Tandem calibration methodology: dual nebulizer sample introduction for ICP-MS
Vanessa
Huxter
,
Jan
Hamier
and
Eric D.
Salin
*
Department of Chemistry, McGill University, 801 Sherbrooke St. W., Montreal, Quebec, Canada H3A2K6
First published on 3rd December 2002
Abstract
The application to inductively coupled plasma mass spectrometry (ICP-MS) of a calibration method called the TCM (tandem calibration method) is described. The TCM involves simultaneous introduction of sample and standard into the plasma by two nebulizers operated in parallel. Figures of merit were determined by comparing the results obtained with those produced using the method of standard additions and external standard calibration was used as a probe of the severity of the matrix effect induced. The test solutions in this study contained Cu, Y, Pt and Pb as analytes and 10 mM Ba and 35 mM Na as matrix effect generators. Classical standard addition verified the validity of the TCM and the two methods were shown to be statistically equivalent. The precision of the results obtained was limited by the noise of the sample introduction device (about 4% RSD on difficult samples versus roughly 1% on clean standards), while the accuracy was only slightly limited by the short and long term stability of the arrangement, typically around 2% relative error. The method was easy to implement on existing equipment, inexpensive and potentially suited to automation.
Introduction
The inductively coupled plasma (ICP) used in atomic emission spectrometry (ICP-AES) or as a source for mass spectrometry (ICP-MS), is routinely used for the analysis of liquids and there is a growing use of ICP-based solid sample analysis techniques, especially in the field of environmental analysis.1,2 ICP-MS3,4 is generally preferred for ultra trace level multi-element analysis owing to good detection limits, simple spectra, high resolution and wide linear dynamic range; however, ICP-MS is significantly hampered by matrix effects.5Matrix effects can be attenuated to some extent by the use of the technique of internal standards (IS) to the point where this technique, along with its most efficient subset, namely isotope dilution (ID), has become a ubiquitous procedure for ICP-MS analyses. The efficiency of IS in terms of matrix effects reduction is highly dependent on the proper choice of the internal standard(s) that closely matches the response of the desired set of analytes. While ID is a perfect match in terms of the “ideal” internal standard, it is limited in its application by the availability of another isotope as well as its cost for each analyte undergoing analysis. On the other hand, the technique of standard additions is one of the most powerful techniques for analytical calibration since it can provide higher accuracy than either external or internal standards with difficult samples regardless of the nature of the matrix. The proper application of this method produces precise, accurate results independent of significant matrix effects induced bias and is therefore very desirable for use in ICP-MS calibrations. However, it is usually employed only as a last resort because it is time consuming, labour-intensive and potentially expensive. The objective is therefore to devise a method that combines the accuracy of standard additions with the ease of use of external standards.
A procedure, called the tandem calibration method (TCM), which combines the desirable aspects of external standards and standard additions, was proposed for ICP-AES.6 The system consists of a tandem nebulizer apparatus in which the sample solution is aspirated through one nebulizer while standards are aspirated through the other. The aerosols from both nebulizers merge into a Y adapter attached to a single transport tube leading to the injector tube of the ICP-MS torch.
The development of new analysis techniques has seen the appearance of unusual sample introduction devices grafted onto the ICP in place of the classical nebulizer in order to reduce matrix effects, reduce sample preparation or increase the sensitivity or analysis throughput. Little has been published on the use of two introduction devices operating in parallel; however, the possibility of using a nebulizer in parallel with an electrothermal vaporizer (ETV)7 or a laser ablation (LA) device8–10 has been proposed. Moenke-Blankenburg et al.11 described quantitative analysis of glass by ICP-AES, laser microanalysis (LM-ICP-AES) and laser ablation (LA-ICP-AES). A similar approach12 was used for comparing spark ablation (SA-ICP-AES) and (LA-ICP-AES) on minerals with a configuration that is very similar to that in the present study. The main advantage offered by these configurations is the ability to substitute certified reference materials (CRMs) with common and relatively affordable liquid standards for the calibration procedure while retaining an acceptable level of accuracy. Comparing the TCM with the methods described by the various authors,9–12 the most obvious difference is that the standards are not run at the same time as the sample. Their proposed method is closer to a sophisticated form of external calibration with “constant” plasma loading than the TCM, which is more analogous to standard additions in terms of its principles and performance. Other efforts have focused on the matrix interference problems without modifying the sample introduction device. Ross and Hieftje13 reduced interference effects by removing initial ion optics, while Tanner14 re-designed the ICP-MS apparatus, applying the known physics of the system. None of these attempts have resulted in a reliable, convenient procedure for analyzing samples with complex matrices.
