Comparison of a direct injection nebulizer and a micronebulizer associated with a spray chamber for the determination of iodine in the form of volatile CH3I by inductively coupled plasma sector field mass spectrometry
Bertrand
Langlois†
a,
Jean-Luc
Dautheribes
a and
Jean-Michel
Mermet
*b
aC. E. A. Valrhô DEN/DRCP/SE2A, BP17171, F-30207 Bagnols sur Cèze Cedex, France
bLaboratoire des Sciences et Stratégies Analytiques, Bât. 308, Université Claude Bernard–Lyon 1, F-69622 Villeurbanne Cedex, France
First published on 2nd December 2002
Abstract
Experiments were performed to study the influence of the volatility of a liquid sample on various sample introduction systems. The test element was iodine using NaI as a non-volatile species and CH3I as a volatile species. A direct injection nebulizer such as the DIHEN was compared to a micronebulizer associated with a cyclonic spray chamber. An ICP-MS was used for detection. A factor, K, was defined as the ratio of the 127I signal obtained for CH3I to that obtained for NaI. K values in the range 4–6 were obtained for a micronebulizer and a conventional nebulizer with spray chambers. Simple experiments showed that most of the explanation was related to evaporation of the aerosol in the spray chamber. When using a DIHEN, the K value was in the range 0.6–0.8, i.e., lower than unity, which would be the value if the DIHEN had fully eliminated the influence of the spray chamber. The departure from unity could not be explained by a change in the plasma characteristics and did not depend on the operating parameters, such as the carrier gas flow rate, the position of the nebulizer tip, or the spatial distribution of the sample.
Introduction
In the nuclear industry, reprocessing is a very significant stage in the nuclear fuel cycle. During this stage, uranium and plutonium are recycled because they can be used as re-usable energy materials from spent fuel. The nuclear material is dissolved in acid solutions, and uranium, plutonium and fission products are separated by means of solvents. Uranium and plutonium are then recycled through fabrication of new fuel elements. The waste undergoes special processing and conditioning for final disposal. Nitric acid is commonly used as the acid medium in the different steps of the recycling process. Spent nuclear fuel contains iodine and, depending on the dissolution conditions, a small amount of iodine could be present in solution1 without knowledge of its chemical form, i.e., organic or inorganic. Preliminary investigations have shown that the signal produced by iodine in an organic form such as CH3I was significantly higher than the signal produced by iodine in an inorganic form such as NaI, when an ICP-MS was used with a conventional sample introduction system, i.e., a pneumatic nebulizer associated with a spray chamber. The introduction of micronebulizers has greatly improved the nebulization efficiency, while the availability of direct injection nebulization avoids the use of a spray chamber. A study was, therefore, conducted by comparing the organic and inorganic iodine signals using micronebulization associated with a spray chamber and a direct injection nebulizer. An ICP-MS was used for detection.Experimental
Instrumentation
Measurements were performed using an ELEMENT inductively coupled plasma sector field mass spectrometer (ICP-SFMS) (Finnigan MAT, Bremen, Germany). The mass analyzer of this instrument consists of magnetic and electric sector fields in a reversed Nier–Johnson geometry. This geometry, combined with preselectable slits, allows the selection of three different resolutions, i.e., 300, 3000 and 9000 (M/ΔM, 10% valley definition). More details about this instrument have been reported in the literature.2–4 The ELEMENT system has been modified so that the torch and interface are set up inside a glove box. No change in the analytical performance was observed when compared with a standard system. Optimization of the system has been performed to obtain the best sensitivity on the 127I signal.Most experiments were performed using a MicroMist micronebulizer and a Cinnabar cyclonic spray chamber (Glass Expansion). The inner volume of the spray chamber was 19 cm3. Additional experiments used a conventional concentric pneumatic nebulizer (Meinhard Associates) with a double pass spray chamber (Finnigan MAT). A double pass spray chamber with a jacket for water circulation was used to study the influence of the temperature of the spray chamber. Nebulizers were run either with a free aspiration mode or by using a peristaltic pump (Minipulse 3, Gilson), depending on the study. A direct injection nebulizer, the DIHEN (Meinhard Associates), was used and was fed with a syringe having a 5 mL volume and an inner diameter of 12.8 mm (Model 100, KD Scientific Inc.). An external mass flow controller (5850s, Brooks Instrument) was used to permit the flush of the DIHEN even during the ignition of the plasma.
