Hot off the press
Robert A.
Hill
and
Marie
Claire Parker
Department of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bobh@chem.gla.ac.uk; m.parker@chem.gla.ac.uk
First published on 9th January 2003
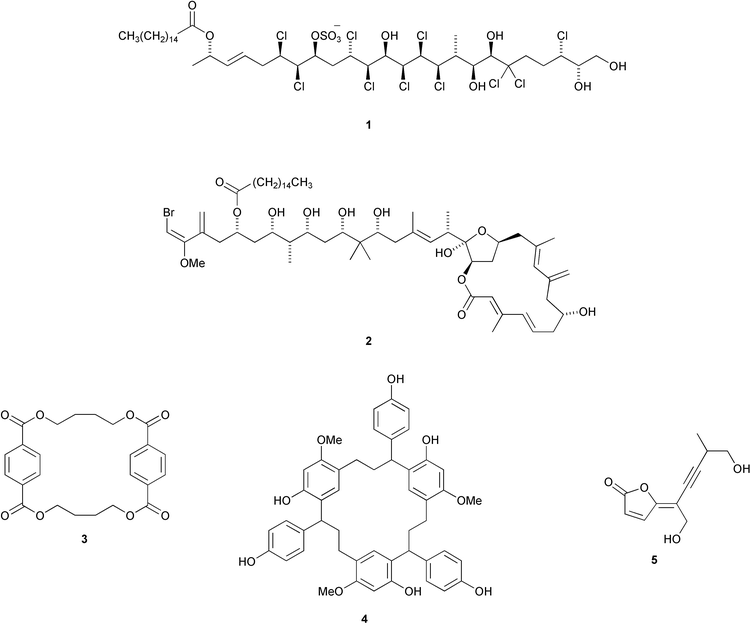
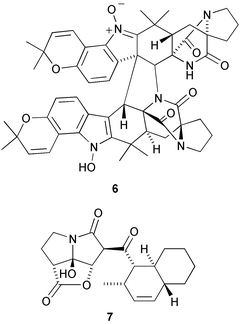
A cytotoxic polychlorinated sulfolipid 1 has been isolated from toxic mussels, Mytilus galloprovincialis (P. Ciminiello et al., J. Am. Chem. Soc., 2002, 124, 13114). Phormidolide 2 is a toxic metabolite of the marine cyanobacterium Phormidium sp. (W. H. Gerwick and co-workers, J. Org. Chem., 2002, 67, 7927). The symmetrical p-cyclophane pharacine 3 is a metabolite of the bacterial strain Cytophaga sp. AM13.1 (H. Laatsch and co-workers, J. Nat. Prod., 2002, 65, 1660). Drocophane 4 is a symmetrical m-cyclophane from the resin of Dracaena cinnabari (D. Veselá et al., Phytochemistry, 2002, 61, 967). Aporpinone A 5, a metabolite of Aporpium caryae, is probably of polyketide origin (G. M. Cabrera and co-workers, Phytochemistry, 2003, 62, 239).A metabolite of Aspergillus ochraceus, stephacidin B 6, shows selective antitumour activity against testosterone-dependent prostate LNCaP cells with an IC50 of 0.06 µM (J. Qian-Cutrone et al., J. Am. Chem. Soc., 2002, 124, 14556). The unusual furanpyrrolizidine ring system is found in UCS1025A 7, an antitumour antibiotic from Acremonium sp. KY4917 (T. Agatsuma et al., Org. Lett., 2002, 4, 4387).
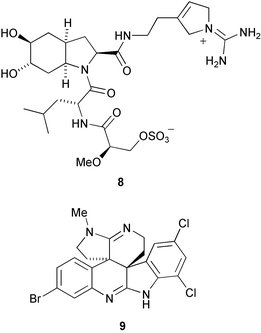
The marine natural product dysinosin A 8, from a sponge of the Dysideidae family, is a potent inhibitor of blood coagulation factor VIIa and an inhibitor of the serine protease thombin (R. J. Quin and co-workers, J. Am. Chem. Soc., 2002, 124, 13340). Perophoramide 9 is an halogenated hexacyclic alkaloid from the Philippine ascidian Perophora namei (C. M. Ireland and co-workers, J. Org., Chem., 2002, 67, 7124).
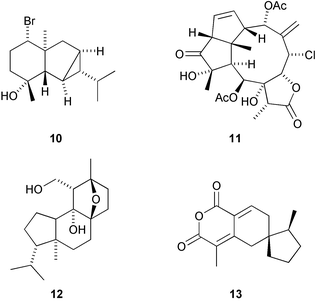
The 6,8-cycloeudesmane sesquiterpenoid 10 has been isolated from Laurencia microcladia (G. Guella et al., Z. Naturforsch., B: Chem. Sci., 2002, 57, 1147). Aquariolide A 11 , from the octacoral Eleutherobia sp., has a novel diterpenoid skeleton, named aquariane, which can formally be derived from the briarane skeleton (R. J. Anderson and co-workers, Org. Lett., 2002, 4, 4085). The norditerpenoid yaretol 12 is a constituent of a compact resinous cushion shrub Azorella madreporica from the high Andes of Central Chile (L. A. Loyola et al., J. Nat. Prod., 2002, 65, 1678). Paniculoid 13 has been isolated from the seeds of Koelreuteria paniculata and has a novel sesquiterpenoid skeleton (W. H. Lin et al., Chin. Chem. Lett., 2002, 13, 1067).