The tandem calibration method (TCM) is a variation on the method of standard additions, the main difference being that the addition of a standard is not performed in the sample itself, but in the transport tube en route to the plasma. The apparatus used, described in detail in the Experimental section, consists of a sample introduction device and a standard introduction device. In this case, both were nebulizers. The two devices are connected to the injector tube of the ICP torch by means of a Y-shaped glass adapter. The principle behind this approach is based on a few simple postulates which have already been studied and published.6–8,10,15
A comparable approach of on-line standard additions was proposed by Wiederin et al.:16 however, instead of relying on two nebulizers in parallel, which are later merged into a single stream to the ICP torch, the sample and standards flows were merged prior to a single direct injection nebulizer (DIN) by means of a flow injection analysis (FIA) setup. Also, the use of an FIA system for sample and standard pumping results in transient signals. The method of Wiederin et al. corrects for nebulizer drift by simultaneously introducing standard and sample into the same sample introduction device. The ratio of their respective intensities should remain constant if no drift is present between the respective sample and standard flow rates. However, it cannot be applied to other types of sample introduction device such as ETV.7
The TCM method has the advantage of employing commonly used nebulizer types and therefore can be implemented with minimal expense or difficulty. The present work uses a dual nebulizer system and concentrates on the analysis of difficult liquid samples. Sodium has been selected as a matrix effect generator since it is an alkaline element commonly present in high concentrations in samples of interest, such as blood plasma or sea-water, which require trace or ultra-trace level determination. Barium was chosen for its high mass and low ionization potential, making it a particularly efficient matrix effect generator in ICP-MS. The object of this study is to demonstrate that the TCM is a potential alternative to classical methods, combining the ease of use and cost effectiveness of external standards with the accuracy of standard additions.
Method
The TCM can calibrate any technique, whether transient or steady state, provided it is able to generate analyte in the form of a dry or wet aerosol, vapor, free atoms or ions. It is assumed that the standard introduction device generates a steady state signal. For the dual nebulizer case, the method can be briefly summarized as follows below. A full description of the method with all the pertaining assumptions and mathematical equations has already been published (Hamier and Salin6) and the reader is invited to refer to it.Brief method description
The proposed method relies on the simultaneous operation of two independent nebulizers, one for the samples and one for the standards, merging into a single ICP torch. The relative efficiencies of the nebulizers (fr) can be calibrated by running a standard in one while the other is aspirating a blank, and vice versa. The procedure can be repeated at regular intervals in order to monitor for drift although it is quite feasible to use distinct internal standards in each nebulizer if severe very-short-term drift is expected. The analysis itself requires the sample to be run continuously in its devoted nebulizer while the standard nebulizer is successively fed with blank and a series of increasing concentration standards. The resulting series of signals is processed mathematically in order to yield the apparent analyte concentration in the sample (ca), which can be converted into the true analyte concentration (cs) with the knowledge of fr. In brief, the method can be seen as an on-line dynamic standard additions without modifications of the original composition of the sample solution.Experimental
Instrumentation
The experiments were carried out using a PE SCIEX Elan 6000 ICP-MS instrument with the modified sample introduction instrumentation illustrated in Fig. 1. A cross flow nebulizer and spray chamber, fed by a peristaltic pump, was used to aspirate the sample solutions while standards were introduced through a type C Meinhard® nebulizer and spray chamber. Both nebulizers were fed by a Gilson Minipuls 3 peristaltic pump. The aerosol from the two nebulizers was combined before the plasma at a Y-joint (Y). The dual nebulizer configuration was chosen over a merging flow system, where the Y joint would have been placed before a single nebulizer and spray chamber, because it allows adjustment of one of the nebulizer parameters with very little effect on the other. This apparatus was similar to that used previously:6 however, the geometry of the configuration was different. In the system devised for the ICP-AES the plasma was located in a vertical position, leading to the vertical orientation of the entire nebulizer system. In ICP-MS, the plasma is oriented horizontally and the nebulizers were placed to ensure that all tubing ran downward to prevent large drops or pools of solution from entering the plasma and causing irreproducible behaviour.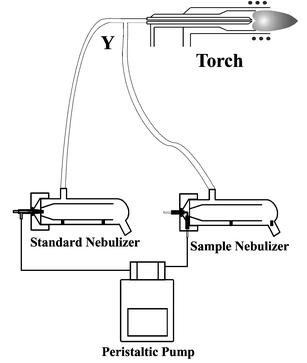 | ||
