New europium(III) complexes containing hybrid ligands with hard and soft complexation centres
Luca
Prodi
*a,
Marco
Montalti
a,
Nelsi
Zaccheroni
a,
Guillaume
Pickaert
b,
Loïc
Charbonnière
b and
Raymond
Ziessel
*b
aDipartimento di Chimica “G. Ciamician”, Università degli Studi di Bologna, Via Selmi 2, 40126, Bologna, Italy. E-mail: lprodi@ciam.unibo.it; Fax: 39 051 2099456
bLaboratoire de Chimie Moléculaire. École de Chimie, Polymères, Matériaux, 25 Rue Becquerel, BP 08, 67087, Strasbourg Cedex 2, France. E-mail: ziessel@chimie.u-strasbg.fr; Fax: 33 390 242689
First published on 20th November 2002
Abstract
The terpyridine–phenylphosphine oxide ligands 2–4 and their Eu3+ complexes have been synthesized and studied. In acetonitrile solutions at room temperature, the complexes show an absorption band in the 300–350 nm region, indicating that the terpy units are coordinated to the metal. In these conditions, the complexes show a metal-centred luminescence, indicative of an energy transfer from the terpy subunits to the Eu centre. The efficiency of such an energy transfer process drastically depends on the nature of the anions: a 20-fold increase of the luminescence intensity could be observed upon adding up to 3 equiv. of nitrate to a solution containing equimolar amounts of ligand 2 and Eu(OTf)3. In other solvents, such as methanol, DMF, and DMSO, the metal ion is coordinated through the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P
O group; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of the energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.
O group can also be performed on adding Zn2+ ions; the luminescence typical of the lanthanide ion disappears, while the typical fluorescence of Zn–terpy complexes is observed.
Introduction
As a result of their unique electronic and photonic properties, lanthanide complexes have found a broad variety of analytical applications, ranging from water proton relaxation agents for NMR imaging1 to luminescent probes in time resolved fluoroimmunoassays.2 In the past decade, in the context of the increasing use of luminescence spectroscopy for facing complex analytical problems in fields of large social and economical impact such as environmental sciences, medical diagnostics, and cell biology,3–6 lanthanide complexes have been the focus of much attention for their possible application as labels and sensors.2,7–11 Eu3+ and Tb3+ ions, which possess luminescence lifetimes in the microsecond–millisecond range,8 offer the opportunity to take advantage of time-resolved spectroscopy, which can efficiently exclude autofluorescence and light scattering, the most relevant interferences, particularly when biological material is involved. To obtain an efficient luminescent frame, the ligand is expected (i) to form stable complexes with the Ln ions in coordinating solvents, (ii) to shield the ion from deactivating solvent molecules, and (iii) to absorb light effectively and to transfer this energy to the metal centre with high efficiency. This latter feature is of particular importance to overcome the intrinsic drawback of low absorption coefficients of the Ln ions.8–10 In previous works,12–14 we have synthesised and studied Eu3+ and Tb3+ complexes of the bis-bipyridine–phenylphosphine oxide ligand 1 (Scheme 1). In particular, we have demonstrated that these complexes, in which the metal ion has a coordination sphere incompletely saturated, can interact with incoming anions, and that this interaction leads to a large increase in luminescence intensity. All these findings suggested that lanthanide complexes of 1 could be very promising for the realisation of luminescent sensors for anions. In this context, trying to synthesise more efficient chemosensors, we have synthesised the ligands 2–4, containing two different coordinating groups: the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O function, which in ligand 1 operated as a valuable anchoring function for lanthanide cations, and the terpy ligand, which was expected to work as an efficient photon antenna. A 20-fold increase of the luminescence intensity could be observed on adding up to 3 equiv. of nitrate to a solution containing [Eu(2)3](OTf)3, an increase which is even larger than that observed for [Eu(1)](OTf)3 under the same conditions. In addition, the use of solvents such as methanol, DMF, and DMSO, or the addition of zinc ions, promotes the translocation of the Eu ion from the terpy moiety to the P
O function, which in ligand 1 operated as a valuable anchoring function for lanthanide cations, and the terpy ligand, which was expected to work as an efficient photon antenna. A 20-fold increase of the luminescence intensity could be observed on adding up to 3 equiv. of nitrate to a solution containing [Eu(2)3](OTf)3, an increase which is even larger than that observed for [Eu(1)](OTf)3 under the same conditions. In addition, the use of solvents such as methanol, DMF, and DMSO, or the addition of zinc ions, promotes the translocation of the Eu ion from the terpy moiety to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, leading to a dramatic quenching of the Eu centred luminescence. All these results are of significant relevance for the design of more and more efficient chemosensors based on lanthanide cations.
