Electrowetting-based droplet mixers for microfluidic systems†
Phil
Paik
*,
Vamsee K.
Pamula
*,
Michael G.
Pollack
and
Richard B.
Fair
Department of Electrical Engineering, Duke University, Durham, North Carolina 27708, USA. E-mail: pyp@ee.duke.edu; vkp@ee.duke.edu
First published on 3rd February 2003
Abstract
Mixing of analytes and reagents is a critical step in realizing a lab-on-a-chip. However, mixing of liquids is very difficult in continuous flow microfluidics due to laminar flow conditions. An alternative mixing strategy is presented based on the discretization of liquids into droplets and further manipulation of those droplets by electrowetting. The interfacial tensions of the droplets are controlled with the application of voltage. The droplets act as virtual mixing chambers, and mixing occurs by transporting the droplet across an electrode array. We also present an improved method for visualization of mixing where the top and side views of mixing are simultaneously observed. Microliters of liquid droplets are mixed in less than five seconds, which is an order of magnitude improvement in reported mixing times of droplets. Flow reversibility hinders the process of mixing during linear droplet motion. This mixing process is not physically confined and can be dynamically reconfigured to any location on the chip to improve the throughput of the lab-on-a-chip.
1. Introduction
Mixing of analytes and reagents in microfluidic devices is a critical step in realizing a μTAS (micro total analysis system) or lab-on-a-chip. Mixing in these systems can be used for pre-processing, sample dilution, or reactions between samples and reagents in particular ratios. The ability to rapidly mix liquids greatly improves the throughput of such systems. However, as microfluidic devices are approaching the sub nano-liter regime, reduced volume flow rates and very low Reynolds numbers make mixing such liquids difficult to achieve in reasonable time scales. Improved mixing thus relies on two principles: the ability to create turbulent flow at such small scales, or alternatively, the ability to increase the interfacial area to achieve fast mixing via diffusion. Most of the research has been focused on the second principle, since turbulent flow would require the liquids to travel at high velocities or to introduce energy into the flow from an external source.Microfluidic systems can be broadly categorized into continuous-flow and droplet-based architectures. Continuous-flow systems rely on liquid that is continually input into the system, whereas droplet-based systems utilize discrete volumes of liquid. Within each architecture mixing can be accomplished by passive or active means. Passive mixing is mediated purely through diffusion without any external energy for the process. Active mixing, however, takes advantage of external energy, through actuation of some sort, to create either dispersed multilaminates or turbulence in the liquid.
Mixing in continuous flow systems is performed passively by merging two separate streams through etched microchannels. Koch et al. have demonstrated passive mixing by either separating a pressure-driven main stream into laterally alternating partial flows, or by superimposing two fluids by injection of one liquid into the other.1 Jacobson et al. have merged two fluid inputs with T-intersecting or cross-intersecting channels to promote parallel and serial mixing in electrokinetically driven flows.2 Bertsch et al. have shown enhanced diffusion in a channel with 3D elements such as helical structures and intersecting channels fabricated with microstereolithography.3 Rohr et al. have simply modified the channel surface to render it porous through photopolymerization to facilitate mixing.4 Some workers have created wells and ridges in the channels to enhance mixing.5,6 Other researchers created intricate channeling systems to promote passive mixing in continuous flow, such as the use of serpentine paths and interweaving channels7–9 or bend-induced vortices when a channel flow encounters an abrupt change in angle.10
Active mixing in continuous flow systems introduces additional energy into the system to create flow instabilities. Oddy et al. used oscillating electroosmotic actuators to create electrokinetic instabilities, which stretch and fold the channel flow to promote mixing.11 Chou et al. have enhanced mixing in continuous flow systems by peristaltically pumping the fluid in a circulating loop fabricated in soft lithography.12 Some researchers have demonstrated turbulent mixing using ultrasound by subjecting discrete mixing chambers to high-frequency vibrations.13,14.
