Diterpenoids
James R.
Hanson
School of Chemistry, Physics and Environmental Science, University of Sussex, Brighton, Sussex, UK BN1 9QJ
First published on 28th November 2002
Abstract
Covering 2001. Previous review: Nat. Prod. Rep., 2002, 19, 125.
The review covers the isolation and structures of diterpenoids including labdanes, clerodanes, pimaranes, abietanes, kauranes, gibberellins, cembranolides, taxanes and marine diterpenoids. The literature from January to December 2001 is reviewed and 140 references are cited.
1 Introduction
This report follows the pattern of its predecessors.1 The variety of diterpenoid structures has made them attractive targets for synthesis. Although synthesis is outside the scope of this review, nevertheless it is worth noting the number of synthetic endeavours which have been reported. The range of diterpenoid carbon skeleta also makes these natural products useful taxonomic markers. A computer-assisted approach to the chemotaxonomy of the Lamiaceae (Labiatae) using diterpenoid skeleta as a guide, has been reported.22 Acyclic and related diterpenoids
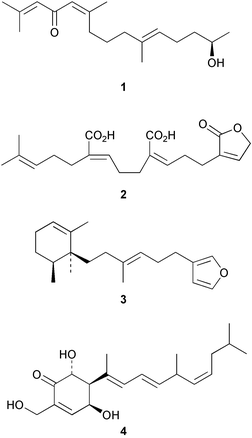
12,13-Dehydrogeranylgeraniol has been isolated3 as an anti-fungal and anti-oxidant agent from the aquatic plant, Saururus cernuus. (S)-12-Hydroxygeranylgeraniol derivatives have been found4 in an Atlantic collection of the brown alga, Bifurcaria bifurcata whilst the hedaol norditerpenes (e.g. hedaol A, 1) were found5 as cytotoxic constituents of a Sargassum species. Inconyzic acid 2 was obtained6 from Conyza incana. Examination of the sponge, Jaspis splendens afforded7 (+)-subersin 3 which is an inhibitor of 15-lipoxygenase. The phorbasins, e.g.4, were obtained8 from a Phorbas species of sponge.3 Bicyclic diterpenoids
3.1 Labdanes
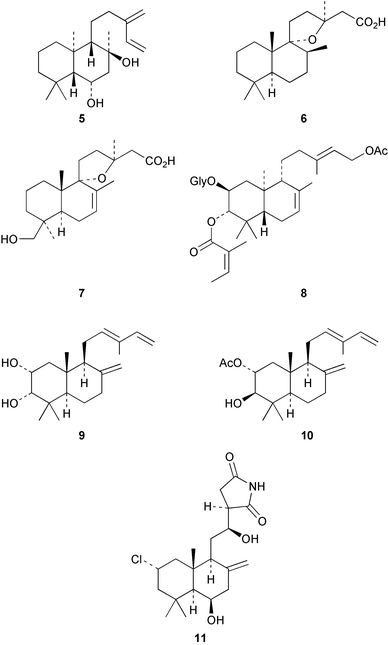
The readily available diterpenoid, larixol, which is isolated from larch oleoresin, has continued to be a useful starting material for diterpenoid partial synthesis. Syntheses of the perfumery constituent ambrox® from larixol, labdanolic acid and sclareol have been reported.9,10 The ent-labdane 5 has been obtained11 from Sideritis argyrea (Labiatae). Dihydrogrindelic acid 6 was obtained12 from Colophosphermum mopane (Leguminoseae) which is a tree that grows in the arid regions of South Africa. The corresponding aldehyde shows some cytotoxicity. 19-Hydroxygrindelic acid 7 was isolated13 from Grindelia integrifolia. Labdanes are widespread amongst the Compositae. Some glycosides, e.g.8, have been detected14 in the flowers of Baccharis medullosa. A number of Croton (Euphorbiaceae) species have been used in South-East Asian folk medicine. The labdanes 9 and 10 were isolated from C. joufra and C. oblongifolius respectively.15,16 Some chlorinated labdane imides, known as the haterumaimides, e.g.11, were extracted from a marine ascidian Lissoclinum species.17,18Hedychium species have previously been the source of diterpenes. Extraction of H. villosum gave19 villosin 12. Pacovatinin A 13 was obtained20 from the seeds of Renealmia exaltata (Zingiberaceae). Vitex rotundifolia (Verbenaceae) is widely distributed in Asia and its fruit, Viticus fructus, is used in folk medicine for the relief of pain. Examination of the fruit has given a number of labdanes exemplified by 14.21 The absolute stereochemistry of limonidilactone 15, which had been isolated from V. limonidifolia, has been established22 by the partial synthesis of its enantiomer from zamoranic acid 16. The Diels–Alder adduct 17 has been obtained23 from Alpinia flabellata.