O group, leading to a dramatic quenching of the Eu centred luminescence. All these results are of significant relevance for the design of more and more efficient chemosensors based on lanthanide cations.
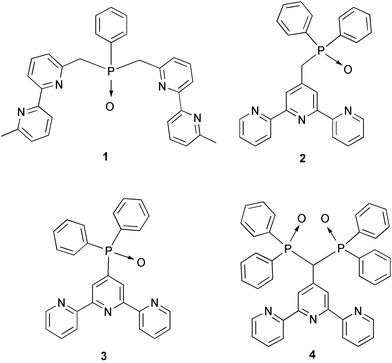 | ||
| Scheme 1 | ||
Experimental
Synthesis and complex characterisation
Ligands 2,15316 and 417 were prepared and purified according to the literature procedures. The Eu(III) complexes were prepared following a general procedure in which a solution of Eu(NO3)3·6H2O (1 equiv.) dissolved in 2 mL anhydrous THF was added dropwise to a stirred solution of the ligands (1 equiv., 100 mg scale) in anhydrous THF (10 mL). During the gradual addition of the lanthanide solution a white precipitate was formed. After additional stirring at rt for one night, the solid was recovered by centrifugation and washed with THF and diethyl ether. The crude sample was dissolved in a minimum amount of MeOH, filtered over celite. Slow diffusion of THF resulted in the precipitation of a white solid. The mother liquor was decanted, the solid was washed twice with 5 mL Et2O and dried under vacuum, affording the analytically pure complexes in fair yields. The triflate complex was prepared under similar conditions by adding Eu(OTf)3 (1 equiv.) as a solid in an acetonitrile solution (10 mL) of ligand 2 (3 equiv. on 50 mg scale). After one hour stirring at rt, the solution was filtered over celite and diethyl ether was allowed to diffuse into the solution. The resulting white compound is recrystallised twice in a mixture of acetonitrile/diethyl ether.[Eu(2)](NO3)3·3H2O 5: (93%). IR (KBr pellets, cm−1): 2910 (m), 1611 (s), 1574 (w), 1484 (m), 1426 (m), 1384 (m), 1323 (m), 1155 (s), 1122 (m), 1095 (m), 1013 (m), 723 (m), 628 (m), 524 (m). Anal. calcd. for C28H22EuN3OP·3H2O (Mr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785.45
785.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.05): C, 40.06; H, 3.36; N, 10.01. Found: C, 39.83; H, 3.12; N, 9.73%.
54.05): C, 40.06; H, 3.36; N, 10.01. Found: C, 39.83; H, 3.12; N, 9.73%.
[Eu(3)](NO3)3·3H2O 6: (90%). IR (KBr pellets, cm−1): 1599 (s), 1480 (vs), 1437 (s), 1404 (s), 1277 (vs), 1225 (m), 1164 (s), 1122 (s), 1097 (m), 1028 (m), 1010 (m), 792 (s), 729 (s). Anal. calcd. for C27H20EuN3OP·3H2O (Mr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 771.43
771.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.05): C, 39.29; H, 3.17; N, 10.18. Found: C, 38.96; H, 2.89; N, 9.98%.
54.05): C, 39.29; H, 3.17; N, 10.18. Found: C, 38.96; H, 2.89; N, 9.98%.
[Eu(4)](NO3)3·4H2O 7: (89%). IR (KBr pellets, cm−1): 1610 (m), 1598 (m), 1476 (vs), 1440 (m), 1425 (m), 1287 (s), 1150 (s), 1125 (m), 1029 (m), 1015 (m), 801 (s), 743 (m), 726 (m). Anal. calcd. for C10H31EuN3O2P2·4H2O (Mr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985.63
985.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72.06): C, 45.42; H, 3.72; N, 7.95. Found: C, 45.18; H, 3.52; N, 7.62%.
