Modulated and intercalated smectic phases formed by dimeric molecules
Damian
Pociecha
*a,
Dorota
Kardas
a,
Ewa
Gorecka
ab,
Jadwiga
Szydlowska
a,
Jozef
Mieczkowski
a and
Daniel
Guillon
b
aWarsaw University, Department of Chemistry, Al. Zwirki i Wigury 101, 02-089 Warsaw, Poland. E-mail: pociu@chem.uw.edu.pl
bGMO, Institute of Physics and Chemistry of Materials of Strasbourg, France
First published on 22nd November 2002
Abstract
A series of molecules having a hydroxyl group attached to the end of one terminal chain were found to form a sequence of smectic antiphases, SmÃ, Sm![[C with combining tilde]](https://www.rsc.org/images/entities/char_0043_0303.gif) , Sm
, Sm![[F with combining tilde]](https://www.rsc.org/images/entities/char_0046_0303.gif) and Cr
and Cr , with a growing degree of in-plane molecular ordering. The bilayer structure of the modulated phases is due to the formation, through hydrogen bonding, of molecular dimers that are linear in shape. Moving the hydroxy group to a position closer to the molecular core resulted in a similar sequence of non-modulated, monolayer smectic phases. The related dimeric compounds in which the same mesogenic cores were covalently linked by an alkyl chain formed intercalated smectic phases.
, with a growing degree of in-plane molecular ordering. The bilayer structure of the modulated phases is due to the formation, through hydrogen bonding, of molecular dimers that are linear in shape. Moving the hydroxy group to a position closer to the molecular core resulted in a similar sequence of non-modulated, monolayer smectic phases. The related dimeric compounds in which the same mesogenic cores were covalently linked by an alkyl chain formed intercalated smectic phases.
Introduction
Apart from monolayer smectic phases, in which rod-like molecules are randomly oriented up and down within each layer, bilayer structures, with antiparallel, i.e., head to head organization of molecules from subsequent layers, are also observed. The formation of molecular dimeric assemblies, which are responsible for the appearance of bilayer smectic phases, can be driven by strong dipole–dipole interactions1 or hydrogen bonding2 between molecules from neighbouring layers. If these interactions are weak the monolayer smectics are formed. In the cross-over region between bilayer and monolayer structures, a variety of phases could appear, e.g., re-entrant nematic phase3 or complex modulated (‘antiphase’) smectic phases.4 Some attempts to correlate the type of phase with the strength of dipole–dipole interactions have been undertaken previously for CN and NO2 derivatives;5 however no systematic studies have been performed. Following this idea, we synthesized series of compounds with molecules having a hydroxyl group at different positions in one of terminal chains. The aim was to influence the strength and the direction of hydrogen bonds, and thus the type of the smectic phase formed.The analogue compounds in which two mesogenic cores are joined by covalent bonds were also prepared.
Experimental
The phase sequence as well as the phase transition temperatures and their thermal effects were mainly determined by calorimetric measurements, performed with a PerkinElmer DSC-7 apparatus. Calorimetric studies were combined with examination of the textures formed by samples prepared between two glass plates or as free suspended films. Microscopic observations were carried out with a Nikon OptiPhot2-POL polarising microscope equipped with a Mettler FP82HT hot stage. The structural studies of the detected LC phases were based on the X-ray diffraction method. The measurements were carried out on powder samples in Lindemann capillaries with Cu-Kα radiation. The patterns were registered with an Inel CPS 120 curved positional sensitive counter and with a Guinier camera set-up. During all the measurements, the sample temperature was controlled within 0.1 K.The molecular structure of the compounds obtained was confirmed by some analytical methods. The NMR spectra were recorded on a Varian Unity Plus spectrometer operating at 125 MHz and at 200 MHz or 500 MHz for 13C and 1H, respectively, with tetramethylsilane as an internal standard. Registered chemical shifts are reported in ppm. Infrared (IR) spectra were obtained using a Nicolet Magna IR 500 spectrophotometer. The elemental analysis of the synthesised compounds was performed to confirm the expected molecular structures. TLC analyses were performed on Merck 60 silica gel glass plates and visualized using iodine vapour. Column chromatography was carried out at atmospheric pressure using silica gel (100–200 mesh, Merck).
