DOI:
10.1039/B206124B
(Paper)
New J. Chem., 2003,
27, 128-133
[MnII2(bpym)(H2O)8]4+ and [MIV(CN)8]4−
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Mo and W) as building blocks in designing bpym- and cyanide-bridged bimetallic three-dimensional networks (bpym
Mo and W) as building blocks in designing bpym- and cyanide-bridged bimetallic three-dimensional networks (bpym![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 2,2′-bipyrimidine)†
2,2′-bipyrimidine)†
Received (in Montpellier, France) 24th June 2002, Accepted 17th September 2002
First published on 22nd November 2002
Introduction
The strategy based on the use of stable mononuclear complexes as ligands, which is known as the building block approach, is one of the best strategies in designing heterometallic compounds. Two recent illustrative examples are the tris-chelated [M(ox)3](m−6)− (m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidation state of the metal ion) species and the hexacyanometalate [M(CN)6](m−6)− unit (M
oxidation state of the metal ion) species and the hexacyanometalate [M(CN)6](m−6)− unit (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trivalent first-row transition metal ion).1–5 Their high negative charge, great stability in solution and versatility as Lewis bases allowed the rational preparation of polyfunctional heterometallic assemblies such as high-Tc molecule-based magnets,6,7 chiral magnets,8–10 systems exhibiting photo-induced magnetic ordering11,12 and high spin molecules.13–15
trivalent first-row transition metal ion).1–5 Their high negative charge, great stability in solution and versatility as Lewis bases allowed the rational preparation of polyfunctional heterometallic assemblies such as high-Tc molecule-based magnets,6,7 chiral magnets,8–10 systems exhibiting photo-induced magnetic ordering11,12 and high spin molecules.13–15In the last decade, the stability of the bpym-bridged dinuclear species {[M(H2O)4]2(bpym)}2m+
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) first row transition metal ion; bpym
first row transition metal ion; bpym![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,2′-bipyrimidine) in aqueous solution and the easy replacement of the terminally coordinated water molecules by potential bridging ligands allowed the design of extended homometallic compounds.16,17 Focusing on the case where M
2,2′-bipyrimidine) in aqueous solution and the easy replacement of the terminally coordinated water molecules by potential bridging ligands allowed the design of extended homometallic compounds.16,17 Focusing on the case where M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn(II), dinuclear complexes,18–20 uniform chains,20,21 honeycomb layered materials18,20,22 and three-dimensional22,23 networks were prepared and magneto-structurally characterised. A weak but significant antiferromagnetic coupling between the manganese(II) ions separated by more than 5 Å through bridging bpym was reported (−J values varying in the range 0.9–1.2 cm−1).21 Very recently, we extended this strategy to the preparation of bpym- and cyanide-bridged heterobimetallic species using the mononuclear low-spin [FeIII(bipy)(CN)4]− as a ligand towards the dinuclear {[Mn(H2O)4]2(bpym)}4+ unit.24 In our attempts to extend this strategy to other cyanide-bearing units, we obtained the isostructural three-dimensional heterobimetallic compounds {(μ-bpym)[Mn(H2O)]2(μ-NC)6M(CN)2} with M
Mn(II), dinuclear complexes,18–20 uniform chains,20,21 honeycomb layered materials18,20,22 and three-dimensional22,23 networks were prepared and magneto-structurally characterised. A weak but significant antiferromagnetic coupling between the manganese(II) ions separated by more than 5 Å through bridging bpym was reported (−J values varying in the range 0.9–1.2 cm−1).21 Very recently, we extended this strategy to the preparation of bpym- and cyanide-bridged heterobimetallic species using the mononuclear low-spin [FeIII(bipy)(CN)4]− as a ligand towards the dinuclear {[Mn(H2O)4]2(bpym)}4+ unit.24 In our attempts to extend this strategy to other cyanide-bearing units, we obtained the isostructural three-dimensional heterobimetallic compounds {(μ-bpym)[Mn(H2O)]2(μ-NC)6M(CN)2} with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2) where the manganese(II) ions are bridged by the bpym molecule and the [MIV(CN)8]4− units. Their preparation, crystal structure characterization and magnetic properties are presented here.
Mo (1) and W (2) where the manganese(II) ions are bridged by the bpym molecule and the [MIV(CN)8]4− units. Their preparation, crystal structure characterization and magnetic properties are presented here.
Experimental
Materials
K4[Mo(CN)8]·2H2O and K4[W(CN)8]·2H2O were prepared as previously described.25 Bpym and Mn(NO3)2·4H2O were purchased from commercial sources and used as received. Elemental analyses (C, H, N) were carried out by the Microanalytical Service of the Universidad Autónoma de Madrid. The M∶Mn [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)] molar ratio was determined by electron probe X-ray microanalysis at the the Servicio Interdepartamental de Investigación de la Universitat de València.
Mo (1) and W (2)] molar ratio was determined by electron probe X-ray microanalysis at the the Servicio Interdepartamental de Investigación de la Universitat de València.Preparation of {μ-(bpym)[Mn(H2O)]2-μ-(NC)6Mo(CN)2}
(1). Compound 1 is obtained as a yellow solid in a practically quantitative yield by mixing concentrated aqueous solutions of K4[Mo(CN)8]·2H2O (0.5 mmol) and Mn2(bpym)(NO3)2
(0.5 mmol)
[mixture of stoichiometric amounts of Mn(NO3)2·4H2O and bpym] in the dark. Yellow needles of 1 suitable for single crystal X-ray diffraction were grown by slow diffusion in an H-shaped tube of aqueous solutions of the octacyanometalate salt in one arm and the Mn(II)/bpym mixture in the other one in the dark. The crystals were washed with water and ethanol and air dried. Yield ca. 90%. Anal. calc. for C16H10Mn2MoN12O2
(1): C, 31.61; H, 1.64; N, 27.63. Found: C, 31.51; H, 1.57; N, 27.54%; Mo/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.
1/2. Preparation of {μ-(bpym)[Mn(H2O)]2-μ-(NC)6W(CN)2}
(2). The preparation of 2 is analogous to that of 1 except for the use of the octacyanotungstate(IV) anion as the cyano-bearing unit. 2 is obtained as an orange solid in a practically quantitative yield by mixing concentrated aqueous solutions of K4[W(CN)8]·2H2O (0.5 mmol) and Mn2(bpym)(NO3)2
(0.5 mmol)
[mixture of stoichiometric amounts of Mn(NO3)2·4H2O and bpym] in the dark. Orange needles of 2 suitable for X-ray diffraction were grown by slow diffusion in an H-shaped tube in the dark as for 1. The crystals of 2 were washed with water and ethanol and air dried. Yield ca. 90%. Anal. calc. for C16H10Mn2N12O2W (2): C, 27.61; H, 1.44; N, 24.14. Found: C, 27.49; H, 1.35; N, 23.98%; W/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.
1/2. Physical techniques
IR spectra (4000–400 cm−1) were recorded on a Bruker IF S55 spectrophotometer with samples prepared as KBr pellets. Variable-temperature (1.9–290 K) magnetic susceptibility measurements on polycrystalline samples were carried out with a Quantum Design SQUID operating at 5000 Oe in the high temperature range (20–290 K) and at 250 Oe at T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 K in order to avoid saturation phenomena. Diamagnetic corrections for the molar units were estimated from Pascal's constants26 as −275
20 K in order to avoid saturation phenomena. Diamagnetic corrections for the molar units were estimated from Pascal's constants26 as −275![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6
(1) and −281
10−6
(1) and −281![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6 cm3 mol−1
(2). The values of the temperature independent paramagnetism (TIP) estimated from the magnetic susceptibility data are 600
10−6 cm3 mol−1
(2). The values of the temperature independent paramagnetism (TIP) estimated from the magnetic susceptibility data are 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6
(1) and 1400
10−6
(1) and 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6 cm3 mol−1
(2).