The interactions between aquatic plants and microalgae form an important facet of aquatic eco-systems. Investigations of the aquatic plant, Potamogeton natans in this context have afforded24,25 the lactone 18 and its furan analogue. Cacofuran A, 19, has been obtained26 from a Cacospongia species of sponge. 11α-Hydroxy-13-epimanoyl oxide has been detected27 in extracts of the medicinal plant, Kyllinga erecta (Cyperaceae). Further examples of the ptychantins have been obtained28 from the liverwort, Ptychanthus striatus. The biotransformation of ribenone 20 by the fungus, Mucor plumbeus leads to hydroxylation at C-1α, C-6 (α and β) and C-12β.29
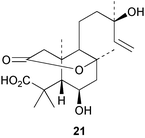
The secolabdane, rhizophorin A 21 has been obtained30 from the roots of the tree, Rhizophora mucronata. The tumour inhibitory activity of the secolabdanes from Excoecaria agallocha has been examined.31
3.2 Clerodanes and related diterpenoids
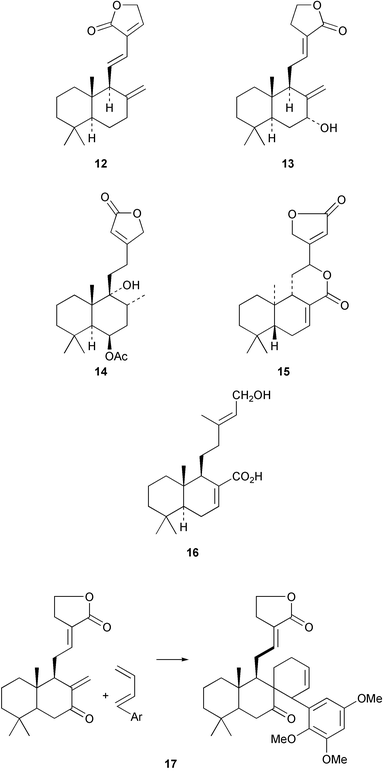
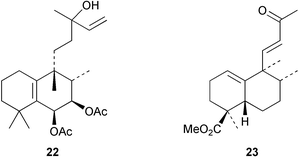
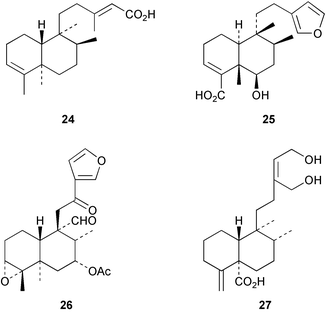
Halima-1(10),14-dien-13-ol has been detected32 in the liverwort, Jungermannia infusca. Some halimanes, the vitetrifolins D–G, e.g. D 22, have been reported33 in the fruit of the Asian medicinal plant, Vitex trifolia. The 14,15-dinorhalimane 23 is a minor diterpenoid in Halimium viscosum.34Assignments of the 1H and 13C NMR spectra of the clerodanes have been reported.35 The seeds of Hymenaea courbaril have been reinvestigated and the clerodane 24 has been reported.36 The thrombin inhibitory constituents of Duranta repens include the clerodane 25.37Croton eluteria (Euphorbiaceae) is a small tree which is indigenous to the Bahamas and which has been used in the treatment of fever. The constituents of the bark include the cascarillins B–D 26.38 The porwenins A and B, e.g.27, have been obtained39 from Portulaca okinawensis.
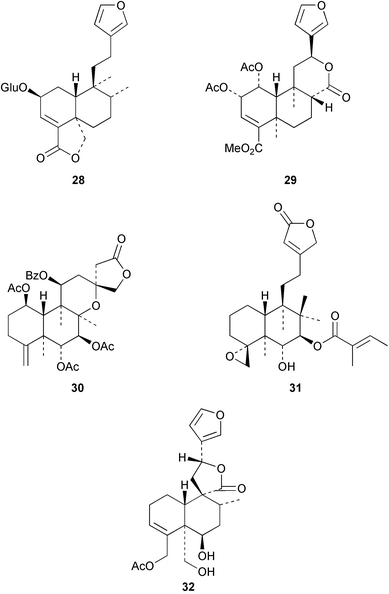
The X-ray crystal structure of amarisolide 28 from Salvia amarissima has been reported.40 Examination of the hallucinogenic Mexican herb, Salvia divinorum, has afforded41 a further clerodane, salvinorin C 29. A number of clerodanes have been isolated from Scutellaria (Labiatae) species. The scuteruleins A–D, e.g.30, have been found42 in S. caerulea and hematochloridin 31 was obtained43 from S. hematuchlora. Teughrebin 32 has been isolated44 from Teucrium maghrebinum whilst 7α-acetoxy-7,8α-dihydrogesnerofolin was detected45 in Salvia gesneraeflora. The structure–activity relationships of neoclerodanes as insect antifeedants has been examined46 and the topic has recently been reviewed.47
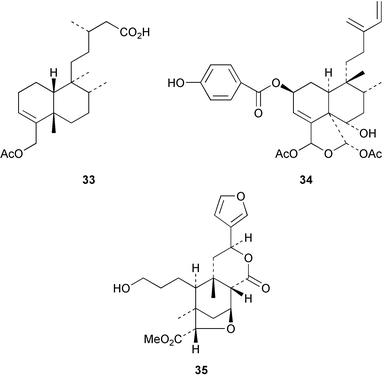
The cis-clerodane 33 has been obtained48 as an anti-bacterial constituent of Cistus monspeiensis whilst the intrapetacins A and B 34, from the roots of Licania intrapetiolaris, have anti-fungal properties.49 The Thai medicinal plant, Tinospora baenzigeri has attracted attention because of its antimalarial properties. Ring A of the clerodane structure has been cleaved, possibly via a hydroxy-epoxide, in the formation of baenzigeride B 35 which is a constituent of this plant.50
4 Tricyclic diterpenoids
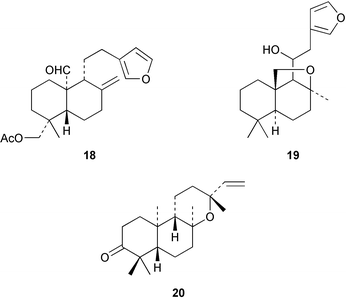
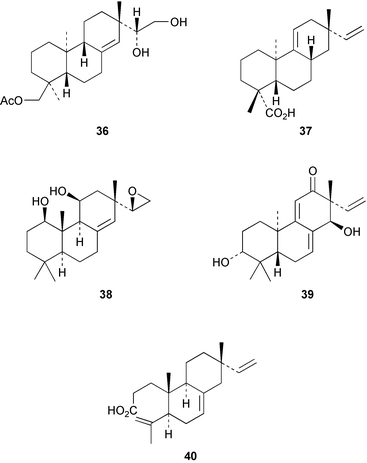
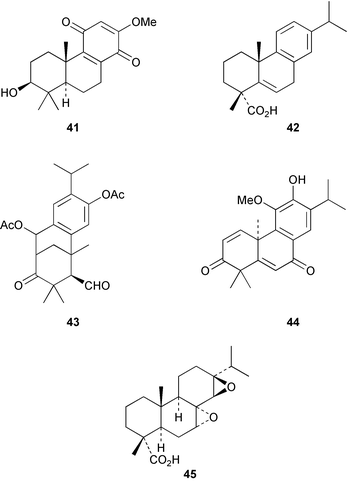
2α,18-Dihydroxysandaracopimaradiene has been obtained51 from the leaves of Guarea rhophalocarpa (Meliaceae). The powdered leaves and bark from this plant have been reported to control bleeding. 6β-Hydroxyisopimaric acid has been identified52 as an anti-bacterial constituent of the roots of Salvia caespitosa. The triol 36 has been isolated53 from the bark of the Chinese tree, Dysoxylum hainanense (Meliaceae). The beneficial analgesic and anti-inflammatory activity of the root-bark of the Korean medicinal plant, Acanthopanax koreanum has been associated with acanthoic acid 37. The absolute stereochemistry of this diterpene has now been established54 by synthesis. A revised structure 38, based on an X-ray crystal structure, has been proposed55 for leucophleoxol from Acacia leucophloea. The pimarane 39 has been obtained56 from Jatropha divaricata. Further examination of Orthosiphon stamineus has afforded57 additional members of the orthosiphol series which possess anti-proliferative activity. The ring A secopimarane 40 from Salvia cinnabarina has been reported58 to possess anti-spasmodic activity.Further investigations of the tree, Taiwania cryptomedioides (Taxodiaceae), which is resistant to fungal decay, have yielded59 some podocarpane quinones including 41. The abietane, centdaroic acid, 42, has been isolated60 from the roots of Cedrus deodara. The 7(6→2)abeo-abietane, obtusanal 43 has been obtained61 from the heartwood of Chamaecyparis obtusa. The drypetenones, e.g.44, are more highly oxidized abietanes which have been obtained62 from Drypetes littoralis (Euphorbiaceae). Abietane diepoxides, e.g.45 with anti-tumour activity, have been isolated63 from the cones of Larix kaempferi.