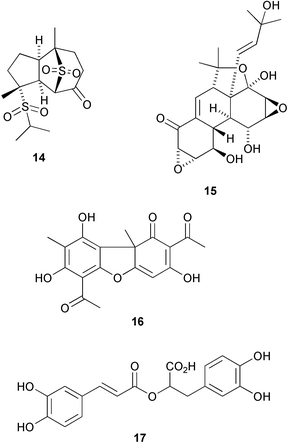
The structure of orientanone 14, from Alisma orientalis, is apparently derived by migration of an isopropyl group of a guaiane derivative from carbon-7 to sulfur (G.-P. Peng et al., Tetrahedron, 2002, 58, 9045). Panepophenanthrin 15, a metabolite of Panus rudis, is an inhibitor of the ubiquitin-activating enzyme (R. Sekizawa et al., J. Nat. Prod., 2002, 65, 1491). A comprehensive review of the insect antifeedant activity of clerodane diterpenoids has identified some interesting trends on the structure of the strongest antifeedants (A. de Groot and co-workers, Phytochemistry, 2002, 61, 737). A series of reviews giving the chemistry, biosynthesis and biological activities of “Molecules of Interest” includes usnic acid 16 from lichens (K. Ingólfsdóttir, Phytochemistry, 2002, 61, 729) and rosmarinic acid 17 found in many medicinal plants (M. Peterson and M. S. J. Simmonds, Phytochemistry, 2003, 62, 121).
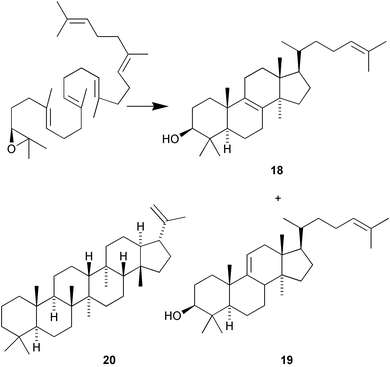
Mutants at cycloartenol synthase residue His477 have been shown to dramatically alter catalysis (S. P. T. Matsuda and co-workers, Org. Lett., 2002, 4, 4459). The His477Asn mutant produced 88% lanosterol 18 and 12% parkeol 19 whereas His477Gln mutant produced 22% lanosterol 18, 73% parkeol 19 and 5% lanosta-7,24-dien-3β-ol. Bacterial squalene cyclase has been used to cyclise a larger analogue (C35) of squalene to the hexacyclic polyprenoid 20 (I. Abe et al., J. Am. Chem. Soc., 2002, 124, 14514).
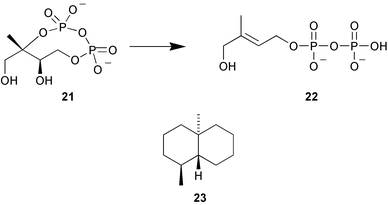
A key enzyme in the methylerythritol phosphate pathway to isoprenoid biosynthesis is 4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) that catalyses the transformation of the cyclodiphosphate 21 into 4-hydroxy-3-methylbut-2-enyl diphosphate 22. This enzyme has been shown to be an air sensitive [4Fe-4S] protein (M. Rohmer and co-workers, Angew. Chem., Int. Ed., 2002, 41, 4337). The oxygen sensitivity of the Fe/S cluster in this enzyme is one of the reasons why cell-free systems have not converted intermediates into IPP or DMAPP and for the late discovery of this pathway. Labelling studies have established that the trisnor-sesquiterpenoid geodesmin 23 is formed by the methylerythritol phosphate pathway in Streptomyces sp. JP95 whereas it is formed by the mevalonate pathway in the liverwort Fossombronia pusilla (W. Boland and co-workers, Phytochemistry, 2002, 61, 827).
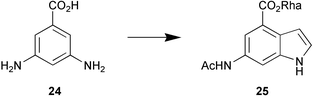
An entire issue of Chemical Reviews has been devoted to 15 review articles covering the important enzymes classed as proteases (Chem. Rev., 2002, 102, issue 12). The δ15N-value of natural nitrogen compounds can give information about primary nitrogen sources (nitrate, ammonium, N2) and about metabolic nitrogen recycling. R. A. Werner and H.-L. Schmidt have reviewed nitrogen isotope discrimination among organic plant compounds (Phytochemistry, 2002, 61, 465). Cultures of Streptomyces griseoviridis when supplemented with 3,5-diaminobenzoic acid 24 transform it into the indole derivative 25 (S. Grond et al., Eur. J. Org. Chem., 2002, 3237). The use of monooxygenase-mediated Baeyer–Villiger oxidations has been reviewed by M. D. Mihovilovic et al. (Eur. J. Org. Chem., 2002, 3711).