10−6 cm3 mol−1
(2).X-Ray data collection and structure refinement
Crystals of dimensions 0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09
0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 (1) and 0.30
0.13 (1) and 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08
0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 mm (2) were mounted on a Bruker R3m/V automatic diffractometer and used for data collection. Diffraction data were collected at room temperature by using graphite-monochromated Mo-Kα radiation (λ
0.12 mm (2) were mounted on a Bruker R3m/V automatic diffractometer and used for data collection. Diffraction data were collected at room temperature by using graphite-monochromated Mo-Kα radiation (λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.71073 Å) with the ω–2θ scan method. The unit cell parameters were determined from least-squares refinement of the setting angles of 25 reflections in the 2θ range 15–30°. A summary of the crystallographic data and of the structure refinements is given in Table 1. Examination of two standard reflections, monitored after every 50 reflections, showed no sign of crystal deterioration. Lorentz-polarization and Ψ-scan absorption corrections27 were applied to the intensity data. The maximum and minimum transmission factors were 0.736 and 0.574 for 1 and 0.342 and 0.270 for 2.
0.71073 Å) with the ω–2θ scan method. The unit cell parameters were determined from least-squares refinement of the setting angles of 25 reflections in the 2θ range 15–30°. A summary of the crystallographic data and of the structure refinements is given in Table 1. Examination of two standard reflections, monitored after every 50 reflections, showed no sign of crystal deterioration. Lorentz-polarization and Ψ-scan absorption corrections27 were applied to the intensity data. The maximum and minimum transmission factors were 0.736 and 0.574 for 1 and 0.342 and 0.270 for 2.
Table 1 Crystallographic data for {μ-(bpym)[Mn(H2O)]2-μ-(NC)6M(CN)2} with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)a
Mo (1) and W (2)a
| | 1 | 2 |
|---|
Details in common: monoclinic, C2/c, Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, U 4, U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2162(1)
Å3 and T 2162(1)
Å3 and T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295 K. Goodness-of-fit 295 K. Goodness-of-fit![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {∑[w(Fo2 {∑[w(Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fc2)2/(No Fc2)2/(No![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Np)]}1/2. I Np)]}1/2. I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) > >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2σ(I). R1 2σ(I). R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∑(|Fo| ∑(|Fo|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)/∑|Fo|, wR2 |Fc|)/∑|Fo|, wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {∑[w(Fo2 {∑[w(Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fc2)2]/∑[w(Fo2)2]}1/2 and w Fc2)2]/∑[w(Fo2)2]}1/2 and w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/[σ2(Fo2) 1/[σ2(Fo2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (mP)2 (mP)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nP] with P nP] with P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fo2 [Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2Fc2]/3, m 2Fc2]/3, m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0625 (1) and 0.0444 (2) and n 0.0625 (1) and 0.0444 (2) and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1 and 2). 0 (1 and 2). |
|---|
| Formula | C16H10Mn2MoN12O2 | C16H10Mn2N12O2W |
| FW | 608.18 | 696.09 |
| a/Å | 14.539(3) | 14.542(2) |
| b/Å | 12.620(2) | 12.608(2) |
| c/Å | 12.114(3) | 12.126(2) |
| β/° | 103.40(1) | 103.46(1) |
| Dc/g cm3 | 1.868 | 2.138 |
| F(000) | 1192 | 1320 |
| μ(Mo-Kα)/cm−1 | 17.63 | 64.99 |
| Refl. colld./indep. | 2019/1918 | 2018/1917 |
| Refl. obs./param. | 1918/156 | 1917/156 |
| Goodness-of-fitb | 0.997 | 1.002 |
| R1/wR2cd | 0.0326/0.0859 | 0.0244/0.0608 |
| R1/wR2(all data) | 0.0386/0.0877 | 0.0263/0.0613 |
| Largest diff. peak/e Å−3 | 0.608 | 1.007 |
| Largest diff. hole/e Å−3 | −2.645 | −0.875 |
The structures of 1 and 2 were solved by standard Patterson methods through the SHELXTL NT package28 and subsequently completed by Fourier recycling. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms of the water molecule were located on a ΔF map and refined with constraints whereas those of the bpym ligand were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter. The final full-matrix least-squares refinement on F2, minimizing the function Σw(|Fo|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)2, reached convergence with values of the discrepancy indices given in Table 1. The final geometrical calculations were carried out with the PARST program.29 The drawings were performed using the XP utility of the SHELXTL NT system. Main interatomic bond distances and angles for 1 and 2 are listed in Table 2.
|Fc|)2, reached convergence with values of the discrepancy indices given in Table 1. The final geometrical calculations were carried out with the PARST program.29 The drawings were performed using the XP utility of the SHELXTL NT system. Main interatomic bond distances and angles for 1 and 2 are listed in Table 2.
Table 2 Selected bond lengths (Å) and angles (°) for compounds 1 and 2ab
| | M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) Mo (1) | M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W (2) W (2) |
|---|
| M(1)–C(5d) | 2.155(4) | 2.146(4) |
| M(1)–C(6b) | 2.164(4) | 2.163(5) |
| M(1)–C(7) | 2.166(4) | 2.175(5) |
| M(1)–C(8) | 2.161(4) | 2.171(5) |
| Mn(1)–O(1) | 2.222(3) | 2.229(4) |
| Mn(1)–N(1) | 2.321(3) | 2.319(4) |
| Mn(1)–N(2a) | 2.360(3) | 2.363(4) |
| Mn(1)–N(5) | 2.186(3) | 2.183(4) |
| Mn(1)–N(6) | 2.196(3) | 2.194(4) |
| Mn(1)–N(7) | 2.155(3) | 2.148(4) |
| C(5d)–M(1)–C(5e) | 101.0(2) | 100.9(3) |
| C(5d)–M(1)–C(6b) | 92.2(1) | 92.3(2) |
| C(5e)–M(1)–C(6b) | 144.6(1) | 144.5(2) |
| C(5d)–M(1)–C(7) | 69.8(1) | 70.1(2) |
| C(5e)–M(1)–C(7) | 75.4(1) | 75.3(2) |
| C(5d)–M(1)–C(8) | 142.3(1) | 142.5(2) |
| C(5e)–M(1)–C(8) | 74.6(1) | 74.2(2) |
| C(6b)–M(1)–C(6c) | 95.7(2) | 95.7(3) |
| C(6b)–M(1)–C(7) | 78.8(1) | 78.6(2) |
| C(6c)–M(1)–C(7) | 145.6(1) | 145.3(2) |
| C(7f)–M(1)–C(7) | 124.0(2) | 124.5(2) |
| C(8)–M(1)–C(6b) | 75.0(1) | 75.3(2) |
| C(8)–M(1)–C(6c) | 72.8(1) | 72.8(2) |
| C(8)–M(1)–C(7) | 73.0(1) | 72.7(2) |
| C(8)–M(1)–C(7f) | 132.9(1) | 132.7(2) |
| C(8)–M(1)–C(8f) | 131.3(2) | 131.7(3) |
| O(1)–Mn(1)–N(1) | 90.8(1) | 90.6(1) |
| O(1)–Mn(1)–N(2a) | 80.7(1) | 80.5(1) |
| N(1)–Mn(1)–N(2a) | 70.5(1) | 70.5(1) |
| N(5)–Mn(1)–O(1) | 168.1(1) | 167.8(1) |
| N(5)–Mn(1)–N(1) | 81.1(1) | 81.4(2) |
| N(5)–Mn(1)–N(2a) | 88.2(1) | 88.2(1) |
| N(5)–Mn(1)–N(6) | 104.2(1) | 104.7(2) |
| N(6)–Mn(1)–O(1) | 85.4(1) | 85.2(1) |
| N(6)–Mn(1)–N(1) | 97.2(1) | 97.4(2) |
| N(6)–Mn(1)–N(2a) | 161.2(1) | 161.0(2) |
| N(7)–Mn(1)–O(1) | 89.0(1) | 89.0(2) |
| N(7)–Mn(1)–N(1) | 162.1(1) | 162.0(2) |
| N(7)–Mn(1)–N(2a) | 91.9(1) | 91.7(1) |
| N(7)–Mn(1)–N(5) | 96.0(1) | 95.8(2) |
| N(7)–Mn(1)–N(6) | 100.6(1) | 100.6(2) |
| Hydrogen bondsc |
|---|
| Compound | A | D | H | A⋯D | A⋯H | A⋯H–D |
|---|
Estimated standard deviations in the last significant digits are given in parentheses. Symmetry code: (a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x −x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y 3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −z 3/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (b) 2; (b)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x −x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y 3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −z 1/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (c)
x 2; (c)
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −y 1/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, z 1/2, z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2; (d) 1/2; (d)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, −y x, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, z 1, z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2; (e) 1/2; (e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x −x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, −y 2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, −z 1, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (f) 2; (f)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x −x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, y, −z 2, y, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2; (g) 5/2; (g)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, y 1/2, y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, z. A 1/2, z. A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor, D acceptor, D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) donor. donor. |
|---|
| 1 | O(1) | N(8g) | H(1w) | 2.930(5) | 2.06(3) | 152(4) |
| 2 | | | | 2.917(6) | 2.00(3) | 158(6) |
| 1 | O(1) | N(8b) | H(2w) | 3.006(5) | 2.08(2) | 163(5) |
| 2 | | | | 3.001(6) | 2.07(2) | 163(6) |
CCDC reference numbers 188051 (1) and 188052 (2). See http://www.rsc.org/suppdata/nj/b2/b206124b/ for crystallographic data in CIF or other electronic format.