A common limitation that continuous flow systems face is that liquid transport is physically confined to permanently etched structures and additional methods are required to enhance mixing. The transport mechanisms used are usually pressure-driven by external pumps or electrokinetically-driven by high-voltage supplies. While large flow rates can be achieved using these mechanisms, these approaches require the use of valves and/or complex channeling, consuming valuable real-estate on a chip and adding complexity to system integration. These restrictions make it difficult to achieve high degrees of functional integration and control in conventional continuous-flow systems.
An alternative approach to microfluidic systems are ones that manipulate discrete droplets rather than continuous liquid streams. Through independent micromanipulation of discrete droplets, complex procedures can be carried out in a manner that directly mimics traditional bench-top protocols. Because each droplet can be independently controlled, highly integrated, scalable and flexible architectures can be implemented.
Only a few droplet-based systems have been presented to date. Burns et al. describe a system in which pressure-driven droplets are metered, transported and thermocycled within glass channels.15 Hosokawa et al. have demonstrated the ability to draw liquid and form droplets using a hydrophobic microcapillary valve device, after which droplets could be transported, merged and actively mixed with the help of air pressure.16 Our group and others have demonstrated devices that use electric fields to manipulate discrete droplets.17–21
We have previously reported results for passive mixing of droplets using an electrowetting-based platform where the droplets are electrically actuated to coalesce only, but no external energy is introduced into the system after droplet coalescence.22 Fowler et al. have claimed fast droplet mixing using a similar electrowetting-based platform to actively roll coalesced droplets to double the number of interfacial layers.23 Both Fowler et al. and Hosokawa et al. assess mixing times by just looking at the top view of the mixing droplets. In this paper, we investigate various strategies to enhance active droplet mixing through electrowetting microactuation. Additionally, we introduce the use of simultaneous top and side imaging which we find to be essential for accurately visualizing mixing in these systems, as well as an objective and standard method to calculate mixing times using MATLAB.
2. Experimental
Chip fabrication
Fig. 1 shows a typical experimental setup. Droplets are sandwiched between two glass plates. The bottom plates were fabricated using standard microfabrication techniques. The bottom glass plate consists of an array of independently addressable control electrodes patterned in a 200 nm thick layer of chrome. It is further coated with Parylene C (800 nm) as an insulator. The top glass plate was coated with a conducting layer of optically transparent indium tin oxide (ITO) to form the ground electrode. Both top and bottom plates are coated with a thin hydrophobic layer of Teflon AF 1600 (50 nm). | ||
| Fig. 1 Schematic of the assembled electrowetting chip. | ||
A glass spacer is used to separate the top and bottom plates, yielding a fixed gap. The height of the gap given a certain electrode pitch size has been shown to have a significant influence on the behavior of droplet motion and mixing. Details of the electrowetting experimental setup have been reported elsewhere.17
Operation of the microfluidic device
Droplets are actuated through an electrostatic method in which the interfacial tension of the droplets can be modulated with voltage, a phenomenon known as electrowetting.18 A droplet of polarizable and/or conductive liquid is sandwiched between two planar plates.The volume of the droplet is chosen so that it is slightly larger than the pitch of the electrodes, ensuring overlap between the droplet and adjacent electrodes. The electrodes are interdigitated to enhance droplet overlap, although in later experiments non-interdigitated structures also worked well. Filler fluid of an immiscible liquid is used to surround the droplet to prevent evaporation and reduce the voltage required for actuation. A custom controller was made to address and switch each electrode independently. Since actuation does not require any fixed channels or moving parts, any electrode on the chip can be designated for mixing.