72.06): C, 45.42; H, 3.72; N, 7.95. Found: C, 45.18; H, 3.52; N, 7.62%.
[Eu(2)3](CF3SO3)3·3H2O 8: (72%). IR (KBr pellets, cm−1): 1661 (m), 1617 (m), 1602 (m), 1466 (m), 1487 (m), 1429 (s), 1300 (s), 1217 (s), 1172 (s), 1123 (m), 1092 (m), 1026 (vs), 796 (m), 727 (m). Anal. calcd. for C87H66EuN9O12F9S3P3·3H2O (Mr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1941.59
1941.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.05): C, 52.36; H, 3.64; N, 6.32. Found: C, 52.05; H, 3.36; N, 6.02%.
54.05): C, 52.36; H, 3.64; N, 6.32. Found: C, 52.05; H, 3.36; N, 6.02%.
Spectroscopic measurements
The solvents used for photophysical measurements were acetonitrile, DMSO, methanol, and DMF from Merck (UVASOL) without further purification. Absorption spectra were recorded with a Perkin Elmer lambda 40 spectrophotometer. UV-Vis spectrophotometric titrations were carried out in acetonitrile according to previously published procedures.14 Uncorrected emission, and corrected excitation spectra were obtained with a Fluorolog spectrofluorimeter equipped with a Hamamatsu R928 phototube. Phosphorescence lifetimes were obtained with a Perkin Elmer LS 50 spectrofluorimeter. Luminescence quantum yields (uncertainty![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) were determined (phosphorescence mode, td
15%) were determined (phosphorescence mode, td![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, tg
0, tg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ms) using [Ru(bipy)3]2+
(Φ
10 ms) using [Ru(bipy)3]2+
(Φ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.028 in aerated water18) as standard. In order to allow comparison of emission intensities, corrections for instrumental response, inner filter effects19 and phototube sensitivity were performed. A correction for differences in the refractive indices was introduced when necessary.
0.028 in aerated water18) as standard. In order to allow comparison of emission intensities, corrections for instrumental response, inner filter effects19 and phototube sensitivity were performed. A correction for differences in the refractive indices was introduced when necessary.
Results and discussion
Photophysical properties
The photophysical properties of the europium complexes in acetonitrile solutions are gathered in Table 1. The absorption spectra of the ligands are largely dominated by the very intense π–π* transitions centred on the terpyridine moiety, which cover the much less intense transitions centred on the phenyl rings (Fig. 1 for 2).| Absorption | Luminescence | ||||
|---|---|---|---|---|---|
| Complex | λ max/nm | ε/M−1 cm−1 | λ max/nm | Φ | τ/ms |
| 5 | 325 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
617 | 0.26 | 1.75 |
| 6 | 330 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
617 | 0.31 | 1.29 |
| 7 | 325 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
617 | 0.041 | 1.55 |
| 8 | 330 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
617 | 0.004 | 0.93 |
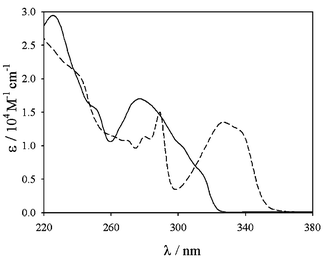 | ||
Fig. 1 Absorption spectra of the ligand 2
(—) and of it Eu3+ complex 5
(![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) in acetonitrile solution at room temperature. ) in acetonitrile solution at room temperature. | ||
The coordination with a metal ion is expected to change dramatically the absorption spectrum of the terpy unit,20 this is indeed what can be observed when the Eu complexes 5–8 are dissolved in acetonitrile solution (see Fig. 1 for 5). In this solvent, a lowering of the absorbance in the 260–300 nm region could be observed on passing from the ligand to the metal complexes, with a concomitant appearance of a new band in the 300–350 nm region. These changes are the result of the displacement of the π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π* transitions centred on the terpy moieties associated with the coordination of a metal ion.20 In contrast, in methanol, DMF, and DMSO only a very weak shoulder appears in this spectral region, while the predominant band remains at ca. 280 nm. This is a clear indication that in acetonitrile the terpy units are mainly coordinated to a metal ion, while this occurs only to a much lesser extent in the other solvents used, the metal ion being coordinated preferentially via the P
π* transitions centred on the terpy moieties associated with the coordination of a metal ion.20 In contrast, in methanol, DMF, and DMSO only a very weak shoulder appears in this spectral region, while the predominant band remains at ca. 280 nm. This is a clear indication that in acetonitrile the terpy units are mainly coordinated to a metal ion, while this occurs only to a much lesser extent in the other solvents used, the metal ion being coordinated preferentially via the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. For instance, in d4-methanol, NMR investigations clearly showed that the methylene doublet at δ