Results and discussion
IR characterisation
The most relevant features of the IR spectra of 1 and 2 concern the presence of two main peaks at 2161m and 2118s cm−1
(1) and at 2160m and 2112s cm−1
(2) which are assigned to νCN stretching vibrations of the cyanide groups. Their significant shift towards higher frequencies when compared with the values in the corresponding mononuclear precursors, multiplets in the frequency ranges 2137–2103 cm−1 for K4[Mo(CN)8]·2H2O and 2138–2096 cm−1 for K4[W(CN)8]·2H2O,30 suggests the occurrence of bridging cyanide in both compounds.31 The very asymmetric doublet at 1570s and 1530w cm−1 in the IR spectra of 1 and 2
(ring stretching vibrations of bpym) indicates the presence of bischelating bpym.32 Finally, a strong and broad absorption centered at 3450 cm−1 in the spectra of both compounds with peaks at 3480 (1 and 2), 3395 (1) and 3385 cm−1
(2) is attributed to the OH stretching of coordinated water involved in hydrogen bonds. These spectral observations on 1 and 2 are confirmed by the X-ray structure determination (see below).Description of the structures
1 and 2 are isostructural compounds where bpym-bridged aquamanganese(II) dinuclear entities are connected through octacyanometalate [MIV(CN)8]4− units [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)]
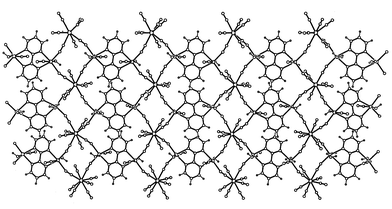
(Fig. 1) to afford a neutral three-dimensional arrangement of manganese and molybdenum (1)/tungsten (2) atoms (Fig. 2). Alternatively, the structure of 1 and 2 can be described as Mn(II)/M(IV) cyanide-bridged chains linked by bpym molecules adopting a bis-bidentate coordination mode towards the Mn(II) ions to afford a bidimensional arrangement (Fig. 3). Adjacent layers are joined by cyanide groups (Fig. 4) to form a three-dimensional network, the octacyanometalate anions acting as hexakismonodentate ligands through six of their eight cyanide ligands. The water molecule which is coordinated to the manganese atom is involved in hydrogen bonds with the cyanide-nitrogen atoms of the two terminally bound cyanides (see end of Table 2) contributing to the stabilization of the structure.
Mo (1) and W (2)]
(Fig. 1) to afford a neutral three-dimensional arrangement of manganese and molybdenum (1)/tungsten (2) atoms (Fig. 2). Alternatively, the structure of 1 and 2 can be described as Mn(II)/M(IV) cyanide-bridged chains linked by bpym molecules adopting a bis-bidentate coordination mode towards the Mn(II) ions to afford a bidimensional arrangement (Fig. 3). Adjacent layers are joined by cyanide groups (Fig. 4) to form a three-dimensional network, the octacyanometalate anions acting as hexakismonodentate ligands through six of their eight cyanide ligands. The water molecule which is coordinated to the manganese atom is involved in hydrogen bonds with the cyanide-nitrogen atoms of the two terminally bound cyanides (see end of Table 2) contributing to the stabilization of the structure.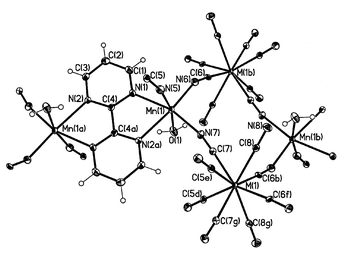 |
| | Fig. 1 Perspective view of a fragment of 1
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo) and 2
(M Mo) and 2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W) with the atom numbering. The thermal ellipsoids are drawn at the 30% probability level. W) with the atom numbering. The thermal ellipsoids are drawn at the 30% probability level. | |
![Projection of the three-dimensional rhombic array of the M(iv)
(cross-hatched circles) and Mn(ii) ions in 1
(M = Mo) and 2
(M = W) along the [001] direction. The bpym ligand has been omitted for clarity.](/image/article/2003/NJ/b206124b/b206124b-f2.gif) |
| | Fig. 2 Projection of the three-dimensional rhombic array of the M(IV)
(cross-hatched circles) and Mn(II) ions in 1
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo) and 2
(M Mo) and 2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W) along the [001] direction. The bpym ligand has been omitted for clarity. W) along the [001] direction. The bpym ligand has been omitted for clarity. | |
 |
| | Fig. 3 A view of the two-dimensional arrangement of the Mn(II) and M(IV) metal ions from 1 and 2 extending in the (−1 0 1) plane. | |
![View along the [010] direction showing the interlayer connection through bridging cyanide groups in the three dimensional network of 1 and 2.](/image/article/2003/NJ/b206124b/b206124b-f4.gif) |
| | Fig. 4 View along the [010] direction showing the interlayer connection through bridging cyanide groups in the three dimensional network of 1 and 2. | |
The manganese atom is six-coordinated: five nitrogen atoms, two from the bpym molecule and three from the cyanide groups, and an oxygen atom from a water molecule form a distorted octahedron around the metal atom. The small bite of the bridging bpym [70.5(1) for N(1)–Mn(1)–N(2a) in 1 and 2] is the main factor accounting for this distortion. The Mn–N(bpym) bond lengths [2.340(3)
(1) and 2.341(4)
Å
(2)] are significantly longer than those of the Mn–N(cyanide)
[average values 2.179(3)
(1) and 2.175(4) A (2)] and that involving the coordinated water molecule [2.222(3)
(1) and 2.229(4)
Å
(2)]. A comparison of the bpym-bridged manganese(II) dinuclear unit of 1 and 2 with the structure of the related dinuclear complex μ-(bpym)[Mn(H2O)3(SO4)]218
(3) reveals a slight increase in the Mn–bpym bonds in this last species [av. value 2.312(3)
Å in 3] but a shorter manganese–manganese separation across bridging bpym [6.123(2)
Å in 3vs. 6.201(3)
(1) and 6.203(4)
Å
(2)]; the larger value of the bite of bpym in 3
[71.4(1)°] accounts for the reduction of the metal–metal separation in this compound.
