Influence of copper oxide on mullite formation from kaolinite
Tomas
Martišius
*a and
Raimondas
Giraitis
b
aChemijos Institutas, Goštauto 4, Vilnius, Lithuania. E-mail: tomas@vtex.lt.
bChemijos katedra, Gamtos mokslų fakultetas, Vilniaus Pedagoginis Universitetas, Studentų 39, Vilnius, Lithuania
First published on 18th November 2002
Abstract
Kaolinite transforms to mullite and crystobalite at 1200 °C. The additive copper oxide decreases the transformation temperature by 200 °C. The effect of copper oxide addition and its concentration and transformed products are reported using a combination of X-ray diffraction and thermal analysis.
1 Introduction
Mullite is a material distinguished for its high chemical resistance and mechanical strength, which remains even at high temperature. Owing to these qualities mullite can be widely used in the production of heat-resistant materials in heat insulation, ceramics, composites, computer chips etc.1,2 The transformation of kaolinite to mullite requires a high thermal input. This process has been investigated by many scientists. The mechanism for mullite formation has not been adequately explained. Some authors affirm that mullite forms through an intermediate spinel-type phase, others suggest that mullite forms directly from metakaolinite.3–5The literature suggests that copper oxide is one of the most effective additives for reducing the formation temperature for the conversion of kaolinite to mullite.6–8 The effect of copper oxide addition results in an exothermal reduction of the transformation temperature by some 50–70 °C.6 The explanation of the mechanism for such temperature lowering requires explanation and opinions differ on such explanations. According to Špokauskas,7 copper oxide reacts with kaolinite at 800 °C by forming a quartz-type phase. At a higher temperature it decomposes, amorphous SiO2 and quartz crystallize to crystobalite, and finally mullite is formed. Segnit and Gelb also affirm that the high temperature process of kaolinite conversion to mullite in the presence of copper oxide during an intermediate quartz-type phase, however, also forms an intermediate spinel-type phase at that time.8 Other researchers link the formation of mullite at a lower temperature to the formation of copper aluminate.6,9 CuO is not stable and decomposes around 1050 °C to Cu2O. This results in Cu+ formation which can diffuse and react with the metakaolinite matrix.6 After dehydration of kaolinite metakaolinite reacts with copper oxide and a copper aluminate (CuAl2O4) is formed. The matrix of metakaolinite loses its stability and the diffusion of the structure elements increases. Therefore, finally, mullite is formed at the lower temperature.9
The main aim of the present paper is to analyze how copper oxide, as an additive, influences the high-temperature process of kaolinite with copper oxide, together with the composition of products formed in this process. Results achieved may be useful not only for determining capacities of aluminium silicates but a wider application of the results as well.
2 Experimental
2.1 Sample materials
In this research kaolin from Prosianov, Ukraine was used and mixed with copper oxide in the following ratios: 1∶39, 1∶19, 1∶9, 1∶4, 3∶7, 4∶6 and 1∶1. The copper oxide was formed by the thermal decomposition of copper nitrate on the kaolinite.2.2 Sample synthesis
The mixture of copper oxide and kaolin was heated at 300–350 °C until the total decomposition of copper nitrate. Thus, in the sample copper oxide was distributed equally and covered the fractions of kaolinite. The heated mixture was graded and then the samples of 6–7 mm diameter were formed in a plastic way. These samples were heated in the tubular furnace at temperatures of 800, 850, 950, 1050 and 1200 °C (heating rate, 25 °C min−1; length in the maximum temperature, 30 min).2.3 Investigation methods