O groups. For instance, in d4-methanol, NMR investigations clearly showed that the methylene doublet at δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.84 ppm and the phenyl groups at δ
3.84 ppm and the phenyl groups at δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.55–7.25 ppm for the free ligand are significantly shifted upfield and broadened due to coordination of the paramagnetic europium centre.21 Concomitantly, all the resonance signals belonging to the terpy protons remain well resolved and unshifted versus the free ligand at δ
7.55–7.25 ppm for the free ligand are significantly shifted upfield and broadened due to coordination of the paramagnetic europium centre.21 Concomitantly, all the resonance signals belonging to the terpy protons remain well resolved and unshifted versus the free ligand at δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.33
8.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.09 ppm. In addition, new very weak peaks spread over all the NMR window (from 17 to −1 ppm) appeared in the spectrum, but they could not be unambiguously attributed. This solvent dependent coordination of the terpyridine unit to the metal is also supported by the analysis of the luminescence spectra. The excitation of the ligand in the terpy absorption bands leads to a weak fluorescence with λmax at 337 nm. This band is completely quenched in complexes 5–8 when dissolved in acetonitrile solution and the typical luminescence of the Eu(III) ions shows up. Excitation spectra performed looking at the metal centred luminescence match in all cases the absorption spectra in acetonitrile, unambiguously indicating the presence of an energy transfer process from the coordinated terpy to the metal centre. Furthermore, the high metal centred luminescence quantum yields shown by 5 and 6 upon ligand excitation (Table 1) indicates that the efficiency of the energy transfer process in these cases is particularly high.
7.09 ppm. In addition, new very weak peaks spread over all the NMR window (from 17 to −1 ppm) appeared in the spectrum, but they could not be unambiguously attributed. This solvent dependent coordination of the terpyridine unit to the metal is also supported by the analysis of the luminescence spectra. The excitation of the ligand in the terpy absorption bands leads to a weak fluorescence with λmax at 337 nm. This band is completely quenched in complexes 5–8 when dissolved in acetonitrile solution and the typical luminescence of the Eu(III) ions shows up. Excitation spectra performed looking at the metal centred luminescence match in all cases the absorption spectra in acetonitrile, unambiguously indicating the presence of an energy transfer process from the coordinated terpy to the metal centre. Furthermore, the high metal centred luminescence quantum yields shown by 5 and 6 upon ligand excitation (Table 1) indicates that the efficiency of the energy transfer process in these cases is particularly high.
In contrast, the quantum yields of 7 and 8 are considerably lower, although the excited state lifetimes are of the same order of magnitude. As far as complex 7 is concerned, it is to be noted that the ligand presents greater steric hindrance, and this is expected to have an effect on the coordination mode of the terpy ligand. It has been in fact already shown14 that even small differences in the distance between the lanthanide ion and the nitrogen atoms of a polypyridine unit can have a large effect on the efficiency of the energy transfer process, thus affecting the luminescence quantum yield but not its excited state lifetime. On the other hand, it is worth pointing out that 8 differs from 5 in the nature of the counter anions, which evidently play a significant role in the overall quantum yield, as already found for 1.13,14 This conclusion finds further support in titration experiments (vide infra).
As reported for the absorption spectra, a completely different behaviour was observed in methanol, DMF, and DMSO. In these cases too, the typical metal centred luminescence could be observed, but with a much lower intensity. In contrast with what was observed in acetonitrile, the excitation spectra in these solvents do not match the absorption ones but, rather, they are very similar to the excitation and absorption spectra recorded in acetonitrile solutions. This finding is a clear indication that only the small percentage of terpy moieties directly bound to the europium ions can transfer to them the excitation energy with good efficiency, leading, as a final step, to the metal centred luminescence. In solvents containing oxygen atoms, the metal ion is coordinated through the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the efficiency of the energy transfer process from the uncoordinated terpys becomes extremely low, suggesting a negligible electronic interaction between the chromophore and the europium ion when they are not directly connected.