Synthesis
The synthetic procedure to obtain the studied materials is presented in Scheme 1. The synthesis of the acid chloride A has already been described.6 As an example of the general procedure used, the synthesis of the 1,3-propanediol derivatives, both mono- and disubstituted, is described.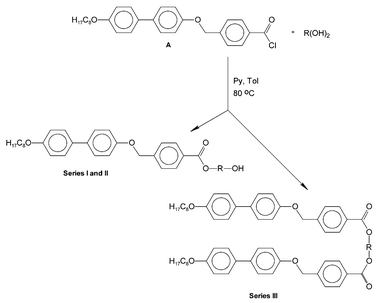 | ||
| Scheme 1 General synthetic route for obtaining the studied materials. | ||
1,3-Propanediol (0.1 g, 1.5 mmol) and pyridine (0.24 g, 3 mmol) were dissolved in toluene (10 ml). The mixture was stirred and kept at 40 °C for 1 h. Then the acid chloride A (2 g, 4.5 mmol) in toluene (10 ml) was added. The mixture was heated to 80 °C and stirred for 6 h, then cooled. The precipitated solid product was filtered and separated by column chromatography with methylene chloride as an eluent. Finally, the products were recrystallized: the monosubstituted from methanol (yield 40%) and the disubstituted from methylene chloride (yield 20%).
Analytical data for monosubstituted compound
Elemental analysis for C31H38O5: calculated C 75.89%, H 7.81%; found C 75.71%, H 7.73%. IR (CCl4), νmax/cm−1: 3600, 2980, 2950, 2900, 2850, 2800, 1710, 1600, 1500, 1280, 1250. 1H NMR (δ, CDCl3): 0.88 (t, 3H, CH3, J = 7.0 Hz), 1.29–1.50 (m, 10H, CH2), 1.79 (m, 2H, CH2), 2.01 (m, 2H, CH2), 3.78 (q, 2H, CH2, J = 6.2 Hz), 3.98 (t, 2H, OCH2, J = 6.6 Hz), 4.49 (t, 2H, HOCH2, J = 6.2 Hz), 5.16 (s, 2H, OCH2), 6.94 (d, 2H, J = 8.5 Hz), 7.00 (d, 2H, J = 8.5 Hz), 7.44 (d, 2H, J = 8.5 Hz), 7.47 (d, 2H, J = 8.5 Hz), 7.48 (d, 2H, J = 8.0 Hz), 8.06 (d, 2H, J = 8.0 Hz). 13C NMR (δ, CDCl3): 14.10, 22.67, 26.08, 29.25, 29.33, 29.38, 31.83, 31.94, 59.21, 61.83, 68.14, 69.44, 114.81, 115.14, 127.00, 127.71, 127.79, 129.64, 133.08, 134.18, 142.53, 157.53, 158.40, 166.74.Analytical data for disubstituted compound
Elemental analysis for C59H68O8: calculated C 78.29%, H 7.57%; found C 78.15%, H 7.44%. IR (CCl4), νmax/cm−1: 2980, 2950, 2900, 2850, 2800, 1710, 1600, 1500, 1280, 1250. 1H NMR (δ, CDCl3): 0.89 (t, 6H, CH3, J = 7.0 Hz), 1.25–1.81 (m, 24H, CH2), 2.01 (m, 2H, CH2), 3.98 (t, 4H, CH2, J = 6.5 Hz), 4.51 (t, 4H, CH2, J = 6.0 Hz), 5.14 (s, 4H, OCH2), 6.93 (d, 4H, J = 8.5 Hz), 6.99 (d, 4H, J = 8.0 Hz), 7.43 (d, 4H, J = 8.5 Hz), 7.46 (d, 4H, J = 8.5 Hz), 7.49 (d, 4H, J = 8.0 Hz), 8.04 (d, 4H, J = 8.0 Hz). 13C NMR (δ, CDCl3): 14.14, 22.67, 26.09, 29.27, 29.38, 29.42, 31.91, 31.99, 61.87, 68.18, 69.49, 114.80, 115.13, 127.02, 127.72, 127.78, 129.66, 129.97, 133.09, 134.16, 142.54, 157.55, 158.43, 166.75.Results
The phase transition temperatures and their thermal effects are collected in Tables 1–3.| R | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 | –CH2-CH(OH)-CH3 | Cr | 135.4 (51.1) | CrK | 148.8 (7.6) | SmI | 167.7 (7.3) | SmC | 172.9 (0.5) | SmA | 186.6 (33.9) | Iso |
| I-2 | –(CH2)2-CH(OH)-CH3 | Cr | 91.2 (45.3) | CrH | 139.8 (5.3) | SmF | 162.2 (7.7) | SmC | 170.8 (1.1) | SmA | 174.5 (33.0) | Iso |
| I-3 | –(CH2)3-CH(OH)-CH3 | Cr | 81.2 (38.4) | CrH | 125.8 (4.5) | SmF | 156.3 (7.2) | SmC | 163.6 (0.8) | SmA | 167.8 (31.9) | Iso |
| R | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II-1 | –CH2-CH2OH | Cr | 141.2 (26.6) | Cr |