The Mo(IV)
(1) and W(IV)
(2) ions show the same distorted dodecahedron geometry, being coordinated to eight cyanide-carbon atoms. Two of these cyanide ligands are monodentate whereas the other six act as bridges towards the manganese atoms. The main differences between the M(IV) cores in 1 and 2 are the values of the metal–carbon bonds which vary in the ranges 2.155(4)–2.166(4)
(1) and 2.146–2.175(5)
Å
(2). These values are close to those observed in other reported structures containing octacyanomolydate(IV)33 and octacyanotungstate(IV)34 units. Structurally characterized polynuclear complexes based on the use of the [M(CN)8]4− unit (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo or W) as a ligand are still rare. In fact, as far as we are aware, structural reports of cyanide-bridged Mn(II)M(IV)
(M
Mo or W) as a ligand are still rare. In fact, as far as we are aware, structural reports of cyanide-bridged Mn(II)M(IV)
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo or W) bimetallic compounds are very recent and concern the following examples: the hexanuclear complex {[Mn(bipy)2]2(μ-CN)2[Mo(CN)6]2[(μ-CN)2[Mn(bipy)2]2}·8H2O,33c the chain {[Mn2(L)2(H2O)][Mo(CN)8]}·5H2O33b and the two-dimensional compound {[Mn(L)]6[MoIII(CN)7][Mo(CN)8]2}·19.5H2O33d
(L
Mo or W) bimetallic compounds are very recent and concern the following examples: the hexanuclear complex {[Mn(bipy)2]2(μ-CN)2[Mo(CN)6]2[(μ-CN)2[Mn(bipy)2]2}·8H2O,33c the chain {[Mn2(L)2(H2O)][Mo(CN)8]}·5H2O33b and the two-dimensional compound {[Mn(L)]6[MoIII(CN)7][Mo(CN)8]2}·19.5H2O33d
(L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,13-dimethyl-3,6,9,12,18-pentaazabicyclo[12.3.1]octadeca-1(18),2,12,14,16-pentaene) in the molybdenum series and the three-dimensional compound [W{(μ-CN)4Mn(H2O)2}2]·4H2O35 in the tungsten family.
2,13-dimethyl-3,6,9,12,18-pentaazabicyclo[12.3.1]octadeca-1(18),2,12,14,16-pentaene) in the molybdenum series and the three-dimensional compound [W{(μ-CN)4Mn(H2O)2}2]·4H2O35 in the tungsten family.
Within the experimental error, there are no significant differences between the mean values for the bridging and terminal cyanide ligands [the values of the C–N bond distance vary in the ranges 1.135(5)–1.159(5)
(1) and 1.132(6)–1.150(6)
Å
(2)]. The M(IV)–C–N angles for both terminal [178.5(3)°
(1) and 178.6(4)°
(2)] and bridging [175.5(3)–178.6(3)°
(1) and 175.1(4)–178.6(4)°
(2)] cyanides are almost in a line, whereas the Mn(1)–N–C(cyanide) linkages depart significantly from 180°
[151.2(3)–166.0(3)°
(1) and 151.9(4)–166.7(4)°
(2)]. The values of the metal-metal separation through bridging cyanide are 5.400(5)
(1) and 5.412(5)
Å
(2) for Mn(1)⋯M(1), 5.316(5)
(1) and 5.305(6)
Å
(2) for Mn(1)⋯M(1b)
[(b)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y
3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −z
1/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2] and 5.390(6)
(1) and 5.397(6)
Å
(2) for Mn(1)⋯M(1e)
[(e)
2] and 5.390(6)
(1) and 5.397(6)
Å
(2) for Mn(1)⋯M(1e)
[(e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, −y
2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, −z
1, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
The pyrimidyl rings are planar as expected [the largest deviation from the mean planes is 0.023(5)
Å at C(2)]. The bpym molecule as a whole is also planar and the manganese atom is 0.180(1)
Å out of this plane. The value of the inter-ring carbon–carbon bond length [C(4)–C(4a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.479(8)
(1) and 1.472(10)
Å
(2)] is very close to that observed in the free bpym in the solid state [1.497(4)
Å].36 The value of the bite distance of the bis-chelating coordination mode of bpym in 1 and 2
[2.702(5)
Å for N(1)⋯N(2a)] is practically identical to that found for the uncoordinated molecule [2.70 and 2.71 Å].36 The bond distances and angles of the pyrimidyl rings of the bpym molecule fall within the range of values found in other bpym-bridged manganese(II) complexes.
1.479(8)
(1) and 1.472(10)
Å
(2)] is very close to that observed in the free bpym in the solid state [1.497(4)
Å].36 The value of the bite distance of the bis-chelating coordination mode of bpym in 1 and 2
[2.702(5)
Å for N(1)⋯N(2a)] is practically identical to that found for the uncoordinated molecule [2.70 and 2.71 Å].36 The bond distances and angles of the pyrimidyl rings of the bpym molecule fall within the range of values found in other bpym-bridged manganese(II) complexes.
Magnetic properties
The temperature dependence of χMT for 1
[χM is the magnetic susceptibility per MnII2MIV unit] is shown in Fig. 5. A quasi identical curve is obtained for 2
(Fig. S1 in ESI†). At room temperature, χMT is 8.70 (1) and 8.40 (2) cm3 mol−1 K, values which are as expected for two magnetically isolated high spin manganese(II) ions (SMn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2). Upon cooling, χMT decreases continuously, slowly until 100 K and more and more steeply at lower temperature to reach 1.1 (1) and 0.98 (2) cm3 mol−1 K at 2.0 K. The suceptibility curve shows a maximum at 4.3 (1) and 4.5 K (2)
(see insert of Figs. 5 and S1 for 1 and 2, respectively). These features are consistent with a weak but significant antiferromagnetic interaction between two single-ion sextuplet states of the manganese(II) ions. The susceptibility data of 1 and 2 were analyzed in terms of an isotropic exchange interaction for a dinuclear species:
5/2). Upon cooling, χMT decreases continuously, slowly until 100 K and more and more steeply at lower temperature to reach 1.1 (1) and 0.98 (2) cm3 mol−1 K at 2.0 K. The suceptibility curve shows a maximum at 4.3 (1) and 4.5 K (2)
(see insert of Figs. 5 and S1 for 1 and 2, respectively). These features are consistent with a weak but significant antiferromagnetic interaction between two single-ion sextuplet states of the manganese(II) ions. The susceptibility data of 1 and 2 were analyzed in terms of an isotropic exchange interaction for a dinuclear species:| | H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −JSA·SB with SA −JSA·SB with SA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SB SB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2 5/2 | (1) |
through the expression:| | 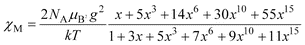 | (2) |
with x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(J/kT). The parameters NA, μB and k have their usual meanings. The values of J and g were determined by least-squares fit minimizing R
exp(J/kT). The parameters NA, μB and k have their usual meanings. The values of J and g were determined by least-squares fit minimizing R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Σ[(χM)obs
Σ[(χM)obs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (χM)calc]2/Σ[(χM)obs]2. The values obtained were J
(χM)calc]2/Σ[(χM)obs]2. The values obtained were J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −1.05 cm−1, g
−1.05 cm−1, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 and R
2.0 and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−5 for 1 and J
10−5 for 1 and J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −1.10 cm−1, g
−1.10 cm−1, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.98 and R
1.98 and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−5 for 2. The calculated curve matches very well the experimental data in both cases.