O group and the efficiency of the energy transfer process from the uncoordinated terpys becomes extremely low, suggesting a negligible electronic interaction between the chromophore and the europium ion when they are not directly connected.
It is also to be noted that all the complexes 5–8 show a very intense metal centred luminescence in the solid state (Fig. 2), with lifetimes of 1.47, 1.50, 1.44, and 1.44 ms, respectively. The analysis of the emission spectra is in agreement with the presence of only one, highly asymmetric luminescent species. The excitation spectra are very similar to those obtained in acetonitrile solution and also show the presence of a band in the 300–340 nm region, leading to the conclusion that in the solid state, excitation also occurs through the coordinated terpyridines.
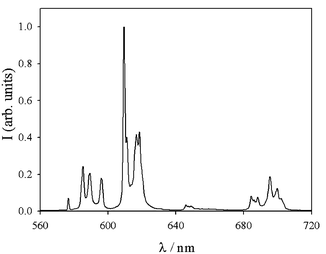 | ||
Fig. 2 Luminescence spectrum (λexc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 nm, td 330 nm, td![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, tg 0, tg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ms) of complex 8 in the solid state at room temperature. 10 ms) of complex 8 in the solid state at room temperature. | ||
Titration experiments
Because of the presence of two different kinds of coordinating groups inside the various ligands, we thought it could be of interest to observe the photophysical properties of acetonitrile solutions with different stoichiometric ratios of the ligand and the Eu ions in the presence of varying counterions. For the same reason, we thought it would be valuable to observe the behaviour of complex 6 upon addition of other metal ions, and in particular of Zn2+, which has good affinity for the terpy moiety.Titration experiments of ligand 2 with nitrate or trifluoromethanesulfonate (OTf) salts of Eu(III) gave interesting insights into the understanding of the nature of the complexes formed and their photophysical properties.
Addition of europium nitrate to an acetonitrile solution of 2 causes, as expected, the appearance in the absorption spectrum of the typical band of coordinated terpys (Fig. 3). Gradual changes of the absorbance are observed until one equivalent of metal ion is added; afterwards a plateau is reached, as can be seen in Fig. 4.
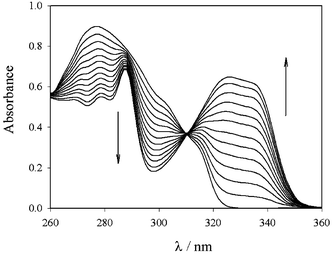 | ||
| Fig. 3 Absorption spectra of the ligand 2 upon addition of increasing amounts (from 0 to 1 equivalent) of Eu(NO3)3. | ||
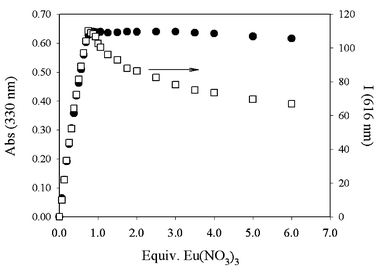 | ||
| Fig. 4 Absorption (330 nm) and corrected intensity (616 nm) of an acetonitrile solution of 2 and increasing amounts of Eu(NO3)3. | ||
The pattern of the luminescence intensity presents some differences (Fig. 4). The maximum of the metal centred luminescence is reached again after the addition of one equivalent of metal ion, but the intensity decreases slowly if a molar excess is added. For addition of 1 equiv. of Eu ions the luminescence lifetime is essentially coincident with that found upon dissolving 5 directly in acetonitrile (1.75 ms), while it is lower (1.10 ms) when a large excess of the salt is added.
From the analysis of the absorption and emission spectra upon addition of Eu nitrate, the formation of three different complexes can be evidenced and the following cumulative association constants could be obtained for the different equilibria:
Eu(NO3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu(2)](NO3)3 logβ11 [Eu(2)](NO3)3 logβ11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.9 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 | (1) |
2 Eu(NO3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu2(2)](NO3)6 logβ21 [Eu2(2)](NO3)6 logβ21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.2 11.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 | (2) |
Eu(NO3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu(2)2](NO3)3 logβ12 [Eu(2)2](NO3)3 logβ12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.1 11.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 | (3) |
According to the above proposed model, the calculated spectra of the species formed can be obtained and are shown in Fig. 5. Whatever the species, the displacement of the π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π* transition indicated that the terpy moieties are coordinated by a europium atom.