| Fig. 1 Experimental configuration for dual nebulizer system. | ||
Samples and standards
All samples and standards were prepared by dilution of a 100 ppm multi-element standard, containing Cu, Ca, Fe, Pb, Mg, Ni, Zn, Al, K, Y, Pt and Na in 5% HNO3 with trace amounts of HCl [SCP Science] with Milli-Q water. The samples (test solutions) were prepared in the following matrices: 10 mM Ba as Ba(NO3)2 and 35 mM Na as NaNO3, both from Alfa Aesar (Puratronic brand). All samples were prepared gravimetrically in order to increase the accuracy with which the concentrations of the solutions were known. The composition of the various samples is indicated in the corresponding tables under Results and discussion. For supplier details see Table 1.| Item | Supplier | Location |
|---|---|---|
| Meinhard nebulizer, type C and low Gas flow type C | J.E. Meinhard Associates, Inc. | Santa-Ana, CA, USA |
| Dell Otiplex GXM 5166 computer | Dell Computers | Round Rock, TX, USA |
| PE Sciex Model Elan 6000 ICP-MS and crossflow nebulizer | PE Sciex | Norwalk, CT, USA |
| Multi-element solution, ref. 900-M03-001 | SCP Science | LaSalle, Qc., Canada |
| Gilson Minipuls 3 peristaltic pump | Mandel Scientific | Guelph, On., Canada |
| Ba(NO3)2 and NaNO3 | Alfa Aesar | Ward Hill, MA, USA |
Operating conditions
The standard conditions listed in Table 2 and Table 3 are those used throughout this study unless noted otherwise. The sample and standards liquid flow rates were selected for optimum signal under the aforementioned gas flow rates/plasma conditions.| RF power: | 1 kW |
| Plasma Ar flow rate: | 15 dm3 min−1 |
| Auxiliary flow rate: | 1.2 dm3 min−1 |
| Lens voltage: | 6 V |
| Scan mode: | Peak hopping |
| Dwell time per u: | 20 ms |
| Sweeps per reading: | 5 |
| Readings per replicate: | 1 |
| Replicates: | 5 |
| Masses used: | Cu 63; Y 89; Pt 195; Pb 208 |
| Sample nebulizer Ar flow rate: | 0.55 dm3 min−1 |
| Sample flow: | 1.62 cm3 min−1 |
| Standard nebulizer Ar flow rate: | 0.70 dm3 min−1 |
| Standard flow: | 4.25 cm3 min−1 |
Procedure
Results and discussion
The initial experiment to test the TCM procedure used a 5% HNO3 blank and a matrix matched blank solution containing 35 mM Na. Analyte standards were introduced through the standard nebulizer while the blank solutions were run through the sample nebulizer. This verified the assumptions that the TCM produced an acceptable calibration curve within the linear range of the detector and that the low concentration of the analyte, with respect to the total matrix concentration, does not influence the observed matrix effect. The slopes of the calibration curves on samples of acid blank and the matrix matched blank TCM were compared to quantify the amount of matrix-induced suppression. The slope ratios were used as an estimate of the suppression. The main assumptions were proven since the intercepts were centered around zero, as expected for properly blank corrected signals (the least squares calibration graphs all had R-squared values better than 0.9999 with no obvious curvature in the residual plots). It should be noted that the correlation coefficient is only an expression of the relationship between the observed and probable data. This alone cannot be taken as an indicator of matrix correction.The standard addition methodology and the TCM give good accuracy and demonstrate a clear advantage over the method of external standards. The results obtained for the TCM and standard additions on the multi-element solution containing Cu, Y, Pt, Pb in a 35 mM Na matrix (Table 4), when tested using a comparison of means t-test (with different estimated standard deviations), were shown to be statistically equivalent. The improvement in accuracy with respect to external calibration ranges from a factor of roughly 3 for Cu to about 45 for Pb. The external standards calibration leads to a severe systematic underestimation of the concentration of all observed analytes. There is no such trend observable with the TCM or standard additions. The mass and ionization potential of the matrix effect generator with respect to the analyte has a well-documented effect on the amount of suppression/enhancement observed.17 Heavier concomitants with lower ionization potentials have a much stronger impact on the analyte signal and affect the lighter elements to a greater extent. This can reflect space charge effects (the mutual repulsion of ions within the ion beam) and the larger inertia of heavier atoms, which influences the trajectory of neighbouring ions3 with lighter species experiencing a greater trajectory change. The presence of an easily ionizable element (EIE) such as Na will tend to suppress the analyte signal, as observed from the external calibration data, directly because of mass charge effects (despite its small mass) and indirectly since EIEs can reduce the ionization of elements in the plasma through increased electron density. Standard additions and TCM linear least-squares regression calibration graphs were obtained with R2 ≥ 0.99 for all elements. The average concentration obtained by standard additions and TCM were statistically indistinguishable from each other and from the true value of the sample analyzed.