The same sampler and skimmer cones and torch remained installed during all the experiments except when the torch was equipped with a guard electrode (CD-option of Finnigan MAT ELEMENT 1) to improve the sensitivity and the acceptance to solvent loading. A power of 1400 W was used with the micronebulizer, while a power of 1250 W was used with the DIHEN. In each case, a plasma gas flow rate of 15 L min−1 and an auxiliary gas flow rate of 0.72 L min−1 were used. Unless otherwise stated, the carrier gas flow rate was 0.97 L min−1 and 0.3 L min−1 for the nebulizers with spray chamber and for the DIHEN, respectively.
Between each introduction of a new iodine compound, total cleaning of the introduction system was carried out with 2% nitric acid and ultrapure water until the observation of a low blank level, and the capillary tubes of the peristaltic pump were systematically changed.
Reagents
High-purity de-ionized water (>18 MΩ, Elga system) and ultra-pure nitric acid (J.T. Baker, Ultrex II) were used for sample preparation and vessel cleaning. Studies were performed with sodium iodide (Orion) and iodomethane (Aldrich).Containers used for the handling and storage of samples and standards were polyethylene (PE) and Pyrex vials. Both these materials could be used for inorganic iodine. In contrast, for iodomethane, there was a risk of adsorption of iodine on any plastic vial. Pyrex material was therefore used. PTFE was also used for capillary tubes. In order to avoid the risk of contamination or sorption effects, vials were cleaned with nitric acid and ultrapure water and then stored and filled with 2% nitric acid.
Standards of iodine were prepared by diluting pure commercially available reagents. All the samples and reagents were prepared daily in order to avoid loss of iodine and any contamination. In the case of iodide standards, 10−6 mol L−1 ascorbic acid, i.e., 176 µg L−1, was added to the 2% nitric acid solution as a reducing agent in order to maintain iodine as iodide. The concentration of iodine was adjusted and specified according to the experiment.
A specific protocol had to be used in order to prepare standards with iodomethane because of its easy volatilization. Iodomethane primary standards were prepared by dissolving an appropriate amount of CH3I (a few mg) in a 100 mL Pyrex bottle with 2% nitric acid. The solubility of iodomethane in aqueous solution is about 14 g L−1. Because the dissolution rate was slow, the following procedure was used. Iodomethane was added after nitric acid. Because of the difference in density between the nitric solution and the iodo-compound, the CH3I product moved down to the bottom of the vial. In this closed environment iodomethane dissolved gently into the solution. Consequently, no loss of iodine was observed. After stirring, the standard solution was stored in the dark at 10 °C. A 2.0% RSD reproducibility was obtained for five different iodomethane standard preparations at a concentration of 1 µg L−1, which was similar to the reproducibility measured for 138Ba and 165Ho solutions at the same concentration for comparison, i.e. 1.7% and 2.2% RSD, respectively.
Results
Study of nebulization efficiency
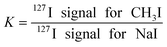
In order to compare the sample introduction efficiency, a K factor was used:Obviously, K should be equal to 1 when the sample introduction system does not introduce any difference in efficiency. Actually, a double pass spray chamber with an inner volume of 110 cm3 led to a K factor in the range 5–6, while a K factor in the range 4–5 was obtained for a MicroMist nebulizer with a delivery rate of 0.2 mL min−1, associated with a Cinnabar spray chamber, which clearly indicates a higher efficiency for CH3I.
Several explanations can be suggested for the overall range of 4–6 for the K value: (i) an influence of the mass analyzer; (ii) a change in nebulization process within the spray chamber, which could be confirmed by changing the type of spray chamber; and (iii) a change in plasma conditions and sample spatial distribution.