π* transition indicated that the terpy moieties are coordinated by a europium atom.
![Calculated UV-Vis spectra of the species formed during the titration of 2 with [Eu(NO3)3].](/image/article/2003/NJ/b207056c/b207056c-f5.gif) | ||
| Fig. 5 Calculated UV-Vis spectra of the species formed during the titration of 2 with [Eu(NO3)3]. | ||
A very different pattern was instead observed upon addition of the Eu(OTf)3 salt. In the absorption spectrum we observed a gradual change up to half an equivalent, and small changes afterwards (Fig. 6). The observed spectral changes (Fig. 7) support terpy coordination as found with nitrate salts. On the other hand, the metal centred luminescence reaches a maximum before the addition of half an equivalent, and then it sharply decreases until one equivalent is added, where a plateau is reached (Fig. 6).
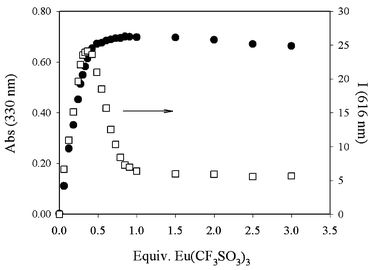 | ||
| Fig. 6 Absorption (330 nm) and corrected intensity (616 nm) of an acetonitrile solution of 2 and increasing amounts of Eu(CF3SO3)3. | ||
![Calculated UV-Vis spectra of the species formed during the titration of 2 with [Eu(OTf)3].](/image/article/2003/NJ/b207056c/b207056c-f7.gif) | ||
| Fig. 7 Calculated UV-Vis spectra of the species formed during the titration of 2 with [Eu(OTf)3]. | ||
From the analysis of the absorption and emission spectra upon addition of Eu(OTf)3, the following model containing three different equilibria was used to calculate cumulative association constants:
Eu(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu(2)](OTf)3 logβ11 [Eu(2)](OTf)3 logβ11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.7 8.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 | (4) |
Eu(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu(2)2](OTf)3 logβ12 [Eu(2)2](OTf)3 logβ12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.7 14.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 | (5) |
Eu(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 2 3 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ↔ ↔![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Eu(2)3](OTf)3 logβ13 [Eu(2)3](OTf)3 logβ13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.9 19.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 | (6) |
It is interesting to note, however, that in this case, one of the equilibria is different, evidencing the formation of a species with three ligands per metal ion. This species, among the different complexes formed during this titration, is the one with the longer lifetime and the higher luminescence quantum yield, while the complex with a 1∶1 stoichiometry is the species presenting the weaker intensity. It is also to note that β11 and β12 are higher in this case compared to those found using europium nitrate.
Furthermore, it is worth noting the large difference in luminescence intensity observed upon addition of one equivalent of europium salt, depending on the nature of the counter anion. This behaviour is indeed very similar to that shown by 1. To test the dependence of the luminescence intensity on the presence of nitrate anions, we added tetraethylammonium nitrate to a solution containing equimolar amounts of the ligand 2 and Eu(OTf)3. As can be seen in Fig. 8, an almost linear increase of the luminescence was observed up to the addition of three equivalents of nitrate, with a 20-fold increase at the end of the titration.
![Increase of the luminescence intensity of an equimolar mixture of [Eu(OTf)3] and 2 upon addition of tetraethylammonium nitrate (0–3 equivalents).](/image/article/2003/NJ/b207056c/b207056c-f8.gif) | ||
| Fig. 8 Increase of the luminescence intensity of an equimolar mixture of [Eu(OTf)3] and 2 upon addition of tetraethylammonium nitrate (0–3 equivalents). | ||
The excited state lifetime in these conditions was found to be 1.7 ms, in good agreement with that found for 5. As far as the absorption is concerned, a small but non negligible decrease in the 300–350 nm region was observed, pointing to a partial decomplexation of the terpy unit, in agreement with the smaller association measured for formation of the 1∶1 complex of Eu and 2 with nitrate anions. The incoming nitrate anions successively fill the first coordination sphere of the europium, thus releasing the water and solvent molecules and thereby reducing non-radiative deactivation. In addition, similarly to what was observed for 1,13–14 insertion of the nitrate anions can influence the distance between the terpy unit and the metal ion, thus affecting the efficiency of the energy transfer process.