171.7 (13.0) | Sm![[F with combining tilde]](https://www.rsc.org/images/entities/char_0046_0303.gif) |
182.7 (9.6) | Sm![[C with combining tilde]](https://www.rsc.org/images/entities/char_0043_0303.gif) |
184.2 (0.5) | Smà | 197.3 (39.3) | Iso |
| II-2 | –(CH2)2-CH2OH | Cr | 136.6 (21.5) | Cr |
150.0 (5.8) | Sm![[F with combining tilde]](https://www.rsc.org/images/entities/char_0046_0303.gif) |
175.0 (7.7) | Sm![[C with combining tilde]](https://www.rsc.org/images/entities/char_0043_0303.gif) |
181.7 (1.4) | Smà | 188.6 (37.7) | Iso |
| II-3 | –(CH2)3-CH2OH | Cr | 121.6 (48.3) | Cr |
141.0 (5.1) | Sm![[F with combining tilde]](https://www.rsc.org/images/entities/char_0046_0303.gif) |
168.9 (7.9) | SmC | 174.7 (0.7) | SmA | 179.7 (34.6) | Iso |
| II-4 | –(CH2)9-CH2OH | Cr | 116.3 (82.4) | CrE | 115.5 (4.2) | SmA | 154.4 (48.5) | Iso | ||||
| R | Phase transitions | |
|---|---|---|
| III-1 | –CH2-CH2-CH2– | Cr 210.5 (42.3) B1 216.9 (26.5) Iso |
| III-2 | –CH2-CH2-CH(CH3)– | Cr 166.8 (32.5) CrE 180.1 (7.8) CrB 196.7 (4.8) SmA 202.2 (30.3) Iso |
| III-3 | –CH2-(CH2)3-CH2– | Cr 170.9 (38.8) SmFA 176.3 (3.6) SmCA 198.6 (26.4) Iso |
| III-4 | –CH2(CH3)-CH2-CH2(CH3)– | Cr 173.4 (35.5) SmFA 180.2 (4.5) SmCA 195.5 (35.4) Iso |
| III-5 | –CH2-CH2– | Cr 200.2 (60.6) CrE 205.8 (41.6) Iso |
| III-6 | –CH2-(CH2)2-CH(CH3)– | Cr 170.1 (22.0) SmC 188.9 (0.3) SmA 197.1 (25.2) Iso |
| III-7 | –CH2(CH3)-(CH2)2-CH2(CH3)– | Cr 178.1 (31.5) SmA 187.4 (20.2) Iso |
| III-8 | –CH2-CH(C6H13)– | Cr 110.3 (16.5) CrB 160.5 (6.1) SmBHex 164.9 (7.7) SmA 168.5 (30.4) Iso |
| III-9 | –CH2-(CH2)8-CH2– | Cr 152.8 (2.3) [CrE 136.4 (4.5)] CrB 173.3 (5.7) SmA 192.6 (31.8) Iso |
Microscopic studies revealed orthogonal SmA, and tilted SmC, hexatic as well as crystalline smectic phases for compounds the molecules of which contained a free hydroxyl group (Series I and II). The presence of the hydroxyl group allowed for the formation of molecular dimers through hydrogen bonding. Relevant materials built of dimeric molecules with two mesogenic cores joined covalently by an alkyl spacer (Series III) form either a sequence of orthogonal smectic phases SmA, CrB and CrE or tilted SmC and SmF phases. Material III-6 shows the orthogonal–tilted smectic phase transition SmA–SmC. Compound III-1 with alkyl joint derived from 1,3-propanediol exhibits the banana phase B1,7i.e., the columnar phase made of broken smectic layers.
Hydrogen bonded dimers
Two types of smectic phases have been observed depending on the position of the hydroxyl group in the terminal chain.Monolayer smectic phases
When the hydroxyl group is attached at the last but one carbon atom of the alkoxyl chain (Series I) the materials form a sequence of monolayer-type smectic phases with a growing degree of intralayer molecular ordering with lowering temperature. The monolayer structure of the phases was determined from the X-ray patterns (Fig. 1) which exhibited, in reciprocal space, one sharp peak at q0 = 2π/d, where d = L cos(θ), L being the molecular length and θ the tilt angle. Higher harmonics of the signal related to the layer spacing were also observed. The increase of the in-plane order could be observed as changes in the wide angle region of X-ray diffraction patterns. In liquid-like SmA