Two analytical techniques were used to determine the transformation of kaolinite to mullite: (a) powder X-ray diffraction and (b) thermal analysis (diffractometer DRON-2 with CoKα radiation: U = 25 kV, I = 12 mA, λ = 1.79020 Å; derivatograph Q-1500D; interval 20–1050 °C, rate 10 °C min−1).3 Results
Samples with various concentrations of CuO were heated at 1000 °C. X-ray data (Fig. 1) showed that formation of mullite depends on the amount of additive (values in figures are in Å). It was established that 5% CuO had the most influence on mullite and crystobalite formation (see Fig. 2). The formation of mullite and crystobalite decreased with increasing amounts from 5 to 50% in the sample. Peaks for copper aluminate were not detected in samples with 2.5 and 5% CuO concentration (Fig. 1). A small amount of copper aluminate formed in the 10% CuO sample. The amount of copper aluminate increased with increasing CuO concentration. Mostly tenorite formed in the sample with a 30% CuO concentration. Cuprite formed in the samples with 40 and 50% CuO.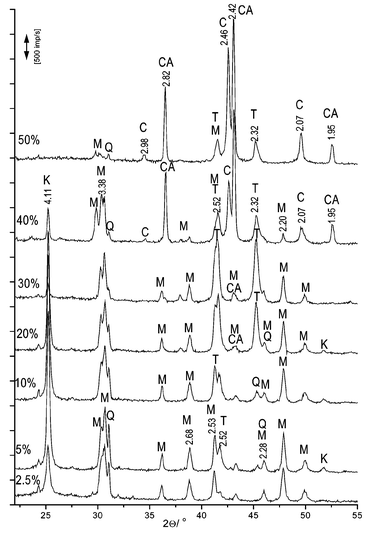 | ||
| Fig. 1 XRD analysis peaks of kaolin with 2.5, 5, 10, 20, 30 and 50% CuO (K crystobalite, Q quartz, M mulite, T tenorite, C cuprite, CA copper aluminate). | ||
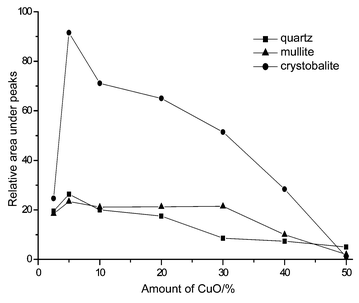 | ||
| Fig. 2 Influence of amount of CuO on formation of phases. | ||
The colour of the heated samples provides information about the phases which form during heating. A green colour is characteristic of copper silicate glasses in which Cu–O–Si bonds exist. In the literature it is not confirmed that the copper silicate forms during the kaolinite heating time. This colour indicates a formation of silicon oxide–copper oxide solid solutions. Copper oxide intervenes between the [SiO4] layer. A dark brown colour is characteristic of tenorite (copper(II) oxide). The sample with 40% CuO had a porous surface and it stuck to the ceramic container. It showed that a liquid phase had formed. The sample with 50% had more fused.
A light green colour showed that the CuO–[SiO4] solid solution formed in the samples with 2.5 and 5% CuO. Samples became darker with increasing CuO concentration. It showed that the amount of tenorite increases in the samples (see peaks of tenorite in Fig. 1). The >30% CuO samples had a lighter and reddish brown colour. Copper oxide reacts with alumina and copper aluminate forms. The amount of tenorite decreases because the amount of copper aluminate increases.
The amount of formed mullite decreases with increasing CuO content because aluminium oxide reacts with copper oxide. The amount of cristobalite should increase, however, we see that the amount of cristobalite decreases (Fig. 2). It shows that the amorphous phase of SiO2 forms. A fusion of 40% and 50% CuO samples confirms this fact. Amorphous SiO2 fuses; the temperature of fusions is decreased by CuO and oxides of alkaline-, alkaline-earth metals and iron (impurities in natural kaolin).
As we expected, 5% CuO additive most influences mullite formation. This sample has been further investigated. X-ray diffraction peaks for this sample are shown in Fig. 3. To compare with- and without-additive-phase formation we also investigated the sample without any additive (see Fig. 4). Mullite is formed at 1200 °C (without CuO). After the additive has been added, the same process can be observed in the 950–1050 °C temperature range (Fig. 3).