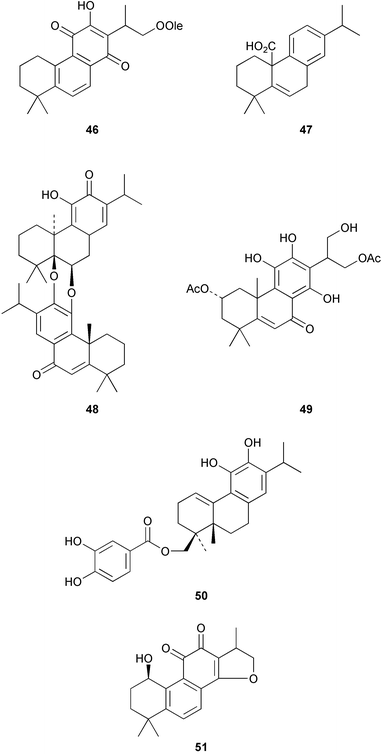
The Chinese medicinal herb, Salvia miltiorrhiza (Tan-shen) has been the source of the tanshinones. Their microwave assisted extraction from the herbal material has been described64 and a number of oleoyl esters, e.g.46, have been reported.65 Blephaein 47 has been obtained66 from the roots of Salvia blepharochlaena. The structure of the dimer, hongencaotone 48 from S. prionitis has been established67 by X-ray crystallography. Many of the leaf pigments of Coleus and Plectranthus species (Labiatae) are diterpenoid quinones. Examination68 of the ornamental medicinal plant, Coleus blumei gave the highly oxidized phenol 49. The rearranged abietane 50, which has anti-oxidative properties, has been obtained69 from the leaves of Plectranthus nummularius. The 1β-hydroxycryptotanshinone 51 has been found70 amongst the constituents of the roots of Perovskia abrotanoides. 7-Oxoabieta-9,12,14-triene, which was isolated71 from the roots of Salvia amplexicaulis, has been reported to have vasodepressor activity.
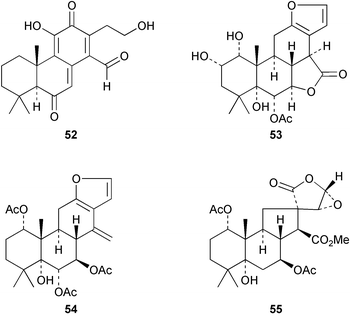
A further anti-fungal cassane quinone methide, 52, has been isolated72 from the roots of Bobgunnia madagascariensis. Plants belonging to the genus Caesalpinia (Leguminosae) have proven to be a rich source of cassane furanoditerpenes. There have been a number of reports on the constituents of Caesalpinia minax, a shrub used in Chinese folk medicine as ‘kushilian’ for the treatment of fever. The structure of caesalmin A 53 was established by X-ray crystallography.73 Caesalmin C 54 possesses anti-viral activity74 whilst the rearranged vouacapane, spirocaesalmin 55 exhibited75 significant activity against the respiratory syncytial virus, a pathogen responsible for a number of respiratory infections.
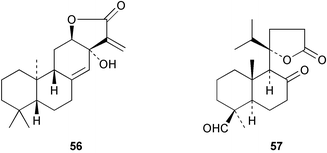
The X-ray crystal structures of jolkinolide D 5676 from Euphorbia jolkini and of the secochinane 5777 from Juniperus chinensis varn. kaizuka have been published.
5 Tetracyclic diterpenoids
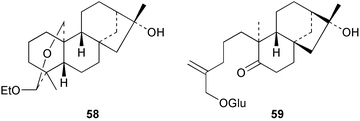
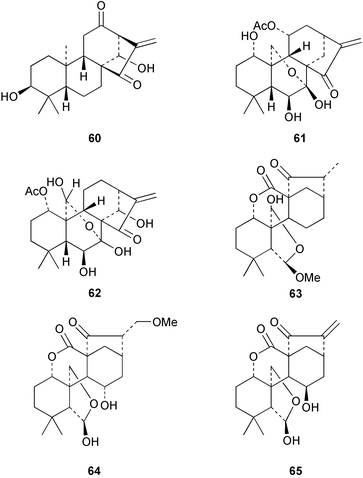
Continuing investigations of Trypterygium wilfordii have yielded some further diterpenoids. Extracts of the roots are used in folk medicine for the treatment of rheumatoid arthritis and other inflammatory diseases. The ethoxyacetal 58 has been obtained78 from the ‘Tπ’ extract. Modification of the readily available ent-kaurene, linearol, ent-3β,7α,18-trihydroxykaur-16-ene gave79 a series of derivatives amongst which the 7,18-ditiglate ester possessed quite high insect anti-feedant activity. ent-7β,18-Dihydroxy-15-oxokaur-16-ene has been isolated80 from Croton tonkinensis. A ring A secokaurene, pierisformoside G 59 has been obtained81 from the leaves of Pieris formosa.Many Isodon (Rabdosia) species are used in Chinese herbal medicines. These plants have continued to attract attention and have been the source of highly oxidized ent-kaurenes and ring B secokaurenes. New compounds which have been reported include pseurata G 60 from R. pseudo-irrorata,82 the taibaihenryiins, e.g.61, from I. henryi,83 the xerophilusins, e.g.62, from I. xerophilus,84,85 irroratin A 63 from I. irrorata,86 and taibaijaonicain A 64 from I. japonica.87 A number of these structures were established by X-ray crystallography. The crystal structure of nodosin 65, from R. serra, has been published.88
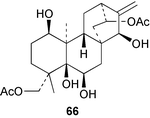
Aphidicolin is a metabolite of the fungus Phoma betae. A range of diterpene hydrocarbons, including aphidicol-16-ene, have been detected89 in the mycelial extract. The NMR data for the scopadulcins have been assigned.90 The bio-transformation of stemodin by the fungus, Beauveria bassiana gave91 2α,13,18-trihydroxystemodane. Some atisanes, e.g.66, which have been obtained92 from the liverwort, Lepidolaena clavigera possess cytotoxic and insecticidal activity.