The basic scheme for mixing is shown in top and side views in Fig. 2. A fluorescent droplet is actuated towards a non-fluorescent droplet. Fluorescence was chosen over absorbing inks or colored dyes for better visualization and higher sensitivity. The fluorescent droplets contain 1 mM fluorescein (JT Baker), 0.125 M KCl to make the droplet conductive, and 0.125 M NaOH to maintain the proper pH for fluorescence. The non-fluorescent droplets contain 0.125 M KCl and 0.125 M NaOH only. A series of control experiments were conducted to verify that over the time scale of our experiments the measured fluorescence levels were stable within the actuated droplets and not affected by either photobleaching or electrochemical effects.
 | ||
| Fig. 2 Schematic side and top views of the electrowetting-based mixing actuator. | ||
The fluorescent droplet is moved toward the non-fluorescent droplet until coalescence occurs. Then, mixing is initiated. In passive mixing, the merged droplet remains on the final electrode throughout the mixing process. In active mixing, the merged droplet is transported over a number of electrodes at various frequencies depending on the mixing scheme.
For all passive mixing experiments demonstrated, the gap between the bottom and top electrodes was fixed at 800 μm. The volume of each droplet was fixed at 1.75 μl, and the actuation voltage to move the droplet was also fixed at 30 V. For all active mixing experiments, a 600 mm gap was found to give optimal mixing times, with 1.32 ml droplets and an actuation voltage of 50 V. (For proper comparison, passive mixing experiments were also performed using 600 mm gaps and 1.32 ml droplets, but were found to give similar results to the 800 mm experiments. The larger gap height, however, provided better visualization from the side view, and thus was used for analysis.)
All experiments were performed with 1 cSt silicone oil as the filler liquid. Surface tension, viscosity, and conductivity measurements were made to ensure the two liquids were closely matched, which represents the ‘worst-case’ for mixing between two liquids, since no surface-tension gradients are present. The interfacial tension of the fluorescent droplet with respect to 1 cSt silicone oil was 37 dyn cm−1 whereas the non-fluorescent droplet was 36 dyn cm−1 with the same oil. The viscosity for the fluorescent and non-fluorescent droplets was 1.396 and 1.373 cP, and the conductivity was 29.7 mMho and 25.9 mMho, respectively (mMho = mS = milliSiemens).
Droplet visualization
In continuous flow geometries, it would be sufficient to obtain only a top view of the mixing process to visualize uniformity of the mixed fluids since it is radially symmetric and there will not be any variation vertically. In the case of visualization of mixing in droplets, assessment from just the top view can result in misleading mixing times. For example, Fowler et al. claimed to have obtained complete mixing in droplets in 1.2 s based solely on a top view.23 By obtaining droplet profiles from both top and side views, we have observed that rapid mixing may appear complete in the top view, but not in the side view.Two CCD cameras were used in all the experiments to view the top and side views of the droplet simultaneously, as shown in Fig. 3. The fluorescent droplets were excited using a tungsten lamp with a blue filter (490 nm). Both cameras were mounted with long pass filters (>510 nm) to collect fluorescence. The video was recorded onto SVHS tape and later digitized at 30 frames per second, such that the top and side views were perfectly synchronized. All experiments were recorded well past a subjective assessment of mixing for later analysis.
 | ||
| Fig. 3 Two CCD-camera setup to view top and side views of droplet mixing. | ||
Image processing
In order to obtain an objective assessment of mixing times, the digitized video was read into a custom-written program using the image processing toolkit in MATLAB. A snapshot of the completely mixed droplet at the end of the video file was obtained. This snapshot is an image of the completely mixed droplet, and is used as an internal reference. Every frame from the start of mixing was then compared to this reference mixed droplet frame by subtracting the two images. A histogram of this difference allowed us to determine the percentage of the droplet which was not mixed. A curve which shows the percentage the droplet was mixed on every frame could then be made, and an arbitrary threshold of 95 percent was used to acquire a final mixing time.3. Results and discussion
Passive mixing
Our initial experiments demonstrated passive mixing where the fluorescent (F) droplet was moved into a non-fluorescent (NF) droplet as shown in Fig. 2. The electrode adjacent to the NF droplet is switched on and off for a small amount of time so that the F droplet merges with the NF droplet onto the electrode the NF droplet initially occupied. The coalesced droplet is held in place throughout the diffusion process. It was observed that even though the droplets looked mixed from the top view in 15 s, the view from the side reveals that the F droplet has simply moved underneath the NF droplet as shown in Fig. 4. Mixing proceeds through diffusion from this point and takes 90 s to complete. It was repeatedly observed that when the droplets merge, the F droplet and the NF droplet are vertically separated. At this point, the mixing is diffusion-limited across this interface. | ||
| Fig. 4 Top and side views of a fluorescein droplet mixing with a non-fluorescein droplet at 15 s after coalescence. In the side view, the top plate and the electrowetting chip are defined by the droplet’s reflection off their respective surfaces. | ||
In a similar experiment, an F droplet was moved towards a NF droplet that contained 0.125 M KCl only. It was repeatedly observed that the F droplet would engulf the KCl droplet in a manner such that the coalesced droplet appeared mixed from the side view, but assumes a donut shape in the top view, as shown in Fig. 5.
 | ||
| Fig. 5 Top and side views of a fluorescent and KCl-only non-fluorescent droplet immediately after coalescence. | ||
Much like the previous experiment, after 10 s uniform fluorescence can be observed from the top view but the droplets appear vertically separated from the side view (shown in Fig. 6). From this point, mixing occurs only by diffusion across this new interface and also takes 90 s to complete. To increase the interfacial area for diffusion, the coalesced droplet was held on two electrodes but we did not observe a significant improvement in mixing times.
 | ||
| Fig. 6 Top and side views of a fluorescent and KCl-only non-fluorescent droplet 10 s after coalescence. | ||
Mixing in linear arrays
The most basic type of active mixing is an oscillation of the coalesced droplet between two electrodes. An F droplet is moved into a NF droplet much like the passive mixing case, but rather than holding the coalesced droplet onto one electrode, it is oscillated between two electrodes at a fixed frequency. To further enhance mixing, this idea can be extended to an n-electrode linear array, where the coalesced droplet would oscillate among those electrodes. Mixing times were measured for 1, 2, 4, 8, and 16 Hz frequencies (the switch time between one electrode and its neighboring electrode) for 2-, 3-, and 4-electrode arrays. The actual speed of the droplet increases with increasing voltage while the switching frequency refers to the amount of time the droplet is at rest on an electrode. In effect, increasing the voltage enables a higher switching frequency. Switching frequency is varied as a parameter and the voltage is fixed at 50 V since beyond this voltage we have observed that the insulator degrades due to charging. Fig. 7 shows the effect of the switching speed of the droplet on the time taken for mixing. Each mixing time datum plotted is an average taken from four experiments. It can be noted that for a given number of electrodes, increasing the frequency of switching results in faster mixing times. Similarly, for a given frequency, increasing the number of electrodes also results in improved mixing. It was observed that the reliability of the assessment of mixing time increases as the number of electrodes increases as the standard deviation becomes smaller with an increasing number of electrodes. The fastest linear array mixing time observed was 4.6 s on a 4-electrode array with the coalesced droplet (volume of 2.64 μl) oscillating at 16 Hz and 50 V. The droplet did not respond to higher frequencies beyond 16 Hz for this volume, voltage, and electrode configuration. However, with smaller electrodes, at the same voltage the switching frequency can be increased since the linear velocity of the droplet was observed to be a constant.17 | ||
| Fig. 7 2, 3, and 4-electrode active mixing times as a function of oscillation frequency. | ||
Fig. 8 shows time lapsed images taken from the top-view in a 2-electrode mixing experiment at 8 Hz, where each column represents the droplet in the same position with the same direction of motion on the array. Each row represents the droplet in motion during a certain loop in the mixing process. This allows us to observe droplet profiles on a specific electrode as a function of time.
During oscillation, the coalesced droplet begins to assume more complex patterns due to the nature of the motion of the droplet. We can see in the left column of Fig. 8 that the F droplet merely engulfs the NF droplet, as shown in the simpler passive mixing case. However, the right column shows that the NF droplet protrudes into the fluorescent droplet, as indicated by the arrows. These protrusions become more pronounced as oscillation continues, giving rise to an increased interfacial surface area between the F and NF parts available for diffusion. The result is a faster rate of mixing than in passive mixing.
By comparing the images in each column, it is observed that the flow patterns within each droplet take on a similar form. For example, the first frame in Fig. 8 shows the fluorescent droplet engulfing the non-fluorescent droplet, and the third and fifth frames show very similar patterns. Likewise, the second frame shows the fluorescent droplet now mainly within the non-fluorescent droplet, with similar patterns in the fourth and sixth frames. This is the result of flow-reversibility which is dominant in low-Reynolds number flow. That is, new patterns the droplet creates are mostly undone as it returns to its original position. In the two-electrode mixing scheme, the droplet is restricted to only two of these configurations, since there are two positions and directions the droplet can assume as shown in Fig. 9. This prevents the creation of even more complex patterns, thus impeding the mixing process. In spite of the flow reversibility, it can be observed that mixing is enhanced due to the transport of the droplets, during which fingers, multilaminates, and other interfacial instabilities form across the interface of the F and NF droplets.
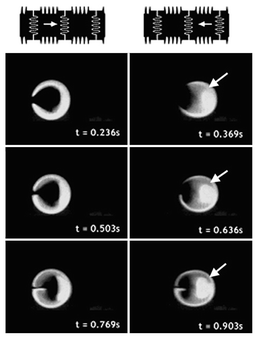
In the case of three-electrode mixing, similar behavior is observed. By adding an extra electrode, the coalesced droplet now has four possible configurations, as shown in Fig. 10a. Time lapsed images of three of the four configurations during three-electrode mixing at 8 Hz are shown in Fig. 10b, which also demonstrates flow reversibility. In the later frames, it can be observed that the interface between F and NF areas looks more diffuse even though flow reversibility can still be observed.
 | ||
| Fig. 10 (a) Possible droplet positions and directions in three-electrode mixing. (b) Time lapse images of three-electrode mixing at 8 Hz. | ||
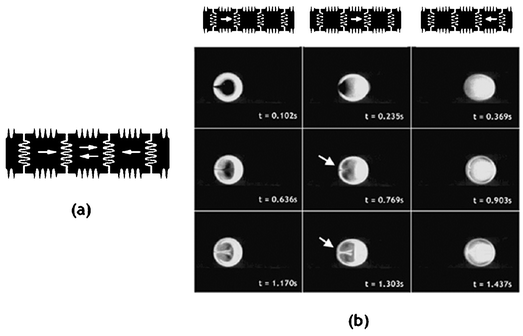
Similarly, there are six possible configurations for the four-electrode mixing scheme as shown in Fig. 11a, with time lapsed images of three of the six configurations shown in Fig. 11b. While flow reversibility is still noticeable, more complex patterns are created compared to the two- and three-electrode mixing schemes.
 | ||
| Fig. 11 (a) Possible droplet positions and directions in four-electrode mixing. (b) Time lapse images of four-electrode mixing at 8 Hz. | ||
By increasing the number of electrodes used in mixing, the number of possible configurations and patterns have been increased. Since diffusion-based mixing depends on the total interfacial surface area generated within the coalesced droplet, increasing the number of possible configurations improves mixing by extending these interfaces. Furthermore, increasing the frequency of oscillation causes this interface to change more quickly, since the switching time among different configurations is reduced.
To achieve even faster mixing times, a greater number of electrodes can be used while operating at higher frequencies. Due to concerns of larger mixing areas, mixing experiments were limited to four electrodes. However, it was observed that flow reversibility reduces as the number of electrodes increases. This observation is based on very complex recirculation patterns in the droplet while it is being transported. These patterns do not seem to be similar to the observations of Hosokawa et al.16 In their system, the droplets were surrounded by solid with a no-slip boundary whereas in the system described here, the droplets are surrounded by a liquid.
To move droplets at higher frequencies, a larger voltage is required in order for a droplet to completely move onto an adjacent electrode. The highest frequency attainable in these experiments at 50 V was 16 Hz. We have fixed 50 V as a maximum, since the insulator layer becomes charged at higher voltages, which renders that electrode unfit for further use by making the insulator surface hydrophilic. While mixing at higher voltages and a greater number of electrodes is possible, flow reversibility will always be present using linear arrays. Alternative methods are being explored that will reduce the effects of flow reversibility, such as circular mixing, where the droplet travels in only one direction.
It should be noted that while images have been obtained from both the top and side view, it has not been possible to obtain a 3D view of the droplet during mixing to determine if there exist any unmixed volumes in the interior of the droplet.
4. Conclusion
Using a simple structure consisting of two electode plates separated by an oil-filled gap, we have previously shown that discrete droplets of a uniform size can be dispensed from an initial larger volume and transported, merged, and split apart under direct electronic control.19 The ability to form, transport and merge droplets in a programmable manner allows a variety of ‘mix-and-read’ type assays to be implemented in this system. However, the time required for complete mixing to occur is likely to become a serious bottleneck in many potential applications.The results presented here demonstrate that mixing in electrowetting systems can be greatly accelerated through active manipulation of the droplets. Mixing in a coalesced droplet increases as the number of electrodes on which the coalesced droplet oscillates increases. Further improvement is obtained by increasing the transport velocity of the droplet. Increasing the number of electrodes over which the droplet is oscillated reduces the problem of flow reversibility associated with laminar flow. The fastest time for mixing two 1.3 microliter droplets in a linear oscillation on 4 electrodes was about 4.6 s which is about 7 times faster than reported for a picoliter droplet mixer.16 The droplets have to be observed in top and side views, otherwise misleading results about the completion of mixing may be obtained. With independent control on individual droplets and the ability to transport omnidirectionally, dynamic reconfigurability of droplet flow is possible thus enabling mixing schemes while transport occurs. The effect of scaling into the nanoliter regime needs to be studied.
Acknowledgements
The authors thank the Biomedical Microsensors Laboratory at North Carolina State University for their assistance with microfabrication and Prof. Edward Shaughnessy, Department of Mechnical Engineering, Duke University, for technical discussions.References
- M. Koch, D. Chatelain, A. G. R. Evans and A. Brunnschweiler, Two simple micromixers based on silicon, J. Micromech. Microeng., 1998, 8, 123–126 CrossRef CAS.
- S. C. Jacobson, T. E. McKnight and J. M. Ramsey, Microfluidic Devices for Electrokinetically Driven Parallel and Serial Mixing, Anal. Chem., 1999, 71, 4455–4459 CrossRef CAS.
- A. Bertsch, S. Heimgartner, P. Cousseau and P. Renaud, Static micromixers based on large-scale industrial mixer geometry, Lab Chip, 2001, 1, 56–60 RSC.
- T. Rohr, C. Yu, M. Davey, F. Svec and J. Frechet, Porous polymer monoliths: Simple and efficient mixers prepared by direct polymerization in the channels of microfluidic chips, Electrophoresis, 2001, 22, 3959–3967 CrossRef CAS.
- T. J. Johnson, D. Ross and L. E. Locascio, Rapid microfluidic mixing, Anal. Chem., 2002, 74, 45–51 CrossRef CAS.
- A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Chaotic Mixer for Microchannels, Science, 2002, 295, 647–651 CrossRef CAS.
- R. H. Liu, M. A. Stremler, K. V. Sharp, M. G. Olsen, J. G. Santiago, R. J. Adrian, H. Aref and D. J. Beebe, Passive Mixing in a Three-Dimensional Serpentine Microchannel, J. Microelectromech. Syst., 2000, 9(2), 190–197 CrossRef.
- B. He, B. J. Burke, X. Zhang, R. Zhang and F. E. Regnier, A Picoliter-Volume Mixer for Microfluidic Analytical Systems, Anal. Chem., 2001, 73, 1942–1947 CrossRef CAS.
- C. Erbacher, F. Bessoth, M. Busch, E. Verpoorte and A. Manz, Towards Integrated Continuous-Flow Chemical Reactors, Microchim. Acta, 1999, 131, 19–24 CrossRef CAS.
- M. Yi and H. H. Bau, The kinematics of bend-induced stirring in micro-conduits, ASME Micro-Electro-Mechanical Systems (MEMS Proc.), 2000, 2, 489–496 Search PubMed.
- M. H. Oddy, J. G. Santiago and J. C. Mikkelsen, Electrokinetic instability micromixing, Anal. Chem., 2001, 73, 5822–5832 CrossRef CAS.
- H.-P. Chou, M. A. Unger and S. R. Quake, A microfabricated rotary pump, Biomed. Microdev., 2001, 3(4), 323–330 Search PubMed.
- Z. Yang, S. Matsumoto, H. Goto, M. Matsumoto and R. Maeda, Ultrasonic micromixer for microfluidic systems, Sens. Actuators A, 2001, 93, 266–272 CrossRef.
- J. C. Rife, M. I. Bell, J. S. Horwitz, M. N. Kabler, R. C. Y. Auyeung and W. J. Kim, Miniature valveless ultrasonic pumps and mixers, Sens. Actuators A, 2000, 86, 135–140 CrossRef.
- M. A. Burns, B. N. Johnson and D. T. Burke, An Integrated Nanoliter DNA Analysis Device, Science, 1998, 282, 484–487 CrossRef CAS.
- K. Hosokawa, T. Fujii and I. Endo, Handling of picoliter liquid samples in a poly(dimethylsiloxane)-based microfluidic device, Anal. Chem., 1999, 71, 4781–4785 CrossRef CAS.
- M. G. Pollack, A. D. Shenderov and R. B. Fair, Electrowetting-based actuation of liquid droplets for integrated microfluidics, Lab Chip, 2002, 2, 96–101 RSC.
- M. G. Pollack, R. B. Fair and A. D. Shenderov, Electrowetting-based actuation of liquid droplets for microfluidic applications, Appl. Phys. Lett., 2000, 77(11), 1725–1726 CrossRef CAS.
- M. Washizu, Electrostatic actuation of liquid droplets for microreactor applications, IEEE Trans. Ind. Appl., 1998, 34, 732 CrossRef CAS.
- J. Lee, H. Moon, J. Fowler, T. Schoellhammer and C. J. Kim, Electrowetting and electrowetting-on-dielectric for microscale liquid handling, Sens. Actuators A—Phys., 2002, 95(2–3), 259–268 CrossRef.
- T. B. Jones, M. Gunji, M. Washizu and M. J. Feldman, J. Appl. Phys., 2001, 89, 1441 CrossRef CAS.
- V. K. Pamula, P. Paik, J. Venkatraman, M. G. Pollack and R. B. Fair, Microfluidic electrowetting-based droplet mixing, Proc. IEEE MEMS, 2001, 8–10 Search PubMed.
- J. Fowler, H. Moon and C. J. Kim, Enhancement of mixing by droplet-based microfluidics, Proc. IEEE MEMS, 2002, 97–100 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: six mpeg videos showing some mixing schemes used in Fig. 7. See http://www.rsc.org/suppdata/lc/b2/b210825a/ |
| This journal is © The Royal Society of Chemistry 2003 |