10−5 for 2. The calculated curve matches very well the experimental data in both cases.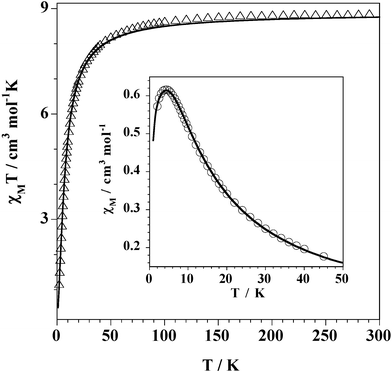 |
| | Fig. 5 χMT
(●)
vs.T plot for complex 1. The insert shows the χMvs.T plot in the vicinity of the maximum. The solid line is the best-fit through eqn. (2)
(see text). | |
The values of J in 1 and 2 are very close to those reported for other bpym-bridged manganese(II) compounds, as shown in Table 3. It is therefore likely that the main exchange interaction occurs within the bpym-bridged manganese(II) dinuclear fragments occurring in 1 and 2 and not through the diamagnetic octacyanomolybdate (1) or tungstate (2) bridging units [the manganese–manganese separation through the octacyanometalate bridging units is larger than 10.6 Å]. The antiferromagnetic coupling observed is mediated by the bis-chelating bpym and the exchange pathways involved are as mentioned in previous works:18,19,21 the main contribution is due to the σ* in-plane overlap between the two dx2−y2 type magnetic orbitals of each manganese(II) ion through the bpym N–C–N bridging skeleton [the x and y axis being roughly defined by the Mn–N(bpym) bonds].
Table 3 Selected magneto-structural data for bpym-bridged manganese(II) complexesa
| Compound | Nuclearity | Donor setb | −J/cm−1c | Mn–N(bpym)/Åd | Mn⋯Mn/Åe | Ref. |
|---|
Abbreviations used: bipy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,2′-bipyridine and dca 2,2′-bipyridine and dca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dicyanamide anion. The two first atoms are those of the bridging bpym. Magnetic coupling between the Mn(II) ions across bridging bpym. Average bond distances are given for each structure. Manganese–manganese separation through bridging bpym. dicyanamide anion. The two first atoms are those of the bridging bpym. Magnetic coupling between the Mn(II) ions across bridging bpym. Average bond distances are given for each structure. Manganese–manganese separation through bridging bpym. |
|---|
| [Mn2(bpym)(H2O)4(SO4)2] | Dinuclear | N2O4 | 1.1 | 2.31 | 6.123(2) | 18 |
| [Mn2(bpym)3(NCS)]4 | Dinuclear | N2N4 | 1.19 | 2.36 | 6.223(1) | 19 |
| [Mn2(bpym)3(NCSe)]4 | Dinuclear | N2N4 | 1.20 | 2.35 | 6.211(1) | 19 |
| {[Mn2(bpym)(H2O)6][Fe(bipy)(CN)4]2}[Fe(bipy)(CN)4]2·12H2O | Tetranuclear | N2NO3 | 1.2 | 2.32 | 6.131(6) | 24 |
| [Mn(bpym)(NCO)2]n | Chain | N2N4 | 1.1 | 2.35 | 6.234(1) | 21 |
| [Mn(bpym)(NO3)2]n | Chain | N2N2O3 | 0.93 | 2.36 | 6.239(1) | 21 |
| | | | | | 6.247(1) | |
| [Mn2(bpym)(dca)4]n | Three-dimensional | N2N4 | 0.86 | 2.33 | 6.156 | 23 |
| 1 | Three-dimensional | N2N3O | 1.05 | 2.34 | 6.201(3) | This work |
| 2 | Three-dimensional | N2N3O | 1.10 | 2.34 | 6.203(4) | This work |
The main conclusion of the present work concerns the synthetic possibilities opened by the availability and stability of the dinuclear {[M(H2O)4]2(bpym)}4+ units in aqueous solution (M being a divalent first-row transition metal ion). These complexes have been prepared and magneto-structurally characterized.18–22,37–40 The easy replacement of the peripheral water molecules by complexes which can act as ligands, as shown in 1 and 2, allows a plethora of n-dimensional (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–3) bimetallic arrays to be foreseen, where the bpym-bridged dinuclear motif could interact magnetically with paramagnetic octacyanometalate entities used as bridging units (work in progress).
0–3) bimetallic arrays to be foreseen, where the bpym-bridged dinuclear motif could interact magnetically with paramagnetic octacyanometalate entities used as bridging units (work in progress).
Acknowledgements
This work was supported by the Ministerio Español de Ciencia y Tecnología (Project BQU2001-2928), the TMR Programme of the European Union (Contract ERBFMRXCT98-0181) and the European Science Foundation through the Molecular Magnets Programme.References
- S. Decurtins, S. Ferlay, R. Pellaux, M. Gross and H. Schmalle, in Supramolecular Engineering of Synthetic Metallic Materials, NATO ASI Ser. C, eds. J. Veciana, C. Rovira and D. B. Amabilino, Kluwer, Dordrecht, 1999, vol. 518, p. 175 and references therein Search PubMed.
- C. Mathonière, C. J. Nuttal, S. G. Carling and P. Day, Inorg. Chem., 1996, 35, 1201 CrossRef CAS.
- H. Tamaki, Z. J. Zhong, N. Matsumoto, S. Kida, M. Koikawa, N. Achiwa, Y. Hashimoto and H. Okawa, J. Am. Chem. Soc., 1992, 114, 6974 CrossRef.
- M. Verdaguer, A. Bleuzen, V. Marvaud, J. Vaissermann, M. Suleiman, C. Desplanches, A. Scuiller, C. Train, R. Garde, G. Gelly, C. Lomenech, I. Rosenman, P. Veillet, C. Cartier and F. Villain, Coord. Chem. Rev., 1999, 190–192, 1023 CrossRef CAS and references therein.
- M. Ohba and H. Okawa, Coord. Chem. Rev., 2000, 198, 313 CrossRef CAS and references therein.
- S. Ferlay, T. Mallah, R. Ouahès, P. Veillet and M. Verdaguer, Nature, 1995, 378, 701 CrossRef CAS.
- W. R. Entley and G. S. Girolami, Science, 1995, 268, 397 CAS.
- S. Decurtins, Philos. Trans. R. Soc. London, Ser. A, 1999, 357, 3025 Search PubMed.
- E. Coronado, J. R. Galán-Mascarós, C. Gómez-García and J. M. Martínez-Agudo, Inorg. Chem., 2001, 40, 113 CrossRef CAS.
- R. Andrés, M. Gruselle, B. Malézieux, M. Verdaguer and J. Vaissermann, Inorg. Chem., 2001, 40, 4633 CrossRef CAS.
- O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Science, 1996, 272, 704 CrossRef CAS.
- G. Champion, C. Cartier dit Moulin, F. Villain, A. Bleuzen, F. Baudelet, E. Dartyge and M. Verdaguer, J. Am. Chem. Soc., 2001, 123, 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544 CrossRef and references therein.
544 CrossRef and references therein. - A. Scuiller, T. Mallah, M. Verdaguer, A. Nivorozkhin, J. L. Tholence and P. Veillet, New J. Chem., 1996, 20, 1 Search PubMed.
- T. Mallah, A. Marvilliers and E. Rivière, Philos. Trans. R. Soc. London, Ser. A, 1999, 367, 3139 Search PubMed.
- R. J. Parker, L. Spiccia, K. J. Berry, G. D. Fallon, B. Moubaraki and K. S. Murray, Chem. Commun., 2001, 333 RSC.
- G. De Munno, F. Lloret and M. Julve, in Magnetism: A Supramolecular Function, NATO ASI Ser. C, ed. O. Kahn, Kluwer, Dordrecht, 1996, vol. 484, p. 555 and references therein Search PubMed.
- G. De Munno and M. Julve, in Metal Ligand Interactions. Structure and Reactivity, eds. N. Russo and D. R. Salahub, Kluwer, Dordrecht, 1996, vol. 474, p. 139 and references therein Search PubMed.
- G. De Munno, R. Ruiz, F. Lloret, J. Faus, R. Sessoli and M. Julve, Inorg. Chem., 1995, 34, 408 CrossRef CAS.
- G. De Munno, G. Viau, M. Julve, F. Lloret and J. Faus, Inorg. Chim. Acta, 1997, 257, 121 CrossRef CAS.
- R. Cortés, M. K. Urtiaga, L. Lezama, J. L. Pizarro, M. I. Arriortua and T. Rojo, Inorg. Chem., 1997, 36, 5016 CrossRef CAS.
