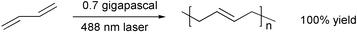News & Views
Some recent trends and problems in green chemistry
Some recent trends and problems in green chemistry
Albert Matlack has been one of the pioneers in green chemistry education and his book Introduction to Green Chemistry (reviewed on the Green Chemistry website) was based on a one semester course at the senior-graduate level interface. Here he critically considers some of the recent developments in green chemistry and some possibilities for improvements especially in areas of solvent replacement, intensive processing, electrochemical synthesis, and catalysis
Green chemistry is coming of age with interest in it increasing in both academic and industrial laboratories.1 Progress has been made in the search for processes that use fewer toxic chemicals and produce less waste while using less energy. However, many challenges remain in the shift to a sustainable future.2The elimination of toxic and / or flammable organic solvents continues to be an area of intense interest.3 A wide variety of reactions have been run in sc carbon dioxide,4 including enzymatic ones. This medium is now being used commercially for the polymerization of tetrafluoroethylene by DuPont, dry cleaning of clothes, cleaning of semiconductor chips, hydrogenations and the extraction of caffeine from coffee. It offers promise for non-aqueous dyeing of textiles and the preparation of fine particles.5 Cheap polyethercarbonates6 might be used instead of the earlier fluorinated surfactants to bring other uses closer to commercialization.
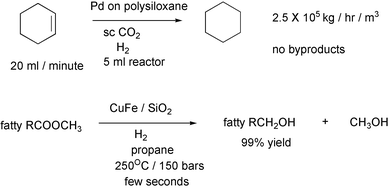
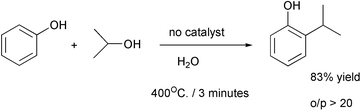
The rates of hydrogenations are limited by the low solubility of hydrogen in the usual solvents. Much higher rates can be obtained in supercritical fluids in which hydrogen is miscible. The first example uses sc carbon dioxide7 and the second uses sc propane.8 The work-up in the second reaction consisted of flashing off the hydrogen, propane and methanol.
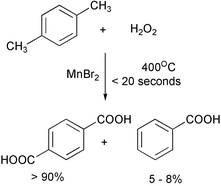
Supercritical water (with a critical temperature of 374 °C.) is too hot for many organic compounds to be stable. Batch reactions with sc water have usually led to mediocre yields and poor selectivity, but recent reactions in sc water with short contact times have been more successful. Phenol has been alkylated with isopropyl alcohol in three minutes in 83% yield.9
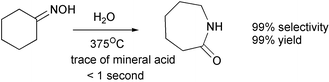
Terephthalic acid has been produced by the oxidation of p-xylene in > 90% yield in < 20 seconds in a continuous process.10
Caprolactam has been made from cyclohexanone oxime in sc water containing a trace of sulfuric acid with 99% selectivity using a continuous flow microreactor in 0.7 second.11
The limitation on the use of supercritical fluids is that they require equipment for use at 2000 p.s.i. or more. Many labs may not be set up for this.
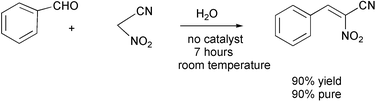
It should be possible to run many more reactions in water at lower temperatures, especially if hydrotropic agents such as sodium xylenesulfonates or cyclodextrins are used to solubilize the organic compounds, or if surfactants are used to form emulsions. Recently, starch, gum arabic and glucosamine have been used to solubilize carbon nanotubes in water.12 Some Knoevenagel reactions have been run in water without catalyst.13 The work-up consisted of filtration of the product after cooling to 0 °C. The filtrate could be used again in additional runs, the yield being the same as in the first and fifth runs. The product could be recrystallized from ethanol (a benign solvent).
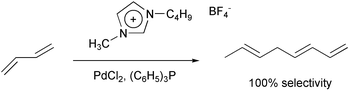
Ionic liquids are being tested as media for a wide variety of reactions.14 In some cases, the organic product can be recovered as a separate layer. However, in far too many cases, workers resort to solvents such as ethyl ether or methylene chloride to recover the product, which obviates the advantage of the ionic liquid as a replacement for an organic solvent.