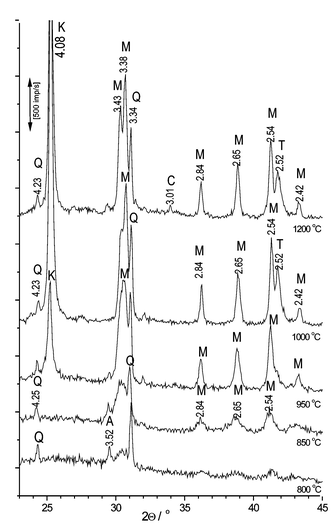 | ||
| Fig. 3 XRD analysis peaks of kaolin with 5% CuO (Q quartz, A TiO2 admixture, M mullite, K crystobalite, T tenorite, C cuprite). | ||
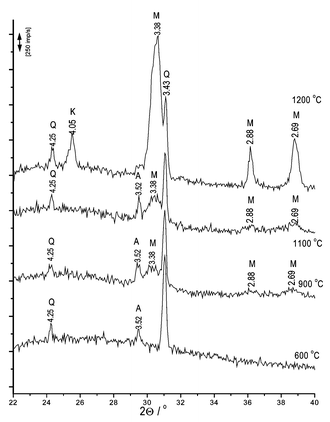 | ||
| Fig. 4 XRD analysis peaks of kaolin. The samples are from kaolin without copper oxide (Q quartz, A TiO2 admixture, M mullite, K crystobalite). | ||
In Fig. 3 only peaks of quartz are detected at the temperature of 800 °C (3.33 and 4.24 Å). The peak (3.504 Å) is bound with TiO2 impurity. Peaks of mullite appear on the lines of diffraction at the 850 °C temperature. Peaks of tenorite are visible as well. The peaks of mullite and crystobalite become intense at a temperature of 1000 °C. Moreover, quite a large amount of tenorite has been formed. At the 1200 °C temperature peaks of cuprite are observed. The amount of tenorite has decreased since copper(II) oxide decomposes to copper(I) oxide, cuprite.
The peaks of copper aluminate were not detected during the process described above (5% CuO). It is possible that the intensity of mullite formation increases due to the reaction of copper ions with SiO2, and consequently, a solid solution is formed. This fact may be proved by the green colour of samples, which were heated at temperatures of 800 and 850 °C. It may be possible that a copper oxide interacts with the [SiO4] tetrahedron and deforms a tetrahedral layer. At the end of this process the matrix of metakaolinite becomes unstable (through the deformed and decomposed chemical combinations). At the 900–1050 °C temperature interval mullite begins to form intensively extruding the surplus of amorphous quartz. It crystallizes to crystobalite.
The quantitative changes of phases are shown in Fig. 5. The intensive transformation of phases was going on at the 850–1000 °C interval. Moreover, DTA analysis confirms that the most intense phase transformation is going on over the mentioned temperature interval (see Fig. 6). In the samples with and without additives the lower temperature endothermic process started approximately at 600 °C. Copper oxide does not influence the endothermic process, but it has a great impact on the exothermic process. When additives were used, the peak of the exothermic process was reached at a temperature of 897 °C, whereas without additives at a temperature of 986 °C. When additives were used, the temperature range of the exothermic process was broad. It can be proved by the fact that two processes–formation of mullite and formation of crystobalite–were going on at the same time. X-ray diffractometry data confirm this fact (see Fig. 3). As shown in Fig. 7, the exothermic effect is dependent on the CuO particle size, the existence of the CuO crystal lattice, and the distribution of additive in the mixture. When CuO was obtained from nitrate, the peak of the exothermic process was observed some 40 °C lower than has been found when using a crystalline CuO. Copper nitrate solution wets the grains of kaolinite. Particles of formed CuO are then evenly distributed on the kaolinite crystals after heating. This way, the mixture was more homogeneous than in a sample prepared from a mechanical mixture of crystalline CuO and kaolinite. The copper oxide obtained from copper nitrate was more dispersed and had more defects of the crystal lattice; Cu2+ ions were more active, and they were slightly more diffused in the metakaolinite lattice. The exothermic process was observed in the mixture of metakaolinite and crystal CuO at the same temperature as in the mixture of kaolinite and crystalline CuO. It shows that CuO diffuses through the metakaolinite matrix after the dehydration of kaolinite.