- G. De Munno, T. Poerio, M. Julve, F. Lloret, G. Viau and A. Caneschi, J. Chem. Soc., Dalton Trans., 1997, 601 RSC.
- G. De Munno, M. Julve, G. Viau, F. Lloret, J. Faus and D. Viterbo, Angew.Chem., Int. Ed. Engl., 1996, 35, 1807 CrossRef CAS.
- S. Martín, M. G. Barandika, J. I. Ruiz de Larramendi, R. Cortés, M. Font-Bardía, L. Lezama, Z. E. Serna, X. Solans and T. Rojo, Inorg. Chem., 2001, 40, 3687 CrossRef CAS.
- L. M. Toma, R. Lescouëzec, L. D. Toma, F. Lloret, M. Julve, J. Vaissermann and M. Andruh, J. Chem. Soc., Dalton Trans., 2002, 3171 RSC.
- J. G. Leipoldt, L. D. C. Bok and P. J. Cilliers, Z. Anorg. Allg. Chem., 1974, 409, 343 CrossRef CAS.
- A. Earnshaw, Introduction to Magnetochemistry, Academic Press, London and New York, 1968 Search PubMed.
- A. C. T. North, D. C. Philips and F. S. Mathews, Acta Crystallogr., Sect. A, 1968, 24, 351.
- SHELXTL NT, Version 5.10, Bruker Analytical X-ray Instruments Inc., Madison, WI, 1998.
- M. Nardelli, Comput. Chem., 1983, 7, 95 Search PubMed.
-
(a) K. O. Hartman and F. A. Miller, Spectrochim. Acta, 1968, 24A, 669 CrossRef CAS;
(b) R. V. Parish, P. G. Simms, M. A. Wells and L. A. Woodward, J. Chem. Soc. A, 1968, 2882 RSC;
(c) T. V. Long and G. A. Vernon, J. Am. Chem. Soc., 1971, 93, 1919 CrossRef CAS.
-
(a) G. F. McKnight and G. P. Haight, Jr., Inorg. Chem., 1973, 12, 3007 CrossRef;
(b) K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 4th ed., 1986, p. 278 Search PubMed.
- M. Julve, M. Verdaguer, G. De Munno, J. A. Real and G. Bruno, Inorg. Chem., 1993, 32, 795 CrossRef CAS.
-
(a) G. Rombaut, M. Verelst, S. Golhen, L. Ouahab, C. Mathonière and O. Kahn, Inorg. Chem., 2001, 40, 1151 CrossRef CAS;
(b) G. Rombaut, S. Golhen, L. Ouahab, C. Mathonière and O. Kahn, J. Chem. Soc., Dalton Trans., 2000, 3609 RSC;
(c) B. Sieklucka, J. Szklarzewicz, T. J. Kemp and W. Errington, Inorg. Chem., 2000, 39, 5156 CrossRef CAS;
(d) A. K. Sra, M. Andruh, O. Kahn, S. Golhen, L. Ouahab and J. V. Yakhmi, Angew. Chem., Int. Ed., 1999, 38, 2606 CrossRef CAS;
(e) B. Nowicka, A. Samotus, J. Szklarzewicz, J. Burguess, J. Fawcetti and D. R. Russell, Polyhedron, 1998, 17, 3167 CrossRef CAS;
(f) S. S. Basson, J. G. Leipoldt and A. J. van Wyk, Acta Crystallogr., 1980, B36, 2025 CAS;
(g) J. G. Leipoldt, S. S. Basson and L. D. C. Bok, Inorg. Chim. Acta, 1980, 38, L99 CrossRef CAS;
(h) B. J. Corden, J. A. Cunningham and R. Eisenberg, Inorg. Chem., 1970, 9, 356 CrossRef CAS.
-
(a) D. F. Li, T. A. Okamura, W. Y. Sun, N. Ueyama and W. X. Tang, Acta Crystallogr., 2002, C58, m280 CAS;
(b) G. Rombaut, C. Mathonière, P. Guionneau, S. Golhen, L. Ouahab, M. Verelst and P. Lecante, Inorg. Chim. Acta, 2001, 326, 27 CrossRef CAS;
(c) J. Szklarzewicz, A. Samotus, B. Nowicka, J. Burguess, J. Fawcetti and D. R. Russell, Transition Met. Chem., 1999, 24, 177 Search PubMed;
(d) N. W. Alcock, A. Samotus and J. Szklarzewicz, J. Chem. Soc., Dalton Trans., 1993, 885 RSC.
- P. Franz, J. M. Herrera, M. Pilkington, M. Biner, S. Decurtins, H. Stoeckli-Evans, A. Neels, A. Bleuzen, Y. Dromzee, M. Verdaguer and K. Hashimoto, J. Am. Chem. Soc. Search PubMed, to be submitted.
- L. Fernholdt, D. Rømming and S. Sandal, Acta Chem. Scand., Ser. A, 1981, 35, 707 Search PubMed.
- E. Andrés, G. De Munno, M. Julve, J. A. Real and F. Lloret, J. Chem. Soc., Dalton Trans., 1993, 2169 RSC.
- G. De Munno, M. Julve, F. Lloret and A. Derory, J. Chem. Soc., Dalton Trans., 1993, 1179 RSC.
- G. De Munno, M. Julve, F. Lloret, J. Faus and A. Caneschi, J. Chem. Soc., Dalton Trans., 1994, 1175 RSC.
- G. De Munno, M. Julve, F. Lloret, J. Cano and A. Caneschi, Inorg. Chem., 1995, 34, 2048 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2003 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Mo and W) as building blocks in designing bpym- and cyanide-bridged bimetallic three-dimensional networks (bpym
Mo and W) as building blocks in designing bpym- and cyanide-bridged bimetallic three-dimensional networks (bpym![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 2,2′-bipyrimidine)†
2,2′-bipyrimidine)†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo and W; bpym
Mo and W; bpym![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,2′-bipyrimidine) in aqueous solution yields the novel heterobimetallic complexes of formula {(μ-bpym)[Mn(H2O)]2-(μ-NC)6M(CN)2} with M
2,2′-bipyrimidine) in aqueous solution yields the novel heterobimetallic complexes of formula {(μ-bpym)[Mn(H2O)]2-(μ-NC)6M(CN)2} with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2). 1 and 2 are isostructural three-dimensional compounds where the manganese atoms are bridged by bisbidentate bpym and hexakismonodentate octacyanometalate units. Variable-temperature magnetic susceptibility data of 1 and 2 show the occurrence of a significant antiferromagnetic coupling between the high spin manganese(II) ions through bridging bpym (Jca.
−1.1 cm−1, the exchange Hamiltonian being defined as H
Mo (1) and W (2). 1 and 2 are isostructural three-dimensional compounds where the manganese atoms are bridged by bisbidentate bpym and hexakismonodentate octacyanometalate units. Variable-temperature magnetic susceptibility data of 1 and 2 show the occurrence of a significant antiferromagnetic coupling between the high spin manganese(II) ions through bridging bpym (Jca.
−1.1 cm−1, the exchange Hamiltonian being defined as H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −JSA·SB).