| Element | ||||
|---|---|---|---|---|
| Cu | Y | Pt | Pb | |
| True value (ppb) | 24.15 | 24.15 | 24.15 | 24.15 |
| Slope | 42.3 | 160 | 39.3 | 73.2 |
| Intercept | 5.52 × 103 | 1.92 × 104 | 4.80 × 103 | 8.77 × 103 |
| f r | 5.00 | 5.00 | 5.00 | 5.00 |
| c a (ppb) | 131 | 120 | 122 | 120 |
| TCM value (cs) (ppb) | 26.1 | 24.1 | 24.4 | 24.0 |
| Error (%) | 7.5 | −0.37 | 1.1 | −0.80 |
| Standard additions (ppb) | 25.3 | 25.3 | 27.0 | 24.6 |
| Error (%) | 1.0 | 1.0 | 7.6 | −1.9 |
| External calibration (ppb) | 19.1 | 21.3 | 14.8 | 15.8 |
| Error (%) | −24 | −15 | −41 | −37 |
A second experiment was performed as described above using the same multi-element solution in a 10 mM Ba matrix (Table 5). Barium, which has a large mass charge ratio and low ionization energy, predictably produced severe matrix effects. The observed RSD for severely interfered samples was typically 2–4% on sample intensity measurements for both the TCM and traditional standard additions versus about 1% for non-interferred samples. These errors are within the limit of the stability of the configuration considering the noise levels from two sample introduction devices as well as the plasma and are insignificant compared to the error caused by external calibration. No significant memory effects were observed as long as thorough flushing was performed after each analysis (approximately 2–3 min). Traditional external standards, standard addition and TCM calibrations were performed in the same manner as described for the Na matrix effect generator sample. Standard additions and the TCM methodology produced marked accuracy improvements over external calibration for all elements, taking into account the instrumental uncertainty. Again, external calibration drastically underestimated the sample solution concentration: however, the error was much greater with the barium matrix since it has a higher mass–charge ratio and one of the lowest first ionization potentials of all stable elements.18 The results obtained by traditional standard additions and the TCM were in excellent agreement with true solution concentration values (Table 5). Both methodologies produced average values that, when tested in the same method used for the Na matrix, were statistically indistinguishable from the true concentration and each other at the 99% confidence level. Linear calibration curves were obtained for all methodologies with a correlation coefficient of R2 > 0.99.
| Element | ||||
|---|---|---|---|---|
| Cu | Y | Pt | Pb | |
| True value (ppb) | 25.73 | 25.73 | 25.73 | 25.73 |
| Slope | 68.6 | 421 | 89.7 | 305 |
| Intercept | 9.95 × 103 | 5.98 × 104 | 1.24 × 104 | 4.20 × 104 |
| f r | 5.51 | 5.51 | 5.51 | 5.51 |
| c a (ppb) | 145 | 142 | 138 | 138 |
| TCM value (cs) (ppb) | 26.3 | 25.8 | 25.0 | 25.0 |
| Error (%) | 2.3 | 0.28 | -2.8 | -2.9 |
| Standard additions | 24.9 | 25.2 | 24.5 | 25.4 |
| Error (%) | -1.4 | -2.3 | -4.9 | -1.4 |
| External calibration (ppb) | 9.19 | 6.85 | 6.85 | 6.01 |
| Error (%) | -64 | -73 | -73 | -77 |
A long-term drift analysis was performed on the instrument in order to evaluate its stability over the course of an experiment. The results indicated that the maximum variations observed were extremely low, of the order of 1–2% over 2 h. A measure of the short- and long-term drift associated with the solution introduction devices can be obtained from the short- and long-term behavior of the fr (relative transport efficiency coefficient) values. The RSD on those average fr values are an indication of the day-to-day stability of the solution introduction devices (nebulizers, transport phenomena, gas pressure and peristaltic pumps). The values of fr used for calculation purposes (reported in the tables) are the average value of the individual values of fr calculated on each element.