and SmC phases only one diffused signal is detected, related to the mean intermolecular distance ∼4.5 Å. In the hexatic smectic phase the peak becomes sharper, indicating a growing correlation length of the positional in-plane molecular order.8 It has been found that the range of positional correlations increases from ∼7 Å in SmC to ∼22 Å in the hexatic phase. In the crystalline smectic phase, a few peaks of instrumental resolution were detected in the wide angle region; these peaks match to a crystallographic unit cell of CrK phase (compound I-1) or CrH phase (compounds I-2 and I-3). The existence of the (210) signal points to a herringbone order of molecules, while the signals coming from the in-plane order with index l = 1 show that there is some positional correlation between molecules from neighbouring layers. | ||
| Fig. 1 Evolution of XRD pattern for I-1 compound. The unit cell dimensions in the CrK phase are: a = 9.20, b = 5.20, c = 31.97 Å, θ = 19.2°. | ||
Modulated smectic phases
Setting the hydrogen group at the terminal position of the alkoxyl chain (Series II) increases the tendency towards the formation of bilayer structures. However, we found that the appearance of a bilayer structure depends also on the length of the terminal chain. For the shortest homologues (compounds II-1 and II-2) the X-ray pattern is characterized by two resolution limited peaks at small angles in all the smectic phases. One of the peaks is at q0 = 2π/d, where d = 2 L cos(θ). The additional signal is incommensurate with d. The pattern obtained with a Guinier camera in the hexatic phase, from a partially oriented sample (Fig. 2), shows that the signals are not co-linear: q0 = (0, 0.193) and q1 = (0.079, 0.096). Such a pattern is characteristic for modulated smectic phases.4,9 The relation q0x ≅ 2q1x suggests a zero tilt angle. The limit of our experimental resolution gives the tilt angle smaller than 5 degrees. However, birefringent optical textures observed for film samples point to higher tilt angle values. The reason for this discrepancy may be the difference in the tilt of mesogenic cores and the tilt of the averaged long molecular axis. The optical birefringence is mainly determined by the mesogenic core inclination, whereas the smectic layer thickness depends on the tilt of the long molecular axis.10 The period of the in-plane modulation calculated from the component of the wavevector q1y is about 78 Å and does not change significantly with temperature. The modulated antiphase structure persists also in the crystalline smectic phase. It seems that the long range antiphase modulations are only weakly influenced by the hexagonal long range molecular structure inside the smectic plane. In the crystalline smectic phase, the reflections visible in the wide angle region matched to the CrH structure with a small tilt angle of ∼5 degrees. In the tilted modulated phases no schlieren texture could be observed, even on free suspended film samples (Fig. 3). Instead, from the homeotropic texture of Smà phase, the fan-like texture with characteristic sharp fringes on the fans developed in the Sm![[C with combining tilde]](https://www.rsc.org/images/entities/char_0043_0303.gif) phase.