−JSA·SB).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidation state of the metal ion) species and the hexacyanometalate [M(CN)6](m−6)− unit (M
oxidation state of the metal ion) species and the hexacyanometalate [M(CN)6](m−6)− unit (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trivalent first-row transition metal ion).1–5 Their high negative charge, great stability in solution and versatility as Lewis bases allowed the rational preparation of polyfunctional heterometallic assemblies such as high-Tc molecule-based magnets,6,7 chiral magnets,8–10 systems exhibiting photo-induced magnetic ordering11,12 and high spin molecules.13–15
trivalent first-row transition metal ion).1–5 Their high negative charge, great stability in solution and versatility as Lewis bases allowed the rational preparation of polyfunctional heterometallic assemblies such as high-Tc molecule-based magnets,6,7 chiral magnets,8–10 systems exhibiting photo-induced magnetic ordering11,12 and high spin molecules.13–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) first row transition metal ion; bpym
first row transition metal ion; bpym![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,2′-bipyrimidine) in aqueous solution and the easy replacement of the terminally coordinated water molecules by potential bridging ligands allowed the design of extended homometallic compounds.16,17 Focusing on the case where M
2,2′-bipyrimidine) in aqueous solution and the easy replacement of the terminally coordinated water molecules by potential bridging ligands allowed the design of extended homometallic compounds.16,17 Focusing on the case where M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn(II), dinuclear complexes,18–20 uniform chains,20,21 honeycomb layered materials18,20,22 and three-dimensional22,23 networks were prepared and magneto-structurally characterised. A weak but significant antiferromagnetic coupling between the manganese(II) ions separated by more than 5 Å through bridging bpym was reported (−J values varying in the range 0.9–1.2 cm−1).21 Very recently, we extended this strategy to the preparation of bpym- and cyanide-bridged heterobimetallic species using the mononuclear low-spin [FeIII(bipy)(CN)4]− as a ligand towards the dinuclear {[Mn(H2O)4]2(bpym)}4+ unit.24 In our attempts to extend this strategy to other cyanide-bearing units, we obtained the isostructural three-dimensional heterobimetallic compounds {(μ-bpym)[Mn(H2O)]2(μ-NC)6M(CN)2} with M
Mn(II), dinuclear complexes,18–20 uniform chains,20,21 honeycomb layered materials18,20,22 and three-dimensional22,23 networks were prepared and magneto-structurally characterised. A weak but significant antiferromagnetic coupling between the manganese(II) ions separated by more than 5 Å through bridging bpym was reported (−J values varying in the range 0.9–1.2 cm−1).21 Very recently, we extended this strategy to the preparation of bpym- and cyanide-bridged heterobimetallic species using the mononuclear low-spin [FeIII(bipy)(CN)4]− as a ligand towards the dinuclear {[Mn(H2O)4]2(bpym)}4+ unit.24 In our attempts to extend this strategy to other cyanide-bearing units, we obtained the isostructural three-dimensional heterobimetallic compounds {(μ-bpym)[Mn(H2O)]2(μ-NC)6M(CN)2} with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2) where the manganese(II) ions are bridged by the bpym molecule and the [MIV(CN)8]4− units. Their preparation, crystal structure characterization and magnetic properties are presented here.
Mo (1) and W (2) where the manganese(II) ions are bridged by the bpym molecule and the [MIV(CN)8]4− units. Their preparation, crystal structure characterization and magnetic properties are presented here.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)] molar ratio was determined by electron probe X-ray microanalysis at the the Servicio Interdepartamental de Investigación de la Universitat de València.
Mo (1) and W (2)] molar ratio was determined by electron probe X-ray microanalysis at the the Servicio Interdepartamental de Investigación de la Universitat de València.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.
1/2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2.
1/2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 K in order to avoid saturation phenomena. Diamagnetic corrections for the molar units were estimated from Pascal's constants26 as −275
20 K in order to avoid saturation phenomena. Diamagnetic corrections for the molar units were estimated from Pascal's constants26 as −275![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6
(1) and −281
10−6
(1) and −281![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6 cm3 mol−1
(2). The values of the temperature independent paramagnetism (TIP) estimated from the magnetic susceptibility data are 600
10−6 cm3 mol−1
(2). The values of the temperature independent paramagnetism (TIP) estimated from the magnetic susceptibility data are 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6
(1) and 1400
10−6
(1) and 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6 cm3 mol−1
(2).
10−6 cm3 mol−1
(2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09
0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 (1) and 0.30
0.13 (1) and 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08
0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 mm (2) were mounted on a Bruker R3m/V automatic diffractometer and used for data collection. Diffraction data were collected at room temperature by using graphite-monochromated Mo-Kα radiation (λ
0.12 mm (2) were mounted on a Bruker R3m/V automatic diffractometer and used for data collection. Diffraction data were collected at room temperature by using graphite-monochromated Mo-Kα radiation (λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.71073 Å) with the ω–2θ scan method. The unit cell parameters were determined from least-squares refinement of the setting angles of 25 reflections in the 2θ range 15–30°. A summary of the crystallographic data and of the structure refinements is given in Table 1. Examination of two standard reflections, monitored after every 50 reflections, showed no sign of crystal deterioration. Lorentz-polarization and Ψ-scan absorption corrections27 were applied to the intensity data. The maximum and minimum transmission factors were 0.736 and 0.574 for 1 and 0.342 and 0.270 for 2.
0.71073 Å) with the ω–2θ scan method. The unit cell parameters were determined from least-squares refinement of the setting angles of 25 reflections in the 2θ range 15–30°. A summary of the crystallographic data and of the structure refinements is given in Table 1. Examination of two standard reflections, monitored after every 50 reflections, showed no sign of crystal deterioration. Lorentz-polarization and Ψ-scan absorption corrections27 were applied to the intensity data. The maximum and minimum transmission factors were 0.736 and 0.574 for 1 and 0.342 and 0.270 for 2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)a
Mo (1) and W (2)a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, U
4, U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2162(1)
Å3 and T
2162(1)
Å3 and T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295 K.b Goodness-of-fit
295 K.b Goodness-of-fit![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {∑[w(Fo2
{∑[w(Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fc2)2/(No
Fc2)2/(No![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Np)]}1/2.c I
Np)]}1/2.c I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >
>![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2σ(I).d R1
2σ(I).d R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∑(|Fo|
∑(|Fo|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)/∑|Fo|, wR2
|Fc|)/∑|Fo|, wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {∑[w(Fo2
{∑[w(Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fc2)2]/∑[w(Fo2)2]}1/2 and w
Fc2)2]/∑[w(Fo2)2]}1/2 and w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/[σ2(Fo2)
1/[σ2(Fo2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (mP)2
(mP)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nP] with P
nP] with P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fo2
[Fo2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2Fc2]/3, m
2Fc2]/3, m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0625 (1) and 0.0444 (2) and n
0.0625 (1) and 0.0444 (2) and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1 and 2).
0 (1 and 2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)2, reached convergence with values of the discrepancy indices given in Table 1. The final geometrical calculations were carried out with the PARST program.29 The drawings were performed using the XP utility of the SHELXTL NT system. Main interatomic bond distances and angles for 1 and 2 are listed in Table 2.
|Fc|)2, reached convergence with values of the discrepancy indices given in Table 1. The final geometrical calculations were carried out with the PARST program.29 The drawings were performed using the XP utility of the SHELXTL NT system. Main interatomic bond distances and angles for 1 and 2 are listed in Table 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1)
Mo (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W (2)
W (2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y
3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −z
3/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (b)
2; (b)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y
3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −z
1/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (c)
x
2; (c)
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −y
1/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, z
1/2, z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2; (d)
1/2; (d)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, −y
x, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, z
1, z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2; (e)
1/2; (e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, −y
2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, −z
1, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (f)
2; (f)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, y, −z
2, y, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2; (g)
5/2; (g)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, y
1/2, y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, z.c A
1/2, z.c A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor, D
acceptor, D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) donor.
donor.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo (1) and W (2)]
(Fig. 1) to afford a neutral three-dimensional arrangement of manganese and molybdenum (1)/tungsten (2) atoms (Fig. 2). Alternatively, the structure of 1 and 2 can be described as Mn(II)/M(IV) cyanide-bridged chains linked by bpym molecules adopting a bis-bidentate coordination mode towards the Mn(II) ions to afford a bidimensional arrangement (Fig. 3). Adjacent layers are joined by cyanide groups (Fig. 4) to form a three-dimensional network, the octacyanometalate anions acting as hexakismonodentate ligands through six of their eight cyanide ligands. The water molecule which is coordinated to the manganese atom is involved in hydrogen bonds with the cyanide-nitrogen atoms of the two terminally bound cyanides (see end of Table 2) contributing to the stabilization of the structure.