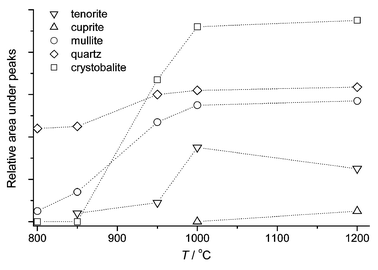 | ||
| Fig. 5 Changes of phases at the heating time (5% CuO). | ||
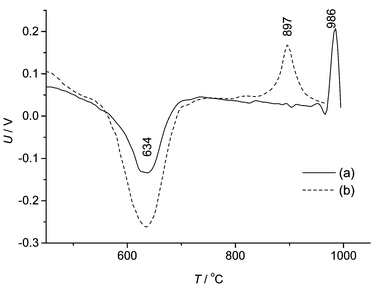 | ||
| Fig. 6 Curves of DTA: (a) kaolin without CuO, (b) kaolin with 5% CuO. | ||
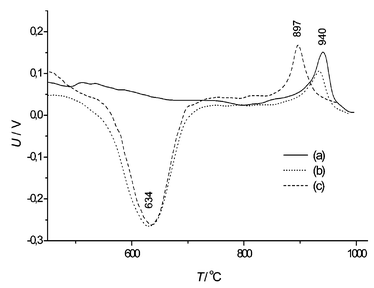 | ||
| Fig. 7 Curves of DTA: (a) metakaolin + CuOcryst, (b) kaolin + CuOcryst, (c) kaolin + CuOnitr. | ||
Copper oxide should react with the layer of aluminium oxide after the dehydration of kaolinite because it is chemically active and part of its chemical combination is breached. Our experiments show that copper aluminate is formed only at a high concentration of copper oxide. Copper oxide interacts with the layer of tetrahedral [SiO4] after the dehydration of kaolinite.
4 Conclusions
Copper oxide decreases the temperature of mullite formation from kaolinite by 150–250 °C. A 5% CuO amount has the greatest influence on mullite formation from kaolinite. Additive doesn't influence the lower temperature endothermic process, however, it does influence the exothermic process: the peak is reached at a temperature of 897 °C with additive and at 986 °C without. The temperature of the exothermic process depends on the CuO particle size and the existence of the CuO crystal lattice.The reason for the formation of mullite at the lower temperature is that the ions of copper interact with the tetrahedral layer of [SiO2] and thus, destabilize the matrix of metakaolinite.
References
- D. C. J. Jain, J. Am. Ceram. Soc., 1998, 77(6), 109 Search PubMed.
- B. Kanka and H. Schneider, J. Mater. Sci., 1994, 29, 1239 CrossRef CAS.
- M. Sarikaya and I. A. Aksay, J. Am. Ceram. Soc., 1987, 70(11), 837–842 Search PubMed.
- P. S. Nickolson and R. M. Furlat, J. Am. Ceram. Soc., 1970, 53(5), 238–240 Search PubMed.
- A. Gualtieri, M. Bellotto, G. Artioli and S. M. Clark, Phys. Chem. Mineral., 1995, 22, 215–222 Search PubMed.
- R. Giraitis, Doctoral Thesis, Vilnius, 1995.
- A. A. Špokauskas and P. V. Kičas, Sbornik Trudov, 1979, 12, 136 Search PubMed (in Russian).
- E. R. Segnit and T. Gelb, Am. Mineral., 1972, 5, 1504 Search PubMed.
- G. N. Maslenikova and T. I. Koneshova, Neorganiceskijie Materjaly, 1989, 25(2), 181 Search PubMed (in Russian).
| This journal is © The Royal Society of Chemistry 2003 |