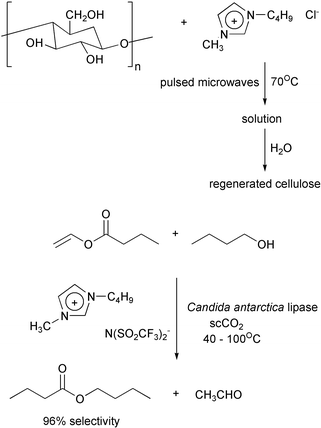
Sometimes the addition of carbon dioxide can promote separation or the product may be removed by vacuum distillation.15 Two recent results are important: The first is the ability to dissolve cellulose in an ionic liquid and to then run reactions on it.16
The second combines an ionic liquid with sc carbon dioxide in an enzymatic esterification in which the enzyme is more stable thermally than it is in water.17 The substrate and the product are in the sc carbon dioxide layer and the enzyme is in the ionic liquid.
The big questions are about the cost and toxicity of ionic liquids. These will vary depending on the structure and the anion. Quaternary ammonium salts used now as bactericides, fabric softeners, hair conditioners and detergents are neither too expensive nor too toxic for general use. For most structures, the LD50 is 100–5000 mg/kg.18 In addition, almost no material is too expensive to use if none is lost and there is a simple method to recycle it for reuse, e.g. the rhodium catalyst in the hydroformylation of propylene.
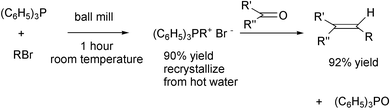
More reactions should be run without solvent in the gas, liquid or solid phases, but only after testing by thermal analysis to see how exothermic they are. Just grinding the reactants together has worked well in some cases. High yields of olefins from the Wittig reaction have been obtained by grinding the reactants in ball mills.19
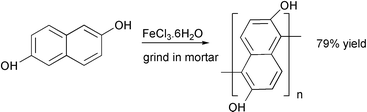
Grinding in a ball mill with iron(III) chloride hexahydrate converted 2,5-napthalenediol to a polymer that cannot be made by a comparable reaction in solution.20
While grinding to perform reactions in the laboratory is not common, grinding is done on a huge scale in the processing of mineral ores prior to benefication.
The vast majority of the reactions that are run without solvent, and are called green, are worked up with solvents such as ethyl ether and methylene chloride. This means that a plant employing such a process still has to handle toxic and/or flammable solvents as well as recycle them. There is an urgent need to study nonsolvent work-ups by means such as vacuum distillation, membrane separations, extraction with hot water (with or without hydrotropic reagents), melt recrystallisation etc—or at least shift to more benign solvents.
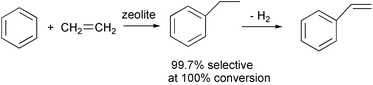
Yields and purity can often be increased by separating products continuously as they are formed. A variety of techniques are available for this, including reactive distillation, continuous extractions, membrane reactors, biphasic reactions, adsorption, crystallizing out as formed and nanoreactors such as zeolites or other molecular sieves. These methods can shift equilibria, and avoid further reaction, isomerization or decomposition. Short contact times in continuous flowing systems are often useful. In a typical reactive distillation, benzene and ethylene are passed into a vertical column of a solid acid catalyst. Any ethylbenzene that forms drops into the pot below, with 99.7% selectivity at 100% conversion in the reaction.
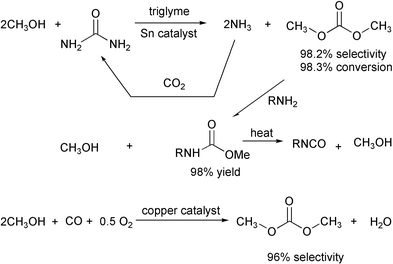
In similar processes, benzene can be alkylated by propylene with 99.6% selectivity and methyl tert-butyl ether can be produced from isobutylene with methanol with nearly 100% selectivity. Dimethyl carbonate can be prepared from urea with 98.2% selectivity.
Dimethyl carbonate can be reacted with amines to produce methylcarbamates which can be pyrolyzed to isocyanates. The ammonia and methanol side products can both be recycled, so that the total sequence from urea to isocyanate uses only the amine and carbon dioxide and produces water as the only useable byproduct. The sequence uses carbon dioxide instead of the toxic carbon monoxide. It should be possible to reduce toxicity further by substituting ethanol for methanol in the synthesis.