A lipid-coated lipase can catalyse the oligomerisation of diethoxydimethylsilane (DEDMS) in isooctane containing 2 wt% water (Scheme 1). The oligomerisation is proposed to occur from the OH head group of the coating lipid (H. Nishino et al., Chem. Commun., 2002, 2684). In mixtures of 10–15% acetonitrile in water, thermolysin is inactive in both synthesis and hydrolysis (R. V. Ulijn et al.,ChemBioChem, 2002, 3, 1112). Upon addition of either acetonitrile or aqueous buffer to a solution containing inactivated thermolysin, full catalytic activity can be recovered. CD and fluorescence studies reveal discontinuous changes in the overall secondary and tertiary structure that correlate with the reversible changes in catalytic activity. The researchers have postulated that the enzyme may be able to access two active conformations which are thermodynamically stable in different solvent environments.
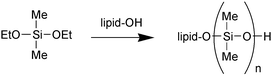 | ||
| Scheme 1 | ||
A contaminant esterase in a commercial preparation of Candida antarctica lipase A (CAL-A) was found to be effective for the enantioselective hydrolysis of ethyl (3RS,4RS)-trans-4-(4′-fluorophenyl)-6-oxopiperidine-3-carboxylate (Scheme 2). The esterase, which was subsequently purified and immobilised showed high enantioselectivity (E > 100) for the production of (−)-paroxetine 26 (J. M. Guisan and co-workers, Tetrahedron: Asymmetry, 2002, 13, 2653). The light-mediated regulation of asymmetric reduction of ketones by a cyanobacterium is reported by K. Nakamura and R. Yamanaka (Tetrahedron: Asymmetry, 2002, 13, 2529). The researchers found that the enantioselectivity of the reduction of α,α-difluoroacetophenone with Synechococcus elongates PCC 7942, a photosynthetic microbe, is improved as a result of illumination with fluorescent light (Scheme 3). A stereoselective sec-alkylsulfatase acting with inversion of configuration was found in Rhodococcus rubber DSM 44541. In contrast to the action of lipases, esterases and proteases, the absolute configuration of the product and the remaining unconverted substrate are identical. This was demonstrated for the enantioselective stereoinversion in the kinetic resolution of rac-sec-alkyl sulfate esters (Scheme 4) (K. Faber and co-workers Angew. Chem., Int. Ed., 2002, 41, 4053).
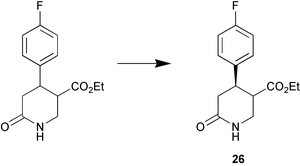 | ||
| Scheme 2 | ||
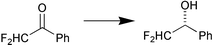 | ||
| Scheme 3 | ||
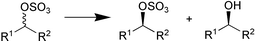 | ||
| Scheme 4 | ||
The chemistry of the antibody molecule is reviewed by P. G. Schultz and co-workers (Angew. Chem., Int. Ed., 2002, 41, 4427). The review is broad-based and covers how the exploitation of the properties of antibodies can be used to test basic theories of enzyme catalysis for the synthesis of new catalysts. H. Lin and V. W. Cornish (Angew. Chem., Int. Ed., 2002, 41, 4402) have reviewed the screening and selection methods for large-scale analysis of protein function. The review covers the types of assays that have been developed and how close we are to the goal of engineering proteins with new activities as well as rapidly assigning function to the thousands of proteins that make up each genome. L. K. Tsou and co-workers have reported that simple cation-π interactions between a phenyl ring and a protonated amine stabilise an α-helix in water (J. Am. Chem. Soc., 2002, 124, 14917). They have observed that the cation–π interaction between phenylalanine and ornithine (Phe–Orn) provides significant stability to the helix, stabilising it by −0.4 kcal mol−1.
A novel molecular imprinting strategy to create amylose-based polymers having high and selective rebinding affinity towards bisphenol A and alkylphenols through a radical polymerisation process is described by Y. Kanekiyo et al. (Chem. Commun., 2002, 2698). Amylose, a (1→4) α-D-glucan, forms helical structures and hydrophobic molecules can be encapsulated within the cavity of the helix. The interaction of polyphenols (tannins) with proline rich proteins (gelatin) have been studied using an automated flow injection system with FT-IR to gain insight into chemical aspects relating to astringency (A. Edelmann and B. Lendl, J. Am. Chem. Soc., 2002, 124, 14741). Significant differences in the affinity of different tannins to the gelatin-agarose system were observed. In addition, white wine showed no interaction whereas for red wine, where tannin is present, a major interaction was found.
G. M. Whitesides et al. (J. Am. Chem. Soc., 2002, 124, 14508) have described visually stunning systems of components in which self-assembly is caused by capillary interactions. The components have the capability of forming two or more assemblies with different structures. The components, which are millimetre scale plates, have edges functionalised into hydrophobic and hydrophilic sets. The structures are able to reconfigure, rather strikingly, when their environment changes.
| This journal is © The Royal Society of Chemistry 2003 |