. Zubia, M. J. Ortega, S. Naranjo and J. Salva, Tetrahedron, 2000, 56, 6031 CrossRef CAS.
- A. Fontana, G. Cimino, M. Gavagnin, M. C. Gonzalez and E. Estornell, J. Med. Chem., 2001, 44, 2362 CrossRef CAS.
- M. A. Exposito, B. Lopez, R. Fernandez, M. Vazquez, C. Debitus, T. Iglesias, C. Jimenez, E. Quinoa and R. Riguera, Tetrahedron, 1998, 54, 7539 CrossRef CAS.
- A. Exposito, M. Fernandez-Suarez, T. Iglesias, L. Munoz and R. Riguera, J. Org. Chem., 2001, 66, 4206 CrossRef CAS.
- L. Garrido, E. Zubia, M. J. Ortega, S. Naranjo and J. Salva, Tetrahedron, 2001, 57, 4579 CrossRef CAS.
- T. Ozawa, S. Aoyagi and C. Kibayashi, J. Org. Chem., 2001, 66, 3338 CrossRef CAS.
- B. Steffan, Tetrahedron, 1991, 47, 8729 CrossRef CAS.
- J. Kubanek, D. E. Williams, E. D. de Silva, T. Allen and R. J. Andersen, Tetrahedron Lett., 1995, 36, 6189 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson, L. K. Cartner, L. K. Pannell and M. R. Boyd, Tetrahedron, 2001, 57, 5751 CrossRef CAS.
- M. C. McCoy and D. J. Faulkner, J. Nat. Prod., 2001, 64, 1087 CrossRef CAS.
- R. M. van Wagoner, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 2001, 64, 1100 CrossRef CAS.
- A. N. Pearce, R. C. Babcock, G. Lambert and B. R. Copp, Nat. Prod. Lett., 2001, 15, 237 Search PubMed.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1454 CrossRef CAS.
- M. Wessels, G. M. König and A. D. Wright, J. Nat. Prod., 2001, 64, 1556 CrossRef CAS.
- T. N. Makarieva, A. S. Dmitrenok, P. S. Dmitrenok, B. B. Grebnev and V. A. Stonik, J. Nat. Prod., 2001, 64, 1559 CrossRef CAS.
- B. R. Copp, J. Jompa, A. Tahir and C. M. Ireland, J. Org. Chem., 1998, 63, 8024 CrossRef CAS.
- D. Skyler and C. H. Heathcock, Org. Lett., 2001, 3, 4323 CrossRef CAS.
- L. A. McDonald, G. S. Eldredge, L. R. Barrows and C. M. Ireland, J. Med. Chem., 1994, 37, 3819 CrossRef CAS.
- N. Lindquist, W. Fenical, G. D. van Duyne and J. Clardy, J. Am. Chem. Soc., 1991, 113, 2303 CrossRef CAS.
- J. Li, S. Jeong, L. Esser and P. G. Harran, Angew. Chem., Int. Ed., 2001, 40, 4765 CrossRef CAS.
- J. Li, A. W. G. Burgett, L. Esser, C. Amezcua and P. G. Harran, Angew. Chem., Int. Ed., 2001, 40, 4770 CrossRef CAS.
- C. Wang, G. Han, J. Su and L. Zeng, Fenxi Huaxue, 2001, 29, 168 CAS.
- I. H. Lee, Y. S. Lee, C. H. Kim, C. R. Kim, T. Hong, L. Menzel, L. M. Boo, J. Pohl, M. A. Sherman, A. Waring and R. I. Lehrer, Biochim. Biophys. Acta, 2001, 1527, 141 CrossRef CAS.
- R. I. Lehrer, I. H. Lee, L. Menzel, A. Waring and C. Zhao, Adv. Exp. Med. Biol., 2001, 484, 71 Search PubMed.
- K. Yamada, R. Matsubara, M. Kaneko, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2001, 49, 447 CrossRef CAS.
- N. V. Ivanchina, A. A. Kicha, A. I. Kalinovsky, P. S. Dmitrenok, N. G. Prokof'eva and V. A. Stonik, J. Nat. Prod., 2001, 64, 945 CrossRef CAS.
- N. Yayli, Indian J. Chem. Sect. B., 2001, 40, 399.
- E. V. Levina, P. V. Andriyashchenko, A. I. Kalinovskii and V. A. Stonik, Russ. Chem. Bull., 2001, 50, 313 CrossRef CAS.
- K. Arao, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2001, 49, 695 CrossRef CAS.
- A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, Russ. Chem. Bull., 2001, 50, 724 CrossRef CAS.
- M. S. Maier, A. J. Roccatagliata, A. Kuriss, H. Chludil, A. M. Seldes, C. A. Pujol and E. B. Damonte, J. Nat. Prod., 2001, 64, 732 CrossRef CAS.
- D. Sato, Y. Ando, R. Tsujimoto and K. Kawasaki, Lipids, 2001, 36, 1371 Search PubMed.
- N. Takada, M. Watanabe, K. Suenaga, K. Yamada, M. Kita and D. Uemura, Tetrahedron Lett., 2001, 42, 6557 CrossRef CAS.
- A. P. Murray, C. Muniain, A. M. Seldes and M. S. Maier, Tetrahedron, 2001, 57, 9563 CrossRef CAS.
- S. Urban, S. J. H. Hickford, J. W. Blunt, M. H. G. Munro and M. Kelly, Curr. Org. Chem., 2000, 7, 765 Search PubMed.
- D. J. Faulkner, Nat. Prod. Rep., 2002, 19, 1 RSC.
| This journal is © The Royal Society of Chemistry 2003 |