phase.
 | ||
Fig. 2 Low angle part of XRD pattern obtained with Guinier camera from partially oriented sample of II-2 compound in Sm![[F with combining tilde]](https://www.rsc.org/images/entities/char_0046_0303.gif) phase. phase. | ||
 | ||
| Fig. 3 Texture of SmC antiphase (compound II-2) on free suspended film, obtained from homeotropic texture of SmA phase. | ||
For the longest homologue (compound II-4) only the monolayer smectic phases are observed. For the intermediate homologue (compound II-3), upon cooling, the evolution from a monolayer to a modulated structure seems to be continuous. The antiphase fluctuations develop gradually, the diffuse peak visible in the upper range of the hexatic phase becoming Bragg-like in the lower temperature range of this phase (Fig. 4). However no DSC signal was observed and the schlieren textures also evolved continuously into a frozen schlieren texture.
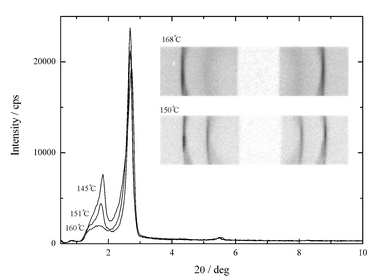 | ||
| Fig. 4 Temperature evolution of low angle part of XRD pattern for compound II-3. In the inset the Guinier camera images. | ||
Covalently bonded dimers (Series III)
For compound III-1, the columnar B1 phase, usually observed with banana molecules, was found. In this material the bent-core shape of the molecules is induced by the propylene spacer between two mesogenic cores. All other covalent dimer materials form intercalated smectic phases;11 the layer spacing measured by X-ray diffractometry in these phases was comparable to half of the dimer length. The appearance of the intercalated smectic phases is not disturbed by the length of the linkage group or the presence of the lateral substituents in this unit. The tilted smectic phases formed by the materials having the linkage chain with odd number of carbon atoms show intercalated tilted smectic phases with the anticlinic arrangement of subsequent layers. The spacer group with odd number of carbon atoms favours the dimer conformation in which two mesogenic cores make an angle instead being parallel. Such conformation strongly promotes the anticlinic intercalated structure. The anticlinic SmCA and SmFA phases have been confirmed by observing the point defects with the strength ½ (dispiration defects) on schlieren texture12 formed in free suspended films (Fig. 5). | ||
| Fig. 5 Schlieren texture of SmCA phase of compound III-3. The presence of the ½ defects confirms the anticlinic structure of the intercalated phase. | ||
Discussion
The influence of the strength and the direction of hydrogen bonds on the type of smectic structure was systematically studied. It is observed that the molecules in which the hydroxyl group is set at the end of the terminal alkyl chain easily form modulated smectic phases, but the temperature range in which the smectic antiphase is stable decreases with increasing alkyl chain length. For sufficiently long chains only monolayer smectics are formed. Thus, it is obvious that increasing the alkyl chain length decreases the concentration of linear dimers formed by molecules from the neighbouring layers. On the other hand, for molecules with long, flexible alkyl chains, the probability of gauche conformations increases and thus increases the probability of hydrogen bonds between molecules from the same layer. This favours monolayer smectics.Moving the OH group from the terminal position by just one carbon atom closer to the mesogenic core also disturbs the formation of linear dimers: thus no bilayer structure is observed.