A thorough GC MS study93 of the gibberellins of Prunus persica (peach) seeds has led to the detection of novel lα-hydroxy- and 1,2-didehydrogibberellins. These include GA11867 and GA12668. GA73 methyl ester 69 has been detected94 as the antheridiogen in the fern Lygodium microphyllum. Gibberellin metabolism has continued to attract attention. The mono-oxygenases involved95 in GA12 and GA14 biosynthesis in Gibberella fujikuroi and the metabolism of GA1 and GA3 in Prunus avium (sweet cherry)96 are amongst the topics which have been studied.
6 Macrocyclic diterpenoids and their cyclization products
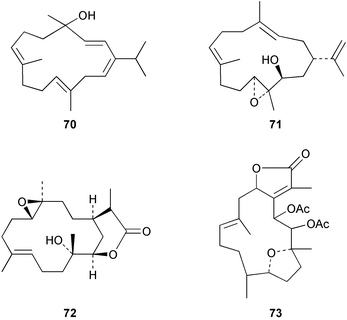
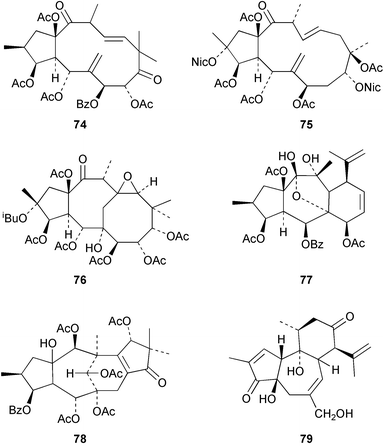
An NMR study of acutanol 70, a cembranoid from Sarcophyton acutangulum, has been reported.97 The absolute configuration of the cembrane epoxide 71 has been established98 by its partial synthesis from (+)-carvone. A number of cembranolides including 72 have been obtained99 from the soft coral, Sinularia tenella. Pachyclavulariolide G 73 was isolated100 from the soft coral, Pachyclavularia violacea.A number of jatrophanes have been isolated from Euphorbia species including 74 from E. turczaninowii101 and euphosalicin 75 from E. salicifolia.102 The latter has multi-drug resistance reversing activity in cancers. The tricyclic salicifoline 76 has been isolated103 from the same plant. A revised structure 77 has been proposed104 for the myrsinane, decipinone B which was obtained from E. decipiens. Segetene A 78 has been isolated105 from E. paralias. Neostellerin 79 has been reported106 as a constituent of the Chinese plant, Stellera chamaejasme.
6.1 Taxanes
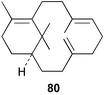
Recent advances in the studies of taxol® and major advances in the genetics of taxol® biosynthesis have been reviewed.107,108 Verticillatriene 80 has been detected109 in the essential oil of Boswellia carterii.Although there are estimated to be about 400 naturally occurring taxoids that are known, new examples continue to be isolated. These include 7-acetyl-10-deacetyltaxol from Taxus canadensis needles,11081 from T. cuspidata,111 some further taxumairols, e.g.82 from the stem bark of T. mairei,11283 from the Himalayan yew, T. wallichiana,113 taxuyunnanine P 84114 and dantaxusin A 85115 from T. yunnanensis. Methods for the large scale separation of taxanes from T. yunnanensis have been described.116 Taxinines NN3 86 and 4 have been reported117 to have multi-drug resistant tumour modulating activity. The crystal structure of the dimethyl sulfoxide solvate of 10-deacetylbaccatin III has been reported.118
7 Miscellaneous diterpenoids
The chabrolols, e.g.87, have been obtained119 from the soft coral, Nephthea chabroli. The prevesols, e.g.88, from the red alga Laurencia obtusa,120 have structures that are reminiscent of bismonoterpenes. Further xenicanes including acalycixeniolide H 89 have been isolated121 from the gorgonian, Acalycigorgia inermis. Continuing investigations on the briaranes of the gorgonian, Briareum excavatum have been reported including work122 on brianthein A 90, which has been shown to reverse multi-drug resistance in human carcinomas, and on briaexcavatolides K 91.123 Milolide A 92 has been obtained124 from the octacoral, Briareum stechei.Examination of the soft corals to obtain a plentiful supply of eleutherobin for preclinical evaluation as an anti-cancer drug led to the isolation of the anti-mitotic compound, caribaeorane 93 from the Caribbean soft coral, Erythropodium caribaeorium.125 The pachyclavulariaenones A–C, e.g.94, have been obtained126 from the soft coral, Pachyclavularia violaceae.