In order to eliminate the possible role of the mass analyzer, some experiments were performed using ICP-AES. A PerkinElmer Optima 3000 system was used with a cross-flow nebulizer and a double pass spray chamber and the 206 nm line was used for iodine. K values in the same range as those obtained by ICP-MS were obtained.
Other iodine species at 1 µg L−1, such as potassium iodate, KIO3, 2-iodoethanol, ICH2CH2OH, and iodoacetic acid, ICH2COOH, were also nebulized. When the iodine signal was normalized to that of 138Ba, the ratios were 0.20, 0.21, 0.20, 0.18, and 1.30 for NaI, KIO3, ICH2CH2OH, ICH2COOH and CH3I, respectively, which confirms the different behavior of CH3I.
Evidence of evaporation of iodomethane
Iodomethane has a very low volatilization temperature of 42 °C. It might therefore be thought that a significant evaporation could occur in the spray chamber so that iodine would be mostly in a gaseous form at the exit of the spray chamber. Two experiments were performed to provide evidence that iodomethane was introduced in the plasma in a gaseous form. In the first experiment, shown in Fig 1, two sample introduction systems based on a MicroMist nebulizer and a Cinnabar cyclonic chamber were used in sequence with a carrier gas flow rate of 1 L min−1. A solution of 3 mg L−1 of iodine and barium was pumped into the first introduction system in order to create a first aerosol. Barium was added as an element with normal behavior. A part of this aerosol was aspirated to the second nebulization system and then sent to the torch. 138Ba and 127I signals were then compared. If the aerosol at the exit of the first spray chamber is mostly in the form of fine droplets, the uptake by the second nebulizer should be negligible. In contrast, if the aerosol turns into a vapour, the uptake should be significant.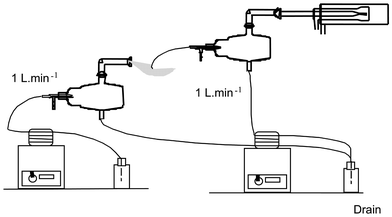 | ||
| Fig. 1 Schematic drawing of the first experimental system to give evidence that iodomethane was introduced into the plasma as a vapor. | ||
When the iodine was in the form of NaI, signals of Ba and I were weak relative to the concentration, 1.5 × 104 and 104 count s−1, respectively, which means that both elements were present mostly in the form of droplets. In contrast, when iodine was present as CH3I, a significant iodine signal could be observed, 3 × 105 count s−1, while the Ba signal remained similar. In this case, the iodine uptake was significant, i.e. iodine was in a gaseous form.
In a second experiment, a 3 µm micro-filter was inserted between the Cinnabar cyclonic chamber and the torch to eliminate the aerosol droplets (Fig. 2). A 10 µg L−1 iodine solution was used in this case. The same conclusions as for the previous experiment were obtained, i.e., no signals for Ba and I when iodine was in the form of NaI, and a signal from iodine when this element was in the form of CH3I. Clearly, these two experiments give evidence that iodine was in a gaseous form when present as iodomethane.
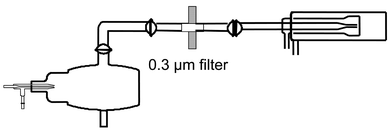 | ||
| Fig. 2 Schematic drawing of the second experimental system to provide evidence that iodomethane was introduced into the plasma as a vapor. | ||
Therefore a K value in the range 4–6 should reflect the difference in efficiency between a vapor and a liquid aerosol. Efficiency of the nebulizer for liquid aerosol was verified by nebulizing de-ionized water and trapping the aerosol with a silica gel trap. Because of the use of a high efficiency micronebulizer working at a delivery rate of 0.2 mL min−1, the efficiency was found to be higher than that of conventional nebulizers, i.e. in the range 10–17% instead of just a few %. This range is comparable to that found for similar nebulizers.5 If a 100% efficiency is assumed for the evaporation of iodomethane, and if the 10–17% range is used for liquid aerosol, a theoretical K factor should then be in the range 6–10, which is higher than the experimental one, 4–6. It could be thought that the silica gel did not trap the vapor that is formed even with the non-volatile iodine species. This would lead to a higher efficiency and, therefore, to a lower theoretical ratio, i.e. closer to the experimental one. However, cooling of a jacketed spray chamber down to 5 °C did not change the experimental K value, which means that the vapour part does not seem to play a significant role for NaI.