The covalent dimers form easily intercalated type smectic phases. The intercalation appears as there is no reason for microsegregation of terminal or central groups. In contrast to the hydrogen bonded dimers, the central chains of the covalent dimers and their terminal chains have very similar chemical structures. The two types of tilted intercalated phases, synclinic and anticlinic, were observed, depending on the length of the linkage group. The dimers in which the mesogenic cores are joined by the alkyl chain with odd number of carbon atoms exhibit anticlinic structures, while synclinic smectic phases are observed for dimers with the joining alkyl chain containing an even number of carbon atoms.
Acknowledgement
This work was supported by the UW grant BW-1522/5/2001.References
- F. Hardouin, A. M. Levelut, J. J. Benattar and G. Sigaud, Solid State Commun., 1980, 33, 337 CrossRef CAS.
- C. M. Paleos and D. Tsiourvas, Curr. Opin. Colloid Interface Sci., 2001, 6, 257 Search PubMed.
- P. E. Cladis, Phys. Rev. Lett., 1975, 35, 48 CrossRef CAS.
- G. Sigaud, F. Hardouin, M. F. Achard and A. M. Levelut, J. Phys., 1981, 42, 107 Search PubMed; F. Hardouin, H. T. Nguyen, M. F. Achard and A. M. Levelut, J. Phys. Lett., 1982, 43, L-32 Search PubMed; A. M. Levelut, J. Phys. Lett., 1984, 45, L-603 Search PubMed; J. Prost, J. Phys (Paris), 1979, 40, 581 Search PubMed; J. Prost and P. Barois, J. Chem. Phys., 1983, 80, 65 CAS.
- H. T. Nguyen, J. Chim. Phys., 1983, 80, 83 Search PubMed.
- J. Mieczkowski, E. Górecka, D. Pociecha and M. Glogarová, Ferroelectrics, 1998, 212, 357 Search PubMed.
- G. Pelzl, S. Diele and W. Weissflog, Adv. Mater., 1999, 11, 707 CrossRef CAS.
- D. Demus, S. Diele, M. Klapperstuck, V. Link and H. Zaschke, Mol. Cryst. Liq. Cryst., 1971, 15, 161 Search PubMed.
- Y. Shi, G. Nounesis, C. W. Garland and S. Kumar, Phys. Rev. E, 1997, 56, 5575 CrossRef CAS; Y. Shi, G. Nounesis and S. Kumar, Phys. Rev. E, 1996, 54, 1570 CrossRef CAS; E. Bialecka-Florjanczyk, A. Orzeszko, I. Sledzinska and E. Gorecka, J. Mat. Chem., 1999, 9, 371 RSC.
- J. W. Goodby, in Ferroelectric Liquid Crystals: Principles, Properties and Applications, Gordon and Breach Science Publishers, New York, 1991 Search PubMed.
- P. J. Le Masurier and G. R. Luckhurst, Chem. Phys. Lett., 1998, 287, 435 CrossRef CAS; A. E. Blatch and G. R. Luckhurst, Liq. Cryst., 2000, 27, 775 CrossRef CAS; A. Krowczynski, E. Gorecka, D. Pociecha, J. Szydlowska and J. Przedmojski, Liq. Cryst., 1996, 20, 607 CAS.
- Y. Takanishi, H. Takezoe, A. Fukuda and J. Watanabe, Phys. Rev. B, 1992, 45, 7684 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