Conclusion
The TCM consistently gave better results than those obtained by the method of external calibration. The results indicate no tendency of the TCM to over- or underestimate the correct concentrations: there are only simple fluctuations about a mean, statistically indistinguishable from the true sample concentration. The TCM values were statistically equivalent to those obtained by standard additions, which is the favoured technique for analytical calibration with difficult samples because it provides the highest accuracy independent of the matrix. The configuration of the instrument was such that it was impossible to install the two spray chambers close to the torch. The experimental configuration required the use of long transport tubes that were sources of analyte loss by drop deposition. No significant memory effects were observed if a flushing time of at least 2 min was used between solutions, and, except for the most concentrated solutions, a flushing time of 45 s to 1 min was sufficient.The TCM can handle samples considered impossible by external calibration standards, and it possesses clear advantages over the traditional use of the method of standard additions. Standard additions is usually employed only as a last resort since it is both time consuming and expensive. The TCM methodology achieves the accuracy of standard additions while maintaining the simplicity of external standards. The addition of standards is not performed in the sample itself and, therefore, any remaining sample is in its original condition and can be re-analyzed by another method. Furthermore, there are no dilution effects due to the absence of “spiking”. Any possible dilution effects from the standard nebulizer are taken into account in the relative transport efficiency factor, fr. A small loss of sensitivity with respect to the use of the classical single nebulizer standard additions method is expected because of sample dilution and additional plasma loading that occurs in the merging adapter when the standard (or blank) stream is added. The sample throughput (number of samples that can be analyzed in a given amount of time) of the TCM is not as efficient as external standards and is equivalent to that of standard additions. However, total analysis time including sample preparation is much shorter for the TCM as compared to standard additions. The TCM is also easy to perform, its main flaw being that the transport efficiency coefficient must be periodically recalibrated.
This method could be applicable to a wide array of instrumentation that relies on the analysis of samples in the form of vapor, whether static or transient sample introduction techniques are used. While the description of the TCM presented here is based on the assumption of a linear behavior of the additions, the method should remain perfectly usable if a non-linear fit model is required. This methodology could be adapted to many existing commercial system with a minimum of modifications and easily automated.
References
- H. Baumann, Fresenius' J. Anal. Chem., 1992, 342(12), 907–916 CrossRef CAS.
- S. J. Hill, T. A. Arowolo, O. T. Butler, S. R. N. Chenery, J. M. Cook, M. S. Cresser and D. L. Miles, J. Anal. At. Spectrom., 2002, 17, 284–317 RSC.
- Inductively Coupled Plasma Mass Spectrometry, ed. A. Montaser, Wiley, New York, 1998, pp. 16–31 Search PubMed.
- J. R. Bacon, J. S. Crain, L. Van Vaeck and J. G. Williams, J. Anal. At. Spectrom., 2001, 16(6), 879–915 RSC.
- J. W. Olesik, Anal. Chem., 1991, 63, 12A–21A CAS.
- J. Hamier and E. Salin, J. Anal. At. Spectrom., 1998, 13, 497–505 RSC.
- J. Hamier and E Salin, 64e congrès de l'ACFAS, Montréal, Qc., Canada, , May 1996..
- M. Thompson, S. Chenery and L. Brett, J. Anal. At. Spectrom., 1989, 4(1), 11–11 RSC.
- S. Chenery and J. M. Cook, J. Anal. At. Spectrom., 1993, 8(2), 299–303 RSC.
- L. A. Allen, R. S. Houk and J. J. Leach, FACCS XXIII Conference, Kansas City, MO, USA, September 29–October 4, 1996.
- L. Moenke-Blankenburg, T. Schumann, D. Günther, H.-M. Kuss and M. Paul, J. Anal. At. Spectrom., 1992, 7, 251–254 RSC.
- L. Moenke-Blankenburg, J. Kammel and T. Schumann, Microchem. J., 1994, 50, 374–384 CrossRef.
- B. S. Ross and G. M. Hieftje, Spectrochim. Acta, 1991, 46B, 1263–1273 Search PubMed.
- S. D. Tanner, Spectrochim. Acta., 1992, 47B, 809–823 Search PubMed.
- J. W. Elgersma, J. Balke and F. J. M. J. Maessen, Spectrochim. Acta, 1991, 46B(6/7), 1073–1088 Search PubMed.
- D. R. Wiederin, R. E. Smyczek and R. S. Houk, Anal. Chem., 1991, 63, 1626–1631 CrossRef CAS.
- E. H. Evans and J. J. Giglio, J. Anal. At. Spectrom., 1993, 8, 1–18 RSC.
- CRC Handbook of Chemistry and Physics, ed. R. C. Weast, CRC Press, Cleveland, OH, USA, 1974 Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |