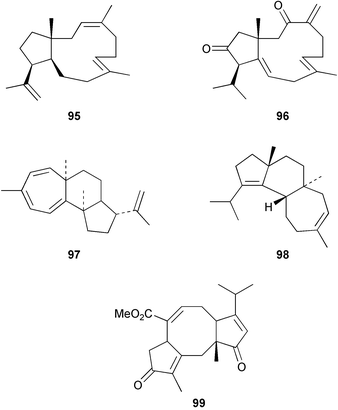
Dolabella-3,7,18-triene 95 was obtained,127 along with some sesquiterpenes, from the essential oil of the rhizomes of Cyperus alopecuroides whilst the dolabellane 96 was isolated128 from Clavularia inflata. 1,3,5,15-Valparatetraene 97 has been obtained129 from Halimium viscosum. The hydrocarbon, cyatha-3,12-diene 98 has been detected130 in the Basidiomycete, Hericium erinaceum where it is a possible intermediate in cyathin biosynthesis. Serpendione 99 has been obtained131 from Hypoestes serpens. The biotransformation of hypoestenone by the fungus, Mucor plumbeus has been examined.132
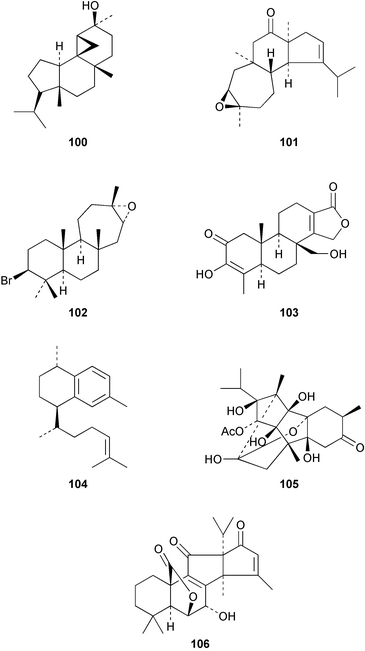
Examination of the aerial parts of Azorella yareta (Umbelliferae) has yielded133 the trichomonocidal diterpene 13β-hydroxyazorellane 100 together with mulinolic acid. Epipolone 101 has been isolated134 from the sponge, Epipolasis reiswigi. The bromoditerpene 102 has been obtained135 from the red alga, Laurencia luzonensis. Amongst the new spongiane diterpenes is zimoclactone B 103 which was isolated136 from Spongia zimocca. The serrulatane, erogorgiaene 104 has been detected137 in Pseudopterogorgia elisabethae. Continued thorough investigations of Persea indica (Lauraceae) for insecticides with the ryanodine skeleton has led to the isolation of garajonone 105.138 An interrelationship between the triquinane diterpenes, laurenene and waihoensene has been reported.139 Some C23 compounds with the apianane skeleton, e.g.106, have been isolated140 from the sage, Salvia officinalis.
References
- J. R. Hanson, Nat. Prod. Rep., 2002, 19, 125 RSC.
- S. A. V. Ivarenga, J. P. Gastmans, G. do Vale Rodriguez, P. R. H. Moreno and V. de Paulo Emerenciano, Phytochemistry, 2001, 56, 583 CrossRef.
- L. Rajbhandari, S. Takamatsu and D. C. Nagle, J. Nat. Prod., 2001, 64, 693 CrossRef.
- G. Culioli, M. Daoudi, A. Ortalo-Magne, R. Valls and L. Piovetti, Phytochemistry, 2001, 57, 529 CrossRef CAS.
- N. Takada, R. Watanabe, K. Suenaga, K. Yamada and D. Uemura, J. Nat. Prod., 2001, 64, 653 CrossRef CAS.
- E. Abdel-Sattar, Monatsh. Chem., 2001, 132, 1095 Search PubMed.
- J. Carroll, E. N. Jonsson, R. Ebel, M. S. Hartman, T. R. Holman and P. Crews, J. Org. Chem., 2001, 66, 6847 CrossRef CAS.
- M. McNally and R. J. Capon, J. Nat. Prod., 2001, 64, 645 CrossRef CAS.
- M. G. Bolster, B. J. M. Jansen and A. de Groot, Tetrahedron, 2001, 57, 56.
- J. Moulines, A. M. Lamidey and V. Desvergnes-Breuil, Synth. Commun., 2001, 31, 749 CrossRef CAS.
- G. Topcu, A. C. Goren, T. Kilic, Y. K. Yildiz and G. Tumen, Fitoterapia, 2001, 71, 1 CrossRef.
- P. P. Mebe, Phytochemistry, 2001, 57, 537 CrossRef CAS.
- A. A. Ahmed, A. A. Mahmoud, U. M. Ahmed, A. A. EI-Bassuony, M. H. Abd.El-Razk, P. W. Pare and J. Karchesy, J. Nat. Prod., 2001, 64, 1365 CrossRef CAS.
- D. A. Cifuente, C. E. Tonn and O. S. Giordano, Nat. Prod. Lett., 2001, 15, 425 Search PubMed.
- S. Sutthivaiyakit, P. Nareeboon, N. Ruangrangsi, S. Ruchirawat, S. Pisutjaroenpong and C. Mahidol, Phytochemistry, 2001, 56, 811 CrossRef CAS.
- S. Roengsumran, A. Petsom, N. Kuptiyanuwat, T. Vilaivan, N. Ngamrojnavanich, C. Chaichantipyuth and S. Phuthong, Phytochemistry, 2001, 56, 103 CrossRef CAS.
- M. J. Uddin, S. Kokubo, K. Ueda, K. Suenaga and D. Uemura, J. Nat. Prod., 2001, 64, 1169 CrossRef CAS.
- M. J. Uddin, S. Kokubo, K. Suenaga, K. Ueda and D. Uemura, Heterocycles, 2001, 54, 1039 Search PubMed.
- P. Xiao, C. Sun, M. Zahid, O. Ishrud and Y. Pan, Fitoterapia, 2001, 72, 837 CrossRef CAS.
- M. Sekiguchi, H. Shigemori, A. Ohsaki and J. Kobayashi, J. Nat. Prod., 2001, 64, 1102 CrossRef CAS.
- M. Ono, M. Yamamoto, T. Yanaka, Y. Ito and T. Nohara, Chem. Pharm. Bull., 2001, 49, 82 CrossRef CAS.
- I. S. Marcos, R. F. Moro, S. Carballares and J. G. Urones, Tetrahedron, 2001, 57, 713 CrossRef CAS.
- S. Tesaki, H. Kikuzaki, S. Yonemori and N. Nakatani, J. Nat. Prod., 2001, 64, 515 CrossRef CAS.
- M. Della Greca, A. Fiorentino, M. Isidori, P. Monaco, F. Temussi and A. Zarrelli, Phytochemistry, 2001, 58, 299 CrossRef CAS.
- T. Cangiano, M. Della Greca, A. Fiorentino, M. Isidori, P. Monaco and A. Zarrelli, Phytochemistry, 2001, 56, 469 CrossRef CAS.
- J. Tanaka, G. Marriott and T. Higa, J. Nat. Prod., 2001, 64, 1468 CrossRef CAS.
- R. Dolmazon, Y. Mahmout and J. M. Bessiere, Flavour Fragrance J., 2001, 16, 100 Search PubMed.
- C. L. Wu, C. J. Wang and M. H. Yin, J. Chin. Chem. Soc. (Taipei), 20011, 48, 241 Search PubMed.
- B. M. Fraga, M. G. Hernandez, P. Gonzalez, M. Lopez and S. Suarez, Tetrahedron, 2001, 57, 761 CrossRef CAS.
- A. S. R. Anganeyulu and V. L. Rao, Nat. Prod. Lett., 2001, 15, 13 Search PubMed.
- T. Konoshima, T. Konishi, M. Tasaki, K. Yamazoe and H. Tokuda, Biol. Pharm. Bull., 2001, 24, 1440 Search PubMed.
- F. Nagashima, M. Suzuki and Y. Asakawa, Fitoterapia, 2001, 72, 83 CrossRef CAS.
- M. Ono, Y. Ito and T. Nohara, Chem. Pharm. Bull., 2001, 49, 1220 CrossRef CAS.
- J. G. Urones, M. J. Sexmero, F. A. Hernandez, A. B. P. P. Basabe and D. Diez, Nat. Prod. Lett., 2001, 15, 387 Search PubMed.
- B. Rodriguez, Magn. Reson. Chem., 2001, 39, 150 CrossRef CAS.
- R. T. Nogueira, G. J. Shepherd, A. Laverde and A. J. Marsaioli, Phytochemistry, 2001, 58, 1153 CrossRef CAS.
- I. Anis, E. Anis, S. Ahmed, G. Mustafa, A. Malik, Z. Amtul and Atta-ur-Rahman, Helv. Chim. Acta., 2001, 84, 649 CrossRef CAS.
- C. Vigor, N. Fabre, I. Fouraste and C. Moulis, Phytochemistry, 2001, 57, 1209 CrossRef CAS.
- A. Ohsaki, M. Ogawa, Y. Imai, T. Shinzato, H. Shigemori and J. Kobayashi, J. Nat. Prod., 2001, 64, 804 CrossRef CAS.
- R. A. Toscano, E. Maldonado, A. Ortega and J. Cardenas, Acta Crystallogr., Sect. C, 2001, 57, 846 CrossRef CAS.
- L. J. Valdes, H. M. Chang, D. C. Visger and M. Koreeda, Org. Lett., 2001, 3, 3935 CrossRef CAS.
- B. Esquivel, R. M. Dominguez and R. A. Toscano, J. Nat. Prod., 2001, 64, 778 CrossRef CAS.
- Y. Takeda, S. Tateoka, T. Matsuda, H. Otsuka, G. Honda, Y. Takaishi, M. Ito, O. K. Khodzhimator and O. A. Ashurmetov, Heterocycles, 2001, 55, 1141 Search PubMed.
- M. Bruno, M. L. Bondi, S. Roselli, F. Piozzi, M. R. Y. Al-Hillo, K. Lamara and S. Ladjel, Eur. J. Org. Chem., 2001, 1669 CrossRef CAS.
- B. Esquivel and E. A. Flores, Heterocycles, 2001, 55, 505 Search PubMed.
- C. Caballero, P. Castanera, F. Ortego, G. Fontana, P. Pierro and G. Savona, Phytochemistry, 2001, 58, 249 CrossRef CAS.
- M. Bruno, F. Piozzi and S. Rosselli, Nat. Prod. Rep., 2002, 19, 357 RSC.
- A. Kolocouris, T. Mavromoustakos, C. Demetzos, A. Terzis and S. G. Grdadolnik, Bioorg. Med. Chem. Lett., 2001, 11, 837 CrossRef CAS.
- N. H. Oberlies, J. P. Burgess, H. S. Navarro, R. E. Pinos, D. D. Soejarto, N. R. Farnsworth, A. D. Kinghorn, M. C. Wani and M. E. Wall, J. Nat. Prod., 2001, 64, 497 CrossRef CAS.
- P. Tuntiwachwuttikul and W. C. Taylor, Chem. Pharm. Bull., 2001, 49, 854 CrossRef CAS.
- M. del Rayo Camacho, J. D. Phillipson, S. L. Croft, G. C. Kirby, D. C. Warhurst and P. N. Solis, Phytochemistry, 2001, 56, 203 CrossRef CAS.
- A. Ulubelen, S. Oksuz, G. Topcu, A. C. Goren, C. Bozok-Johansson, C. Celik, G. Kokdil and W. Voelter, Nat. Prod. Lett., 2001, 15, 307 Search PubMed.
- X.-D. Lu, S. H. Wu, Y. B. Ma and D. G. Wu, Phytochemistry, 2001, 57, 131 CrossRef.
- T. Ling, C. Chowdhury, B. A. Kramer, B. G. Vong, M. A. Palladino and E. A. Theodorakis, J. Org. Chem., 2001, 66, 8843 CrossRef CAS.
- M. del Carmen Apreda Rojas, F. H. Cano and B. Rodriguez, J. Nat. Prod., 2001, 64, 899 CrossRef CAS.
- R. W. Denton, W. W. Harding, C. I. Anderson, H. Jacobs, S. McLean and W. F. Reynolds, J. Nat. Prod., 2001, 64, 829 CrossRef CAS.
- S. Awale, Y. Tezuka, A. H. Banskota, K. Kouda, K. M. Tun and S. Kadota, J. Nat. Prod., 2001, 64, 592 CrossRef CAS.
- G. Romussi, G. Ciarallo, A. Bisio, N. Fontana, F. de Simone, N. de Tommasi, N. Mascolo and L. Pinto, Planta Med., 2001, 67, 153 CrossRef CAS.
- Y. H. Kuo and S. C. Chien, Chem. Pharm. Bull., 2001, 49, 1033 CrossRef CAS.
- S. Srivastava and D. K. Kulshreshtha, Indian J. Chem., Sect. B, 2001, 40, 348 Search PubMed.
- Y. H. Kuo and C. H. Chen, Tetrahedron Lett., 2001, 42, 2985 CrossRef CAS.
- M. T. Lin, L. C. Chen, C. K. Chen, K. C. S. C. Liu and S. S. Lee, J. Nat. Prod., 2001, 64, 707 CrossRef CAS.
- H. Ohtsu, R. Tanaka, Y. In, S. Matsunaga, H. Tokuda and H. Nishino, Planta Med., 2001, 67, 55 CrossRef CAS.
- X. Pan, G. Niu and H. Liu, J. Chromatogr., 2001, 922, 371 CrossRef CAS.
- H. C. Lin, H.-Y. Ding and W. L. Chang, J. Nat. Prod., 2001, 64, 648 CrossRef CAS.
- A. Ulubelen, S. Oksuz, G. Topcu, A. C. Goren and W. Voelter, J. Nat. Prod., 2001, 64, 549 CrossRef CAS.
- M. Li, J. S. Zhang and M. Q. Chen, J. Nat. Prod., 2001, 64, 971 CrossRef CAS.
- C. Y. Ragasa, V. F. Templora and J. A. Rideout, Chem. Pharm. Bull., 2001, 49, 927 CrossRef CAS.
- Y. Narukawa, N. Shimizu, K. Shimotohno and T. Takeda, Chem. Pharm. Bull., 2001, 49, 1182 CrossRef CAS.
- M. Sairafianpour, J. Christensen, D. Staerk, B. A. Budnik, A. Kharazmi, K. Bagherzadeh and J. W. Jaroszewski, J. Nat. Prod., 2001, 64, 1398 CrossRef CAS.
- U. Kolak, S. Ari, H. Birman, S. Hasancebi and A. Ulubelen, Planta Med., 2001, 67, 761 CAS.
- F. Schaller, J. L. Wolfender, K. Hostettman and S. Mavi, Helv. Chim. Acta, 2001, 84, 222 CrossRef CAS.
- R. W. Jiang, P. P. H. But, S. C. Ma and T. C. W. Mak, Phytochemistry, 2001, 57, 517 CrossRef CAS.
- R. W. Jiang, P. P. H. But, S. C. Ma and T. C. W. Mak, J. Nat. Prod., 2001, 64, 1266 CrossRef CAS.
- R. W. Jiang, P. P. H. But, S. C. Ma and T. C. W. Mak, J. Chem. Soc., Perkin Trans. 1, 2001, 2920 RSC.
- H. Kigoshi, T. Ichino, K. Yamada, Y. Ijuin, S. F. Makita and D. Uemura, Chem. Lett., 2001, 581.
- C. K. Lee and Y. S. Cheng, J. Nat. Prod., 2001, 64, 511 CrossRef CAS.
- H. Duan, Y. Takaishi, H. Momota, Y. Ohmoto, T. Taki, M. Tori, S. Takaoka, Y. Jia and D. Li, Tetrahedron, 2001, 57, 8413 CrossRef CAS.
- M. Bruno, S. Rosselli, I. Pibiri, F. Piozzi, M. L. Bondi and M. S. J. Simmonds, Phytochemistry, 2001, 58, 463 CrossRef CAS.
- P. T. Son, P. M. Giang and W. C. Taylor, Aust. J. Chem., 2000, 53, 1003 CrossRef CAS.
- L. Q. Wang, G. W. Qin, S. N. Chen and C. J. Li, Fitoterapia, 2001, 72, 779 CrossRef CAS.
- H. Zhou, L. He, Y. Pan and O. Ishrud, Fitoterapia, 2001, 72, 946 CrossRef CAS.
- B. Li, Y. Pan and W. Pan, Helv. Chim. Acta, 2001, 84, 3418 CrossRef CAS.
- A. J. Hou, Q. S. Zhao, M. L. Li, B. Jiang, Z. W. Lin, H. D. Sun, Y. P. Zhou, Y. Lu and Q. T. Zheng, Phytochemistry, 2001, 58, 179 CrossRef CAS.
- A. J. Hou, H. Yang, Y. Z. Liu, Q. S. Zhao, Z. W. Lin and H. D. Sun, Chin. J. Chem., 2001, 19, 365 Search PubMed.
- H. D. Sun, S.-X. Qiu, E. B. Lobkovsky, L.-Z. Lin, N. R. Farnsworth, J. Clardy and H. S. Fong, Tetrahedron, 2001, 57, 65 CrossRef CAS.
- B. Li and X. Tian, Phytochemistry, 2001, 58, 543 CrossRef CAS.
- P. J. Cox, Y. Meng, S. D. Sarker, Q. Deng and G. Xu, Acta Crystallogr., Sect. C, 2001, 57, 203 CrossRef CAS.
- H. Oikawa, H. Toshima, S. Ohashi, W. A. Konig, H. Kenmoku and T. Sassa, Tetrahedron Lett., 2001, 42, 2329 CrossRef CAS.
- M. Arno, M. A. Gonzalez, M. L. Marin and R. J. Zaragoza, Magn. Reson. Chem., 2001, 39, 414 CrossRef CAS.
- G. O. Buchanan and P. B. Reese, Phytochemistry, 2001, 56, 141 CrossRef CAS.
- N. B. Perry, E. J. Burgess, S.-H. Baek and R. T. Weavers, Org. Lett., 2001, 3, 4243 CrossRef CAS.
- M. Nakayama, M. Koshioka, H. Matsui, H. Ohara, L. N. Mander, S. K. Leitch, B. Twitchin, P. Kraft-Klavnzer, R. P. Pharis and T. Yokota, Phytochemistry, 2001, 57, 749 CrossRef CAS.
- M. Kurumatani, K. Yagi, T. Murata, M. Tezuka, L. N. Mander and M. Nishiyama, Biosci. Biotechnol. Biochem., 2001, 65, 2311 Search PubMed.
- O. Urrutia, P. Hedden and M. C. Rojas, Phytochemistry, 2001, 56, 505 CrossRef CAS.
- M. Huanpu, P. S. Blake, G. Browning and J. M. Taylor, Phytochemistry, 2001, 56, 67 CrossRef CAS.
- K. Mada, T. Oui and T. Kusumi, Spectroscopy, 2001, 15, 177 Search PubMed.
- T. Zhang, Z. Liu and Y. Li, Synthesis, 2001, 393 CrossRef CAS.
- C.-W. Lin, J.-Y. Su, L. M. Zheng, S. J. Qi, W. L. Pen and A. L. Xu, Chin. J. Org. Chem., 2001, 21, 56 Search PubMed.
- J. H. Sheu, G. H. Wang, P. J. Sung, C. Y. Duh and M. C. Chiang, Tetrahedron, 2001, 57, 7639 CrossRef CAS.
- L. G. Liu and R. X. Tan, J. Nat. Prod., 2001, 64, 1064 CrossRef CAS.
- J. Hohmann, F. Evanics, G. Dombi, J. Molnar and P. Szabo, Tetrahedron, 2001, 57, 211 CrossRef CAS.
- J. Hohmann, F. Evanics, G. Dombi and P. Szabo, Tetrahedron Lett., 2001, 42, 6581 CrossRef CAS.
- M. Zahid, S. R. Husani, M. Abbas, Y. Pan, A. R. Jassbi and V. U. Ahmad, Helv. Chim. Acta, 2001, 84, 1980 CrossRef CAS.
- S. A. M. Abdelgaleil, S. M. I. Kassem, M. Doe, M. Baba and M. Nakatani, Phytochemistry, 2001, 58, 1135 CrossRef CAS.
- F. Baomin, P. Yuehu and H. Huiming, J. Chin. Pharm. Sci., 2001, 10, 65 Search PubMed.
- D. G. I. Kingston, Chem. Commun., 2001, 867 RSC.
- F. Walker and R. Croteau, Phytochemistry, 2001, 58, 1 CrossRef.
- S. Basar, A. Koch and W. A. Konig, Flavour Fragrance J., 2001, 16, 315 Search PubMed.
- J. Zhang, F. Sauriol, O. Mamer, X. L. You, M. A. Aloui-Jamali, G. Batist and L. O. Zamir, J. Nat. Prod., 2001, 64, 450 CrossRef CAS.
- Q. W. Shi, T. Oritani, H. Kiyuta and R. Murakami, Nat. Prod. Lett., 2001, 15, 55 Search PubMed.
- Y. C. Shen, C. V. S. Prakash, Y. J. Chen, J. F. Hwang, Y. H. Kuo and C. Y. Chen, J. Nat. Prod., 2001, 64, 950 CrossRef CAS.
- J. K. Prasain, P. Stefanowicz, T. Kiyota, F. Habeichi and Y. Konishi, Phytochemistry, 2001, 58, 1167 CrossRef CAS.
- S. Li, H. Zhang, P. Yao, H. Sun and H. H. S. Fong, Phytochemistry, 2001, 58, 369 CrossRef CAS.
- Y. Shinozaki, N. Fukamiya, M. Fukushima, M. Okano and T. Nehira, J. Nat. Prod., 2001, 64, 1073 CrossRef CAS.
- J. Xue, C. Y. Cao, J. M. Chen. H. S. Bu and H. M. Wu, Chin. J. Chem., 2001, 19, 82 Search PubMed.
- J. Sakai, H. Sasaki, K. Kosugi, S. Zhang, N. Hirata, K. Hirose, A. Tomida, T. Tsuruo and M. Ando, Heterocycles, 2001, 54, 999 Search PubMed.
- J. K. Harper, N. K. Dalley, A. E. Mulgrew, F. G. West and D. M. Grant, Acta Crystallogr., Sect. C, 2001, 57, 64 CrossRef CAS.
- W. H. Zhang, I. D. Williams and C. T. Che, Tetrahedron Lett., 2001, 42, 4681 CrossRef CAS.
- N. Mihopoulos, C. Vagias, E. Mikros, M. Scoullos and V. Roussis, Tetrahedron Lett., 2001, 42, 3749 CrossRef CAS.
- J. R. Rho, M. S. Oh, K. H. Jang, K. W. Cho and J. Shin, J. Nat. Prod., 2001, 64, 540 CrossRef CAS.
- S. Aoki, M. Okano, K. Matsui, T. Itoh, R. Satari, S. Akiyama and M. Kobayashi, Tetrahedron, 2001, 57, 8951 CrossRef CAS.
- P. J. Sung, J. H. Su, C. Y. Duh, M. Y. Chiang and J. H. Sheu, J. Nat. Prod., 2001, 64, 318 CrossRef CAS.
- J. H. Kwak, F. J. Schmitz and G. C. Williams, J. Nat. Prod., 2001, 64, 754 CrossRef CAS.
- R. Britton, M. Roberge, H. Berisch and R. J. Andersen, Tetrahedron Lett., 2001, 42, 2953 CrossRef CAS.
- G. H. Wang, J. H. Sheu, M. Y. Chiang and T. J. Lee, Tetrahedron Lett., 2001, 42, 2333 CrossRef CAS.
- M. M. Sonwa and W. A. Konig, Phytochemistry, 2001, 56, 321 CrossRef CAS.
- C. Y. Duh, M. C. Cha, S. K. Wang, H. J. Chen and A. A. H. El Gamal, J. Nat. Prod., 2001, 64, 1028 CrossRef CAS.
- J. M. L. Rodilla, D. I. de Mendonca, M. I. G. Ismael, J. A. Figueiredo, M. L. A. Silva and E. Lopes, Nat. Prod. Lett., 2001, 15, 401 Search PubMed.
- H. Kenmoku, N. Kato, M. Shimada, M. Omoto, A. Mori and W. Mitsuhashi, Tetrahedron Lett., 2001, 42, 7439 CrossRef CAS.
- B. Andriamihaja, M. T. Martin, P. Rasoanaivo and F. Frappier, J. Nat. Prod., 2001, 64, 217 CrossRef CAS.
- K. A. El Sayed, J. Nat. Prod., 2001, 64, 373 CrossRef CAS.
- L. A. L. Loyola, J. Borquez, G. Morales, J. Araya, J. Gonzalez, I. Neira, H. Sagua and A. San Martin, Phytochemistry, 2001, 56, 177 CrossRef CAS.
- A. D. Rodriguez and B. Vera, J. Org. Chem., 2001, 66, 6364 CrossRef CAS.
- M. Kuniyoshi, M. S. Marma, T. Higa, G. Bernardinelli and C. W. Jefford, J. Nat. Prod., 2001, 64, 696 CrossRef CAS.
- L. M. Zeng, Z. Guan, J. Y. Su, X. L. Feng and J. W. Cai, Huaxue Xuebao, 2001, 59, 1675 Search PubMed.
- A. D. Rodriguez and C. Ramfrez, J. Nat. Prod., 2001, 64, 100 CrossRef CAS.
- B. M. Fraga, D. Terrero, G. Gutierrez and A. Gonzalez-Coloma, Phytochemistry, 2001, 56, 315 CrossRef CAS.
- A. F. O. Chin, D. B. Clarke, S. F. R. Hinckley, N. B. Perry and R. T. Weavers, Aust. J. Chem., 2001, 54, 205 CrossRef CAS.
- K. Miura, H. Kikuzaki and N. Nakatani, Phytochemistry, 2001, 58, 1171 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |