Integrating advanced functionality in a microfabricated high-throughput fluorescent-activated cell sorter
A.
Wolff
*a,
I. R.
Perch-Nielsen
a,
U. D.
Larsen
b,
P.
Friis
c,
G.
Goranovic
a,
C. R.
Poulsen
a,
J. P.
Kutter
a and
P.
Telleman
a
aMikroelektronik Centret (MIC), Technical University of Denmark (DTU), Ørsteds Plads, DTU Bldg 345east, DK-2800, Lyngby, Denmark. E-mail: aw@mic.dtu.dk
bChempaq Microinstruments, Symbion Science Park, Box 38, 2100, Copenhagen, Denmark
cGIGA ApS, Mileparken 22, DK-2740, Skovlund, Denmark
First published on 23rd January 2003
Abstract
The integration of complete analyses systems “on chip” is one of the great potentials of microfabricated devices. In this study we present a new pressure-driven microfabricated fluorescent-activated cell sorter chip with advanced functional integration. Using this sorter, fluorescent latex beads are sorted from chicken red blood cells, achieving substantial enrichments at a sample throughput of 12000 cells s−1. As a part of the sorter chip, we have developed a monolithically integrated single step coaxial flow compound for hydrodynamic focusing of samples in flow cytometry and cell sorting. The structure is simple, and can easily be microfabricated and integrated with other microfluidic components. We have designed an integrated chamber on the chip for holding and culturing of the sorted cells. By integrating this chamber, the risk of losing cells during cell handling processes is eliminated. Furthermore, we have also developed integrated optics for cell detection. Our new design contributes to the ongoing efforts for building a fully integrated micro cell sorting and analysing system.
Introduction
Conventional fluorescent-activated cell sorters (FACS) are widely used in clinical medicine, basic biological and material sciences. FACS provides impressively efficient sorting. However, a FACS is expensive, requires relatively large sample volumes, is difficult to sterilize, is mechanically complex, and can only be operated and maintained by trained personnel. Therefore, inexpensive devices that rapidly sort live cells, particles, and even single molecules would greatly facilitate screening of combinatorial chemistry libraries or cell populations.1Microfabricated fluorescent activated cell sorting devices (μFACS) offer a number of advantages over conventional FACS. Conventional FACS normally applies sorting of droplets in an open system. In contrast, a micro-sorter structure can be fabricated as a closed system, reducing the risk of infecting the sorted cells, and of working with biohazardous materials.
Several groups are developing microfabricated devices for cell analysis by flow cytometry2,3 or for cell sorting.1,4–8 Most of this work is presented as “proof of concept” and, with a few exceptions, no numbers are given on sample throughput or sorting efficiency. Fu et al. were the first to develop a microfabricated elastomeric FACS (μFACS) based on electro-osmotic flow.1 They demonstrated sorting of particle and bacteria cells with enrichments of up to 80–96 fold. However, the sample throughput of the system (10–20 cells s−1) was orders of magnitudes lower than what a conventional FACS can offer (10000 to 20000 cells s−1). A later pressure driven μFACS from the same group had essentially the same enrichment and a 2 fold increased sample throughput.7
The most important advantage of microsystems is the possibility to create complete analytical microsystems by integrating various functional modules on the same chip. Such functional modules may include sample preparation,9,10 integrated optics for excitation and detection of fluorescent labeled cells,11–13 cultivation of sorted cells,14,15 DNA amplification by the polymerase chain reaction (PCR),16–21 or single cell enzymatic analysis.22 In other words, system integration opens up interesting possibilities that cannot be envisaged in conventional machines.23 However, there have been only few attempts to integrate μFACS with other microfabricated compounds: Fu et al.7 have integrated microfluidic functionalities such as peristaltic pumps and switch valves, and Krüger et al.8 have integrated micro-optic compounds for cell detection.
In this paper, we describe a μFACS with a sample throughput comparable to conventional FACS. Furthermore we present and discuss a second generation of this μFACS with several integrated functionalities. These functionalities include a novel microfluidic structure for sheathing and hydrodynamic focusing of the cell-sample stream, a chip-integrated chamber for holding and culturing of the sorted cells, and integrated optics for detection of cells.
Methods
Fabrication of the first generation μFACS
The channels for the sorter structure were made on the front side of a four-inch silicon wafer. A double-mask was composed of a patterned 1.8 μm SiO2 layer and a patterned and hard-baked 2.6 μm photoresist. The oxide and photoresist masks were used during anisotropic reactive ion etching (RIE) of the silicon. Channels with depths between 50 and 200 microns were manufactured by this procedure. Similarly, holes for fluidic interconnection were RIE etched from the backside of the wafer, using a patterned SiO2 layer (3 μm) as mask. For sealing of the channels, a sodium-lime glass plate was anodically bonded to the front of the silicon wafer, yielding a good durable bond. This glass cover allowed external observation and detection of the cells in the channels. Quartz capillaries for fluid in- and outlets were connected through holes in the backside of a polymer holder and sealed to the chip with tiny O-rings.Fabrication of the second generation μFACS
The second generation of the μFACS was fabricated in a similar way as the first generation, except that a thicker oxide (3 μm) and a thicker photoresist (4.2 μm) were used for the front side masks. Holes for fluid interconnection were RIE etched from the back side of the wafer, using a combination of patterned SiO2 (3 μm) and hard-baked photoresist (9 μm) as masks. The integrated wave-guides were fabricated as described elsewhere.12 As for the first generation chip the channels were sealed by anodically bonding a sodium-lime glass plate to the front of the silicon wafer. On the second-generation chip a filter structure was integrated. A narrow gap between the wafer and glass top acted as the filter. Bonding between wafer and glass lid in the filter area could efficiently be prevented by small gold dots on the glass lid. These gold dots (0.5 μm high) were microfabricated using standard sputtering and lift-off techniques.System setup
The cell-sorting device was mounted on an inverted microscope (Leica DMIRB, Leica Microsystems, Wetzlar, Germany) with a 20× objective. A 100 W mercury lamp provided epifluorescence excitation. The fluorescence was collected through the same objective and detected by a photo multiplier tube (PMT) (Leica Microsystems, Wetzlar, Germany). The electrical signal from the PMT was sent via the PMT control unit (Leica Microsystems, Wetzlar, Germany) and a preamplifier (Stanford Research Systems SR560, Sunnyvale, California USA) to a custom-made control box that controlled a flow-switching valve. The flow switching valve (Lee LHDA1202025H, The Lee Company, USA) had a response time of 2.5 ms. Switching of this valve forced the beads of interest to the collecting channel (Fig. 1). Two syringe pumps (Harvard Apparatus Inc, Holliston, Ma, USA) were used for pumping the sample and the sheathing buffer.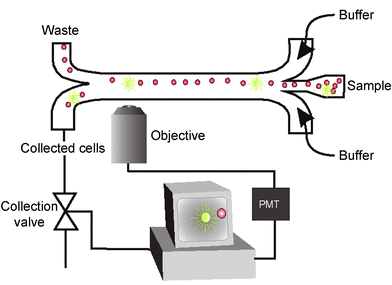 | ||
| Fig. 1 Schematic set-up for first generation micro cell sorter. | ||
Sample preparation
In the sorting experiments, a 10-fold dilution of EDTA stabilized chicken red blood cells (CRBC, Statens Serum Institut, Copenhagen, Denmark) was used in phosphate buffer saline (PBS, pH 7.4, Sigma, St Louis, Missouri, USA). The CRBC were mixed with 10 μm fluorescent latex beads (Polysciences Inc, Warrington, Philadelphia, USA) to a final concentration of 2.6 × 103 beads ml−1 and 1.1 × 108 cells ml−1, respectively. The PBS was used for sample sheathing.Determination of cell and bead concentrations
The concentration of CRBC and fluorescent beads was determined using a counting chamber (Bürker Bright line, KEBO Lab, Denmark). Very low concentrations of fluorescent beads were determined as follows: four aliquots of 20 μl of fluorescent beads were spotted on a microscope slide using a micropipette (Eppendorf, Germany). The slide was dried at 70 °C for 20 min and the number of fluorescent beads per spot was counted under a microscope.Simulation
Simulations were performed on a multiple-physics software package based on the Finite-Volume Method (CFD-ACE + 6.2, CFD Research Corporation, Huntsville Alabama, USA). The microfluidic structure was operated in laminar flow regime. It has a complex three-dimensional geometry and therefore requires a fine grid. Grid-independent simulation solutions were achieved at 48000 grid cells.Results and discussion
First generation of μFACS
| Input | Collection | Waste | Enrichment | |
|---|---|---|---|---|
| Fraction of beads | 2.4 × 10−5 | 2.4 × 10−3 | 8.2 × 10−6 | 100 fold |
Previously, an electrokinetic switching scheme has been used for μFACS.1 In the μFACS presented here, a hydrodynamic switching scheme was used. The advantage is that it does not require high voltage and is more robust for longer runs.1 We have successfully operated the chip sorter for up to 2–3 h. Moreover, problems with cell damage or cell death, caused by exposure to high electric fields, are avoided in our system. In a flow system cell viability and integrity may be compromised by high hydraulic pressure difference. However, the pressure drop through the sorting system presented here can be calculated to be less than 100 Pa, and pressure should therefore not be a problem in the sorter.
Functional integration in second generation μFACS
Cell sorting has become an indispensable part in the studies of cellular metabolism on the single cell level.24,25 For future applications, these single-cell analyses will demand immediate treatment of cells either before or after sorting with minimal time variation and sample loss. Thus, there is a need for a microfabricated cell sorter with additional functionalities.8 We have designed several integrated functionalities “on-chip” in a second generation of μFACS. The functionalities include a novel microfluidic structure for sheathing and hydrodynamic focusing of the cell-sample stream, a chip-integrated chamber for holding and culturing of the sorted cells, and integrated optics for detection of cells. The results of this integration are presented and discussed below.Sheathing and hydrodynamic focusing of the cell sample stream in the second generation μFACS
In the first generation of μFACS, the sample was only sheathed on two sides (Fig. 2A). This partial hydrodynamic focusing had a number of disadvantages. First, fluorescence signals from beads with identical amounts of fluorochrome showed great variation depending on whether the beads were in the focal plane or not. Second, as a result of hydrodynamic laminar flow, the speed of the cells or beads moving through the structure depended on the position of the cell in the channel. Miyake et al.26 have previously miniaturized the design used in conventional FACS for sample sheathing and hydrodynamic focusing on four sides. However, this microstructure was a complicated multi-layer structure that required a number of process steps to fabricate. Our new structure for coaxial sample sheathing and hydrodynamic focusing was build on the principle of “the smoking chimney”, where the sample is sheathed and carried downstream from the inlet like a wisp of smoke from a chimney (Fig. 2B). This chimney structure can be fabricated in a simple process applying standard micromachining methods.27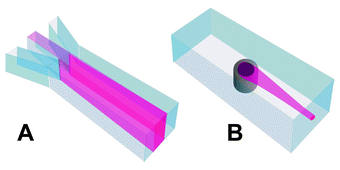 | ||
| Fig. 2 Sample inlet in first and second generation μFACS. A: In the first generation of our cell sorter the sample was only laminated from the flanking sides not from the top or bottom. B: In the second generation the sample is introduced into the buffer stream through a “chimney” like structure. This gives a coaxial flow profile and hydrodynamic focusing of the sample. | ||
The sample sheathing “chimney” structure was designed and optimised with the aid of computer simulation. In Fig. 3 a three-dimensional computer simulation of the “chimney” structure is shown. The colour graduation indicates the probability that a given cell will be inside the volume limited by that colour. The probability for blue and light blue is 95% and 99%, respectively. The simulation predicts a hydrodynamic focusing of the sample both vertically and horizontally (insert Fig. 3). The “chimney” structure was tested as follows: a fluorescein solution and PBS were pumped through the “chimney” and the sheathing channel, respectively. The transparent fluorescein was excited and the fluorescence light was recorded using a CCD camera. In the resulting CCD image, the fluorescence intensity was determined at a line across the channel 2 mm downstream from the “chimney” (Fig. 4A–C). The fraction of the channel height filled with fluorescein at any given point was calculated by comparing this fluorescence intensity to the intensity of a channel totally filled with fluorescein (Fig. 4D–F, solid line). Increasing the flow ratio of sheathing buffer to sample results in an increased focusing of the sample not only from the sides (reduced peak width) but also from the top and bottom (reduced peak height). These results are in good agreement with simulation predictions (Fig. 4D–F, dotted line). These experimental results clearly demonstrated the ability of the “chimney” structure to generate coaxial sheathing flow and hydrodynamic focusing of sample (Fig. 4). The region of the channel occupied by the sample can be controlled by varying the flow ratio of sheathing buffer to sample (Fig. 4). A high ratio will focus the sample in the centre of the channel (Fig. 4C and F). The “chimney” sheathing structure for hydrodynamic sample focusing ensured a low variation in fluorescent signal from equivalent beads, owing to the more uniform velocity distribution across the sample.28
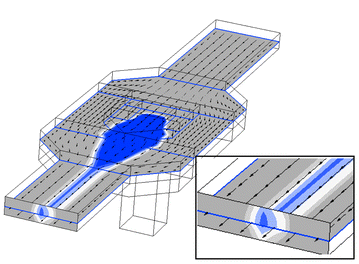 | ||
| Fig. 3 Three-dimensional computer simulation of the “chimney” structure. The colour graduation indicates the probability that a given cell will be inside the volume limited by that colour. The probability for blue and light blue is 95% and 99%, respectively. Insert: detail showing the hydrodynamic focusing of the sample. | ||
 | ||
| Fig. 4 A–C: The chimney seen from above with fluorescein flowing through. The controlling parameter σ is defined as the ratio between the sheathing and sample flow and is set to 0.5, 5 and 25, respectively. D–F: Solid blue line: the relative fluorescence intensity measured along the white lines in A–C. The relative intensity is a direct measure of the fraction of the channel height filled with sample (here fluorescein, see text). Dotted red line: fraction of the channel height filled with sample as predicted in simulations. | ||
In other micro cell sorter designs channels were so shallow and narrow that no additional sample focusing was required.1,7 However, using such shallow and narrow channels increases the risk of clogging the channels, especially when biological material is used. Another group has used electrokinetic focusing in a miniature flow cytometer.3 This focusing was only vertical, analogous to the focusing shown in Fig. 2A. In this sample-focusing scheme broader channels could be used. However, the channels still have to be shallow and sample clogging may therefore still be a problem. In our second μFACS we could use channels large enough to prevent clogging, because the “smoking chimney” was hydrodynamically focusing the sample stream, both vertically and horizontally (Fig. 2B). Recently, dielectrophoresis has been shown to give similar results for 3D focusing in microflow cytometry.6 However, this approach has a number of drawbacks: microfabrication of electrodes on both the bottom and top of the channel, precision alignment of these electrodes, electronics for generating electric AC field, and limitations on the conductivity of the liquid. All these disadvantages are avoided in the simple “smoking chimney” concept.
Integrated chamber for holding and culturing of cells in the second generations μFACS
We have developed a chip-integrated chamber for holding and culturing the sorted cells. This holding/culturing chamber is placed at the end of the collection channel (Fig. 5). A cross-sectional view of the holding/culturing chamber is shown in Fig. 6. The holding/culturing chamber is separated from the draining channel by a “wall”. Small gold dots on the glass lid prevented anodic bonded between the lid and the “wall”, creating a small gap. This gap between the lid and the “wall” acted as a filter. The filter allowed draining of excess liquid during sorting, and feeding fresh medium during culturing. The chamber is sealed to the chip holder with an O-ring for easy access to the sorted cells. Using normal yeast cells and yeast cells containing green fluorescent protein (GFP) in preliminary sorting experiments, we have found that it was possible not only to sort cells of interest into the chamber but also to culture them. When supplying the sorted cells with a flow of fresh medium we observed that the cells were growing and dividing for several days. The integration of holding and culturing chamber with the μFACS on the chip level eliminates the risk of losing cells due to dead volumes in interconnects or during cell handling. This is a crucial factor in rare-event cell sorting. Furthermore, the cells are sorted and cultured in a closed system, providing advantages for critical applications, such as working with biohazardous materials or when absolute sterile conditions are required. The chip-integrated chamber described in the present study provides interesting prospects for on-chip single cell enzymatic analysis, amplification of DNA by polymerase chain reaction (PCR),16–21 and interfacing to DNA chips.29 Such studies are in progress. | ||
| Fig. 5 A: Schematic set-up for the second generation μFACS. B: Scanning electron microscope (SEM) image of the second-generation micro cell sorter chip with integrated holding/culturing chamber: (a) sheathing buffer inlet, (b) “chimney” sample inlet, (c) detection zone, (d) holding /culturing chamber, (e) sieve to allow diffusion of nutrients and confinement of cells, (f) channel for draining excess liquid during sorting and for feeding fresh media to the cells during cultivation, (g) waste outlet. | ||
 | ||
| Fig. 6 Cross-sectional view of the holding/culturing chamber. The holding/culturing chamber is separated from the draining channel by a “wall”. Small gold dots on the anodic bonded glass lid create a small gap between the lid and the “wall”. This allowed draining of excess liquid during sorting and for feeding fresh nutrients during culturing. The chamber is sealed to the chip holder with an O-ring for easy access to the sorted cells. | ||
Integrated optics in the second generations μFACS
The majority of flow cell sorters rely on optical methods for cell detection and cell analysis. Optical cell analysis methods have proven to be versatile, reliable and very sensitive.30 The traditional bulk optics for cell analysis require precision alignment and are expensive. Integrated optics may be adapted for cell analysis and can provide a cheaper and more reliable alternative to traditional optics. In the μFACS we monolithically integrated waveguides with a fluidic micro channel (Fig. 7A). Light from an argon ion laser (488 nm) is coupled into a waveguide that subsequently couples the light into the microfluidic channel. The channel contains a solution of fluorescein that emits green fluorescent light. To test the feasibility of using waveguides for cell analysis, latex calibration beads was guided through such a sorting structure with integrated waveguides Emitted and scattered light were collected perpendicular to the waveguide through the cover glass lid and registered using PMTs. The calibration beads (LinearFlow™, Molecular Probes) are a mixture of beads labelled with varying amount of fluorochrome. The different subpopulations of beads can clearly be identified as three distinguished peaks in the histogram shown in Fig. 7B. The preliminary results obtained clearly demonstrate the potential of integrated optics for optical detection of cells in microsystems. The alignment problems known from bulk optics are avoided because the waveguides are monolithically integrated with the fluidic system. Moreover, it is possible to have a whole array of waveguides for delivering and collecting light. However, we have yet to take full advantage of manipulating of light with waveguides. Different geometries of microchannels and waveguides for delivery and collection of light as well as filtering of specific wavelengths by means of Bragg gratings, and integration of photodiodes for conversion of optical signals to electronic signal are under investigation. | ||
| Fig. 7 A: Optical microscope image of waveguides monolithically integrated with fluidic micro channel. Light from an argon ion laser (488 nm) is coupled into a waveguide that subsequently couples the light into the microfluidic channel. The channel contains a solution of fluorescein that emits green fluorescent light. B: Histogram of fluorescence signal from latex calibration beads flowing through the same microchannel at a rate of 1400 beads s−1. The calibration beads (LinearFlow™, Molecular Probes) are a mixture of beads labeled with varying amount of fluorochrome. The different subpopulations of beads can clearly be identified. | ||
Conclusion
We have developed a microfabricated fluorescent-activated cell sorter (μFACS) with several novel integrated, functional structures. A novel structure for hydrodynamic focusing of sample in flow cytometry and cell sorting was designed. The structure is simple, can easily be fabricated using standard microfabrication methods, and can be monolithically integrated with other microfluidic structures. The hydrodynamic focusing allows channel dimensions large enough to prevent clogging. Sharp sample focusing in both the vertical and the horizontal dimension can be controlled by manipulation of the flow ratio between the sheathing buffer and the sample. A new holding and culturing chamber has been integrated with the μFACS. The integration of holding and culturing chamber with the μFACS on the chip level eliminates the risk of losing cells due to dead volumes in interconnects or during cell handling. The preliminary experiments with waveguides in our microfluidic system clearly demonstrate the potential of integrated optics for optical detection of cells in chip-based systems. Using these microfabricated structures we have realized fluorescent activated cell sorting at a sample throughput as high as 12000 cells s−1 at 100-fold enrichment.Acknowledgements
We thank Sophion Bioscience A/S, Ballerup, Denmark for supplying us with GFP producing yeast cells. We thank Prof. Francois Grey (MIC, DTU), M.Sc. Per Wolff (Chempilots A/S, Farum, Denmark) and Dr. D. D. Bang (Danish Veterinary Institute, Aarhus, Denmark) for their critical review of the manuscript. This work received financial support from the Danish Technical Research Council (grant no. 9901659).References
- A. Y. Fu, C. Spence, A. Scherer, F. H. Arnold and S. R. Quake, A microfabricated fluorescence-activated cell sorter, Nature Biotechnol., 1999, 17, 1109–1111 CrossRef CAS.
- E. Altendorf, D. Zebert, M. Holl and P. Yager, Differential blood cell counts obtained using a microchannel based flow cytometer, in Transducers 97. 1997 International Conference on Solid-State Sensors and Actuators, 1997, Digest of Technical Papers (Cat. No.97TH8267) Search PubMed.
- D. P. Schrum, C. T. Culbertson, S. C. Jacobson and J. M. Ramsey, Microchip flow cytometry using electrokinetic focusing, Anal. Chem., 1999, 71(19), 4173–4177 CrossRef CAS.
- G. Blankenstein, A micro flow system for particle separation and analysis, World Pat. wo9810267, 1997 Search PubMed.
- G. Blankenstein and U. D. Larsen, Modular concept of a laboratory on a chip for chemical and biochemical analysis, Biosens. Bioelectron., 1998, 13(3–4), 427–438 CrossRef CAS.
- S. Fiedler, S. G. Shirley, T. Schnelle and G. Fuhr, Dielectrophoretic sorting of particles and cells in a microsystem, Anal. Chem., 1998, 70(9), 1909–1915 CrossRef CAS.
- A. Y. Fu, H. P. Chou, C. Spence, F. H. Arnold and S. R. Quake, An integrated microfabricated cell sorter, Anal. Chem., 2002, 74(11), 2451–2457 CrossRef CAS.
- J. Krüger, K. Singh, A. O′Neill, C. Jackson, A. Morrison and P. O′Brien, Development of a microfluidic device for fluorescence activated cell sorting, J. Micromech. Microeng., 2002, 12(July 2002), 486–494 CrossRef.
- J. Cheng, L. J. Kricka, E. L. Sheldon and P. Wilding, Sample preparation in microstructured devices, Top. Curr. Chem., 1998, 194, 215–231 CAS.
- Y. Huang, E. L. Mather, J. L. Bell and M. Madou, MEMS-based sample preparation for molecular diagnostics, Anal. Bioanal. Chem., 2002, 372(1), 49–65 CrossRef CAS.
- O. Leistiko and P. F. Jensen, Integrated bio/chemical microsystems employing optical detection: the clip-on, J. Micromech. Microeng., 1998, 8(2), 148–150 CrossRef CAS.
- P. Friis, K. Hoppe, O. Leistiko, K. B. Mogensen, J. Hubner and J. P. Kutter, Monolithic integration of microfluidic channels and optical waveguides in silica on silicon, Appl. Opt., 2001, 40(34), 6246–6251 CAS.
- L. Cui, T. Zhang and H. Morgan, Optical particle detection integrated in a dielectrophoretic lab-on-a-chip, J. Micromech. Microeng., 2002, 12(1), 7–12 CrossRef CAS.
- I. Inoue, Y. Wakamoto, H. Moriguchi, K. Okano and K. Yasuda, On-chip culture system for observation of isolated individual cells, Lab Chip, 2001, 1(1), 50–55 RSC.
- H. Moriguchi, Y. Wakamoto, Y. Sugio, K. Takahashi, I. Inoue and K. Yasuda, An agar-microchamber cell-cultivation system: flexible change of microchamber shapes during cultivation by photo-thermal etching, Lab Chip, 2002, 2(2), 125–132 RSC.
- J. Cheng, M. A. Shoffner, G. E. Hvichia, L. J. Kricka and P. Wilding, Chip PCR. 2. Investigation of different PCR amplification systems in microfabricated silicon-glass chips, Nucleic Acids Res., 1996, 24(2), 380–385 CrossRef CAS.
- A. T. Woolley, D. Hadley, P. Landre, A. J. deMello, R. A. Mathies and M. A. Northrup, Functional integration of PCR amplification and capillary electrophoresis in a microfabricated DNA analysis device, Anal. Chem., 1996, 68(23), 4081–4086 CrossRef CAS.
- T. M. H. Lee, I. M. Hsing, A. I. K. Lao and M. C. Carles, A miniaturized DNA amplifier: Its application in traditional Chinese medicine, Anal. Chem., 2000, 72(17), 4242–4247 CrossRef CAS.
- B. C. Giordano, J. Ferrance, S. Swedberg, A. F. R. Huhmer and J. P. Landers, Polymerase chain reaction in polymeric microchips: DNA amplification in less than 240 seconds, Anal. Biochem., 2001, 291(1), 124–132 CrossRef CAS.
- M. U. Kopp, A. J. de Mello and A. Manz, Chemical amplification: Continuous-flow PCR on a chip, Science, 1998, 280(5366), 1046–1048 CrossRef CAS.
- I. Schneegaß, R. Bräutigam and J. M. Köhler, Miniaturized flow-through PCR with different template types in a silicon chip thermocycler, Lab Chip, 2001, 1(1), 42–49 RSC.
- G. Ocvirk, H. Samimi-Moosavi, R. J. Szarka, E. Arriaga, P. E. Andersson, R. Smith, N. J. Dovichi and D. J. Harrison, Single cell enzymatic analysis on a microchip. lysing of single cells and identification of their b-galactosidase activity, in uTAS ′98, Micro Total Analysis Systems ′98, Banff, Canada, Kluwer Academic Publisher, Dordrecht, Holland, 1998 Search PubMed.
- J. M. Ramsey, The burgeoning power of the shrinking laboratory, Nature Biotechnol., 1999, 17, 1061–1062 CrossRef CAS.
- J. L. Zabzdyr and S. J. Lillard, New approaches to single-cell analysis by capillary electrophoresis, Trends Anal. Chem., 2001, 20(9), 467–476 CrossRef CAS.
- G. D. Meredith, C. E. Sims, J. S. Soughayer and N. L. Allbritton, Measurement of kinase activation in single mammalian cells, Nature Biotechnol., 2000, 18(3), 309–312 CrossRef CAS.
- R. Miyake, H. Ohki, I. Yamazaki and T. Takagi, Investigation of sheath flow chambers for flow cytometers – (Micro machined flow chamber with low pressure loss), JSME Int. J. Ser. B, 1997, 40(1), 106–113 Search PubMed.
- U. D. Larsen, A. Wolff and P. Telleman, Method of establishing at least one enveloped flow in a channel, Patent No. WO0169203, 2001 Search PubMed.
- H. Klank, G. Goranovic, J. P. Kutter, H. Gjelstrup, J. Michelsen and C. H. Westergaard, PIV measurments in a microfluidis 3D-sheating structure with three-dimentional flow behaviour, J. Micromech. Microeng., 2002, 12(6), 862–869 CrossRef.
- Nature Genetics Supplement, The Chipping Forecast, Supplement to Nature Genetics, 1999, 21 (January) Search PubMed.
- S. A. Soper, I. M. Warner and L. B. McGown, Molecular fluorescence, phosphorescence, and chemiluminescence spectrometry, Anal. Chem., 1998, 70(12), 477R–494R CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