Interesting results were also observed upon addition of Zn2+ metal ions to an acetonitrile solution of 5. In this case, relatively small, but not negligible, changes were observed in the absorption spectra, showing that the terpy moiety remains coordinated to a metal ion.
Much more dramatic changes could be instead observed in the luminescence spectra (Fig. 9), since the europium centred luminescence decreased linearly upon addition of up to two equivalents of the Zn2+ ion, a situation in which almost complete quenching was observed. In strict concomitance, a new, very intense band, typical of the fluorescence of Zn2+ complexes with terpy,20 shows up in the 340–460 nm region. This finding is consistent with the translocation of the Eu3+ ion from the terpy unit to the P(O) binding site, while Zn2+ ions are complexed to the terpy subunit, leading to a system in which the energy transfer from the terpy unit to the Eu centre, although thermodynamically feasible, is not efficient.
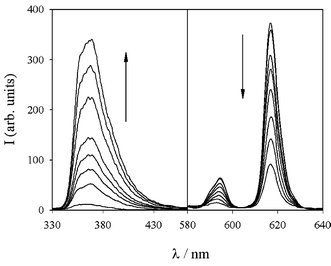 | ||
Fig. 9 Luminescence spectra of a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × ×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−5 M acetonitrile solution of 5 upon addition of increasing amounts (0–2 equiv.) of Zn(ClO4)2. 10−5 M acetonitrile solution of 5 upon addition of increasing amounts (0–2 equiv.) of Zn(ClO4)2. | ||
Conclusions
The terpyridine–phenylphosphine oxide ligands 2–4 form Eu3+ complexes whose photophysical properties drastically depend on the solvent and on the presence of different anions and cations. In acetonitrile solutions at room temperature, the complexes 5–8 show in fact an absorption band in the 300–350 nm region, indicating that the terpy units are coordinated to the europium ion. A metal-centred luminescence, indicative of an energy transfer from the terpy moieties to the Eu centre, also supported by excitation spectra, could be observed in all cases. As found for ligand 1,13,14 the efficiency of such an energy transfer process drastically depends on the nature of the anions: a 20-fold increase of the luminescence intensity could be in fact observed on adding up to 3 equivalents of nitrate to a solution containing equimolar amounts of the ligand 2 and the triflate salt of Eu(III). An additional effect on the luminescence intensity is also caused by the solvent: in methanol, DMF, and DMSO, the metal ion is coordinated through the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, while the terpy moieties remain uncoordinated; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of an energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P
O group, while the terpy moieties remain uncoordinated; in these conditions almost no metal-centred luminescence can be observed, showing that the efficiency of an energy transfer process from uncoordinated terpy units is almost negligible. The translocation of the Eu ion from the terpy unit to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group can also be performed by adding Zn2+ ions; the luminescence typical of the lanthanide ion drastically decreases, while the typical fluorescence of Zn terpy complexes can be observed.
O group can also be performed by adding Zn2+ ions; the luminescence typical of the lanthanide ion drastically decreases, while the typical fluorescence of Zn terpy complexes can be observed.
The large changes observed upon addition of nitrate anions are an important and promising result for the development of new luminescent chemosensors for coordinating species, such as nitrate anion, deserving, in our opinion, further studies that will be presented in due course.
Acknowledgements
This work was supported by MIUR (Solid Supermolecules Project), by the University of Bologna (Funds for Selected Topics) in Italy and by the CNRS and the French Ministry of Research in France.References
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293 CrossRef CAS.
- A. Mayer and S. Neuenhofer, Angew. Chem., Int. Ed. Engl., 1994, 33, 1044 CrossRef.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, New York, 1999 Search PubMed.
- P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef.
- (a) Fluorescent Chemosensors for Ion and Molecule Recognition, ed. A. W. Czarnik, American Chemical Society, Washington, DC, 1992 Search PubMed; (b) Chemosensors for Ion and Molecule Recognition, eds. J.-P. Desvergne and A. W. Czarnik, NATO ASI Series, Kluwer Academic Publisher, Dordrecht, 1997 Search PubMed.