Besides, no change in the plasma characteristics probed by the signals of ArO+, Ar2+, CeO+/Ce+ and Ce2+/Ce+ was observed when moving from NaI to CH3I in the ranges 900–1500 W and 0.6–1.2 L min−1. An example is given in Table 1.
| MicroMist | MicroMist | DIHEN | DIHEN | |
|---|---|---|---|---|
| Ions | NaI | CH3I | NaI | CH3I |
| I+ | 289 × 103 | 1060 × 103 | 110 × 103 | 61 × 103 |
| ArO+ | 4.86 × 106 | 4.66 × 106 | 5.07 × 106 | 5.81 × 106 |
| Ar2+ | 2.29 × 106 | 2.24 × 106 | 1.48 × 106 | 1.53 × 106 |
| CeO+/Ce+ | 0.08 | 0.08 | 0.2 | 0.2 |
| Ce2+/Ce+ | 0.04 | 0.04 | 0.1 | 0.1 |
Use of direct injection nebulization
Because the evaporation of iodomethane occurs in the spray chamber, the use of a device that does not necessitate a spray chamber could solve the problem. Direct injection nebulization has been described6–8 and made commercially available. A DIHEN was selected and equipped with a syringe to avoid pulses from a peristaltic pump and adsorption by the tubing. Reduction of the carrier gas flow rate was necessary to avoid too large a solvent loading in the plasma, along with degradation of the plasma robustness.9–11 The standard position of the DIHEN tip was an 8 mm recess from the top of the intermediate tube of the torch. A power of 1250 W was used with the guard electrode, and iodine was at a concentration of 1 µg L−1.Actually, four DIHENs had to be used during the duration of the experiments. Although they led to the same optimum carrier gas flow rate at 1400 W, i.e., 0.3 and 0.2 L min−1 with and without a guard electrode, respectively, and an optimum delivery rate of 50 µL min−1, they slightly differed in some aspects. In particular, for a given carrier gas flow rate measured with a mass flow controller, a variation in the back pressure was observed for the different nebulizers. To obtain, for instance, a flow rate of 0.3 L min−1, i.e., the optimum with a guard electrode, back pressures of 3.4, 4, 4.3 and 4.4 were necessary for the four nebulizers, respectively. This spread in characteristics was reflected by the K value that was in the range 0.6–0.8. However, for a given DIHEN, the reproducibility between experiments of the K value was around 10% RSD. Uranium was used as an internal standard between experiments. In any case, this 0.6–0.8 range was significantly lower than unity and, therefore, lower than expected.
Because a high solvent loading is obtained with a DIHEN, it was verified that there was no change in the plasma characteristics, similarly probed by measuring the signals of ArO+, Ar2+, CeO+/Ce+ and Ce2+/Ce+ (Table 1). Note that the CeO+/Ce+ and Ce2+/Ce+ ratios were significantly higher than those obtained for the MicroMist nebulizer.
For similar reasons, it was verified that an increase in the carrier gas flow rate did not lead to a variation of the K value in the range 0.2–0.4 l min−1, although the iodine signal decreased by a factor of 2. Clearly, the discrepancy from unity cannot be explained by a change in the plasma characteristics.