Mo (1) and W (2)]
(Fig. 1) to afford a neutral three-dimensional arrangement of manganese and molybdenum (1)/tungsten (2) atoms (Fig. 2). Alternatively, the structure of 1 and 2 can be described as Mn(II)/M(IV) cyanide-bridged chains linked by bpym molecules adopting a bis-bidentate coordination mode towards the Mn(II) ions to afford a bidimensional arrangement (Fig. 3). Adjacent layers are joined by cyanide groups (Fig. 4) to form a three-dimensional network, the octacyanometalate anions acting as hexakismonodentate ligands through six of their eight cyanide ligands. The water molecule which is coordinated to the manganese atom is involved in hydrogen bonds with the cyanide-nitrogen atoms of the two terminally bound cyanides (see end of Table 2) contributing to the stabilization of the structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo) and 2
(M
Mo) and 2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W) with the atom numbering. The thermal ellipsoids are drawn at the 30% probability level.
W) with the atom numbering. The thermal ellipsoids are drawn at the 30% probability level.![Projection of the three-dimensional rhombic array of the M(iv)
(cross-hatched circles) and Mn(ii) ions in 1
(M = Mo) and 2
(M = W) along the [001] direction. The bpym ligand has been omitted for clarity.](/image/article/2003/NJ/b206124b/b206124b-f2.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo) and 2
(M
Mo) and 2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W) along the [001] direction. The bpym ligand has been omitted for clarity.
W) along the [001] direction. The bpym ligand has been omitted for clarity.
![View along the [010] direction showing the interlayer connection through bridging cyanide groups in the three dimensional network of 1 and 2.](/image/article/2003/NJ/b206124b/b206124b-f4.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo or W) as a ligand are still rare. In fact, as far as we are aware, structural reports of cyanide-bridged Mn(II)M(IV)
(M
Mo or W) as a ligand are still rare. In fact, as far as we are aware, structural reports of cyanide-bridged Mn(II)M(IV)
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo or W) bimetallic compounds are very recent and concern the following examples: the hexanuclear complex {[Mn(bipy)2]2(μ-CN)2[Mo(CN)6]2[(μ-CN)2[Mn(bipy)2]2}·8H2O,33c the chain {[Mn2(L)2(H2O)][Mo(CN)8]}·5H2O33b and the two-dimensional compound {[Mn(L)]6[MoIII(CN)7][Mo(CN)8]2}·19.5H2O33d
(L
Mo or W) bimetallic compounds are very recent and concern the following examples: the hexanuclear complex {[Mn(bipy)2]2(μ-CN)2[Mo(CN)6]2[(μ-CN)2[Mn(bipy)2]2}·8H2O,33c the chain {[Mn2(L)2(H2O)][Mo(CN)8]}·5H2O33b and the two-dimensional compound {[Mn(L)]6[MoIII(CN)7][Mo(CN)8]2}·19.5H2O33d
(L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,13-dimethyl-3,6,9,12,18-pentaazabicyclo[12.3.1]octadeca-1(18),2,12,14,16-pentaene) in the molybdenum series and the three-dimensional compound [W{(μ-CN)4Mn(H2O)2}2]·4H2O35 in the tungsten family.
2,13-dimethyl-3,6,9,12,18-pentaazabicyclo[12.3.1]octadeca-1(18),2,12,14,16-pentaene) in the molybdenum series and the three-dimensional compound [W{(μ-CN)4Mn(H2O)2}2]·4H2O35 in the tungsten family.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, −y
3/2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, −z
1/2, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2] and 5.390(6)
(1) and 5.397(6)
Å
(2) for Mn(1)⋯M(1e)
[(e)
2] and 5.390(6)
(1) and 5.397(6)
Å
(2) for Mn(1)⋯M(1e)
[(e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −x
−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, −y
2, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, −z
1, −z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.479(8)
(1) and 1.472(10)
Å
(2)] is very close to that observed in the free bpym in the solid state [1.497(4)
Å].36 The value of the bite distance of the bis-chelating coordination mode of bpym in 1 and 2
[2.702(5)
Å for N(1)⋯N(2a)] is practically identical to that found for the uncoordinated molecule [2.70 and 2.71 Å].36 The bond distances and angles of the pyrimidyl rings of the bpym molecule fall within the range of values found in other bpym-bridged manganese(II) complexes.
1.479(8)
(1) and 1.472(10)
Å
(2)] is very close to that observed in the free bpym in the solid state [1.497(4)
Å].36 The value of the bite distance of the bis-chelating coordination mode of bpym in 1 and 2
[2.702(5)
Å for N(1)⋯N(2a)] is practically identical to that found for the uncoordinated molecule [2.70 and 2.71 Å].36 The bond distances and angles of the pyrimidyl rings of the bpym molecule fall within the range of values found in other bpym-bridged manganese(II) complexes.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2). Upon cooling, χMT decreases continuously, slowly until 100 K and more and more steeply at lower temperature to reach 1.1 (1) and 0.98 (2) cm3 mol−1 K at 2.0 K. The suceptibility curve shows a maximum at 4.3 (1) and 4.5 K (2)
(see insert of Figs. 5 and S1 for 1 and 2, respectively). These features are consistent with a weak but significant antiferromagnetic interaction between two single-ion sextuplet states of the manganese(II) ions. The susceptibility data of 1 and 2 were analyzed in terms of an isotropic exchange interaction for a dinuclear species:
5/2). Upon cooling, χMT decreases continuously, slowly until 100 K and more and more steeply at lower temperature to reach 1.1 (1) and 0.98 (2) cm3 mol−1 K at 2.0 K. The suceptibility curve shows a maximum at 4.3 (1) and 4.5 K (2)
(see insert of Figs. 5 and S1 for 1 and 2, respectively). These features are consistent with a weak but significant antiferromagnetic interaction between two single-ion sextuplet states of the manganese(II) ions. The susceptibility data of 1 and 2 were analyzed in terms of an isotropic exchange interaction for a dinuclear species:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −JSA·SB with SA
−JSA·SB with SA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SB
SB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5/2
5/2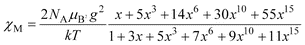
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(J/kT). The parameters NA, μB and k have their usual meanings. The values of J and g were determined by least-squares fit minimizing R
exp(J/kT). The parameters NA, μB and k have their usual meanings. The values of J and g were determined by least-squares fit minimizing R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Σ[(χM)obs
Σ[(χM)obs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (χM)calc]2/Σ[(χM)obs]2. The values obtained were J
(χM)calc]2/Σ[(χM)obs]2. The values obtained were J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −1.05 cm−1, g
−1.05 cm−1, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 and R
2.0 and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−5 for 1 and J
10−5 for 1 and J![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −1.10 cm−1, g
−1.10 cm−1, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.98 and R
1.98 and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−5 for 2. The calculated curve matches very well the experimental data in both cases.
10−5 for 2. The calculated curve matches very well the experimental data in both cases.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,2′-bipyridine and dca
2,2′-bipyridine and dca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dicyanamide anion.b The two first atoms are those of the bridging bpym.c Magnetic coupling between the Mn(II) ions across bridging bpym.d Average bond distances are given for each structure.e Manganese–manganese separation through bridging bpym.
dicyanamide anion.b The two first atoms are those of the bridging bpym.c Magnetic coupling between the Mn(II) ions across bridging bpym.d Average bond distances are given for each structure.e Manganese–manganese separation through bridging bpym.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–3) bimetallic arrays to be foreseen, where the bpym-bridged dinuclear motif could interact magnetically with paramagnetic octacyanometalate entities used as bridging units (work in progress).
0–3) bimetallic arrays to be foreseen, where the bpym-bridged dinuclear motif could interact magnetically with paramagnetic octacyanometalate entities used as bridging units (work in progress).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544 CrossRef and references therein.
544 CrossRef and references therein.