- (a) L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59 CrossRef CAS; (b) C. Bargossi, M. C. Fiorini, M. Montalti, L. Prodi and N. Zaccheroni, Coord. Chem. Rev., 2000, 208, 17 CrossRef CAS.
- I. Hemmilä and R. Harju, in Bioanalytical Applications of Labeling Technologies, eds. I. Hemmilä, T. Stählberg and P. Mottram, Wallac Oy and EG&G Cie Pl, Turku, Finland, 1995 Search PubMed.
- (a) J.-C. G. Bünzli, in Lanthanide Probes in Life, Chemical, and Earth Sciences. Theory and Practice, eds. J.-C. G. Bünzli and G. R. Choppin, Elsevier Sciences Publications B. V., Amsterdam, 1989, p. 219 Search PubMed; (b) N. Sabbatini, M. Guardigli and J.-M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS; (c) N. Sabbatini, M. Guardigli and I. Manet, in Handbook on the Physics and Chemistry of Rare Earth, eds. K. A. Gschneidner Jr. and L. Eyring, Elsevier, Amsterdam, 1996, vol. 23 and references therein Search PubMed; (d) N. Sabbatini, M. Guardigli and I. Manet, in Advances in Photochemistry, eds. D. C. Neckers, D. H. Volman and G. von Bünau, John Wiley & Sons, New York, 1997, vol. 23 Search PubMed.
- (a) W. T. Carnall, in Handbook on the Physics and Chemistry of Rare Earth, eds. K. A. Gschneidner Jr. and L. Eyring, North-Holland, Amsterdam, 1979, vol. 3, p. 171 Search PubMed; (b) L. C. Thompson, in Handbook on the Physics and Chemistry of Rare Earth, eds. K. A. Gschneidner Jr. and L. Eyring, North-Holland, Amsterdam, 1979, vol. 3, p. 209 Search PubMed.
- G. Blasse, in Handbook on the Physics and Chemistry of Rare Earth, eds. K. A. Gschneidner Jr. and L. Eyring, North-Holland, Amsterdam, 1979, vol. 4, p. 237 Search PubMed.
- (a) O. Reany, T. Gunnlaugsson and D. Parker, Chem. Commun., 2000, 473 RSC; (b) T. Yamada, S. Shinoda and H. Tsukube, Chem. Commun., 2002, 1218 RSC.
- L. Douce, L. J. Charbonnière, M. Cesario and R. Ziessel, New J. Chem., 2001, 25, 1024 RSC.
- M. Montalti, L. Prodi, N. Zaccheroni, L. Charbonnière, L. Douce and R. Ziessel, J. Am. Chem. Soc., 2001, 123, 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 CrossRef CAS.
694 CrossRef CAS. - L. J. Charbonnière, R. Ziessel, M. Montalti, L. Prodi, N. Zaccheroni, C. Boehme and G. Wipff, J. Am. Chem. Soc., 2002, 124, 7779 CrossRef CAS.
- G. Pickaert and R. Ziessel, Tetrahedron Lett., 1998, 39, 3497 CrossRef CAS.
- E. C. Constable, C. E. Housecroft, M. Neuburger, A. G. Schneider and M. Zehnder, J. Chem. Soc., Dalton Trans., 1997, 2427 RSC.
- G. Pickaert, M. Cesario, L. Douce and R. Ziessel, Chem. Commun., 2000, 1125 RSC.
- K. Nakamaru, Bull. Chem. Soc. Jpn., 1982, 55, 2697 CAS.
- A. Credi and L. Prodi, Spectrochim. Acta, Part A, 1998, 54, 159 Search PubMed.
- F. Loiseau, C. Di Pietro, S. Serroni, S. Campagna, A. Licciardello, A. Manfredi, G. Pozzi and S. Quici, Inorg. Chem., 2001, 40, 6901 CrossRef CAS.
- (a) I. Bertini, P. Turano and A. J. Vila, Chem. Rev., 1993, 93, 2833 CrossRef CAS; (b) A. D. Sherry and C. F. G. C. Geroldes, in Lanthanide Probes in Life, Chemical, and Earth Sciences. Theory and Practice, eds. J.-C. G. Bünzli and G. R. Choppin, Elsevier Sciences Publications B. V., Amsterdam, 1989, p. 93 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2003 |