The tip of the nebulizer is usually located a few mm off the base of the plasma so as to avoid its possible melting, i.e., 8 mm in our experiments. Because of a possible evaporation of iodomethane between the tip of the nebulizer and the base of the plasma, it was verified that the location of the tip of the nebulizer had no influence on the K value (Table 2).
| Position | 15 | 14 | 12.5 | 10 | 8.5 | 7.5 | 6 | 4 | 3 |
| K | 0.59 | 0.61 | 0.64 | 0.64 | 0.61 | 0.62 | 0.59 | 0.61 | 0.57 |
The spatial distribution of the sample in the plasma was studied by moving the torch along the axis of the cones, i.e., the z-direction, and perpendicular to the axis, i.e., the x- or y-direction. A change in the z-direction over 10 mm did not modify the K value (Table 3). A lack of variation was similarly found for the x-direction.
| Position | 0 | 2 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| K | 0.55 | 0.53 | 0.55 | 0.54 | 0.55 | 0.57 | 0.55 | 0.56 | 0.56 |
Because of the high load with a DIHEN, it was also verified that the CH3I+ ion was not present, which would have resulted from an incomplete dissociation of iodomethane. No signal was observed at a m/z of 142 corresponding to the presence of 142CH3I+.
Conclusions
Iodine is a useful element for the study of sample introduction systems, because it can be present in a volatile form, e.g. CH3I, and a non-volatile form, e.g. NaI. In this work, direct injection nebulization was compared with micronebulization associated to a spray chamber. Clearly, the use of a direct injection nebulizer such as the DIHEN leads to a significant change in the nebulization processes when a volatile compound is involved. The use of a spray chamber facilitates the evaporation of a volatile compound, which enhances the overall efficiency of the sample introduction system. Most of the difference in nebulization efficiency between a volatile and a non-volatile compound can be explained by evaporation within the chamber. A similar conclusion has been previously reported for Os, which also exhibits volatile forms.12,13 When using a DIHEN, nebulization of a volatile compound leads to a signal lower than that observed for a non-volatile compound. It could be expected that the explanation is related to a higher plasma load and, consequently, to a degradation in the plasma characteristics. However, this explanation was not confirmed by measuring the signals of several test species. The phenomenon was also not related to a change in the spatial distribution of the ions or to the position of the nebulizer tip.The use of a DIHEN leads to a substantial reduction in the influence of the sample volatility, but not to a full suppression. It remains to identify the processes not taken into account in this work.
References
- N. Boukis and E. Henrich, Radiochim. Acta, 1991, 54, 103 Search PubMed.
- L. Moens, F. Vanhaecke, J. Riondato and R. Dams, J. Anal. At. Spectrom., 1995, 10, 569 RSC.
- I. Feldmann, W. Tittes, N. Jakubowski, D. Stuewer and U. Giessmann, J. Anal. At. Spectrom., 1994, 9, 1007 RSC.
- U. Giessmann and U. Greb, Fresenius' J. Anal. Chem., 1994, 350, 186 CrossRef CAS.
- J. L. Todoli, V. Hernandis, A. Canals and J. M. Mermet, J. Anal. At. Spectrom., 1999, 14, 1289 RSC.
- D. R. Wiederin, F. G. Smith and R. S. Houk, Anal. Chem., 1991, 63, 219 CrossRef CAS.
- D. R. Wiederin, R. E. Smyczek and R. S. Houk, Anal. Chem., 1991, 63, 1626 CrossRef CAS.
- J. A. McLean, H. Zhang and A. Montaser, Anal. Chem., 1998, 70, 1012 CrossRef CAS.
- E. Björn and W. Frech, J. Anal. At. Spectrom., 2001, 16, 4 RSC.
- J. L. Todoli and J. M. Mermet, J. Anal. At. Spectrom., 2001, 16, 514 RSC.
- J. R. Chirinos, K. Kahen, S. A. E. O'Brien and A. Montaser, Anal. Bioanal. Chem., 2002, 372, 128 Search PubMed.
- J. M. Bazan, Anal. Chem., 1987, 59, 1066 CrossRef CAS.
- A. Lopez-Molinero, J. R. Castillo and J. M. Mermet, Talanta, 1990, 37, 895 CrossRef CAS.
Footnote |
| † Present address: Rockwood Electronic Materials, F-50620 Saint Fromond, France. |
| This journal is © The Royal Society of Chemistry 2003 |