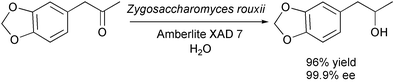
The reduction below is possible only because the inhibition of the yeast by the starting material and the product is removed by adsorbing both on a resin:
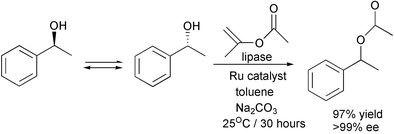
The maximum yield in the resolution of a racemate is 50%. However, an increasing number of resolutions which achieve higher yields by in situ racemization of the unwanted isomer are being done. The following example uses a ruthenium catalyst for the racemization:21
The techniques for separating a product as formed should be tried in any case where an initial product can react a second time. They should be tried in a reaction where % selectivity declines with increasing % conversion. For example, they might increase yields in the reaction of ethylene oxide with water or ammonia and in the base-catalyzed self condensation of acetone. Reactive distillation should be tried in the reaction of isobutane with butenes using solid acid catalysts.
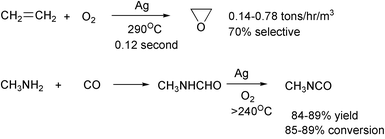
Process intensification offers the prospects of higher yields and purities with less capital expense and smaller plants.22 Problems of scale-up, heat transfer, mass transfer and safety are reduced greatly in these continuous flow systems. The techniques include microreactors with channels, spinning disc reactors, spinning tube in tube reactors, use of microwaves, extruders with or without ultrasound or microwaves and hydrogenation in sc carbon dioxide. Channeled microreactors have been used in the preparation of ethylene oxide23 and in the synthesis of methyl isocyanate.
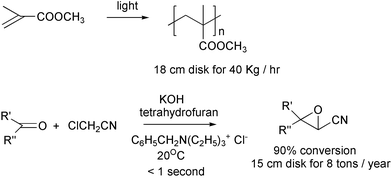
Spinning disc reactors, in which the reagents drop on to a disc spinning at several hundred r.p.m., have been used for the polymerization of methyl methacrylate24 and in a Darzens Reaction.25 In the second reaction, the reaction time was reduced by 99.9%, the inventory by 99% and the impurity level by 93%.
The spinning tube in tube reactor of Holl Technologies consists of a rotor spinning at 3000 r.p.m. inside a stator with an annular gap of 0.25–1.5 mm. which ensures excellent mixing of reagents.6 Sizes vary from desktop units that can process pounds per day to one about 15 feet long that can handle a ton per hour.
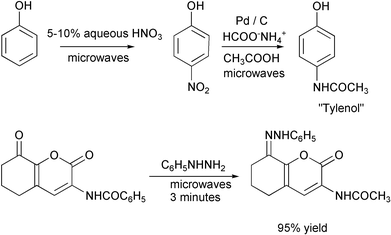
Reactions that take several hours under conventional conditions can be done in a few minutes with microwaves.26 Examples include the preparation of ″Tylenol″27 and phenylhydrazones.28 The work-up in the second case consisted of cooling, addition of methanol and filtration.
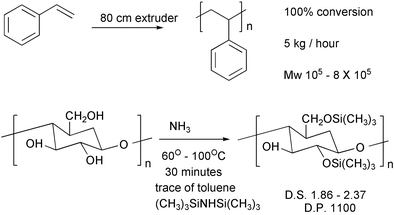
Extruders offer another route to continuous processing. Styrene can be polymerised in an extruder29 and cellulose can be derivatized in one.30 Polymers based on renewable cellulose cost more than those derived from petrochemicals. If it can be made more general, derivatization in extruders should lower the cost of the former appreciably. For reactions without ammonia, it may be possible to achieve higher rates by going to higher temperatures.
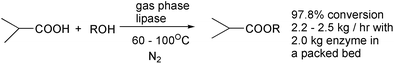
Gas phase biocatalysis in a tubular reactor offers possible reductions in plant size of up to 1000 times. Enzymes are more stable thermally when restricted amounts of water are present.31
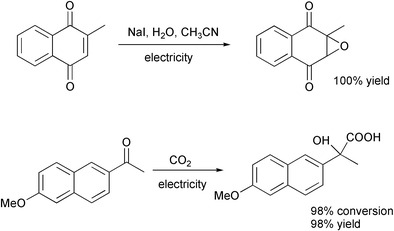
Electricity is a cheap and potentially very clean reagent ($0.06 per ″mole″). There is no catalyst to remove or recycle. It can also be used to regenerate expensive reagents in situ. It deserves to be used more often in organic chemistry.
There are some cases where the desired result can be accomplished by doing without a chemical with a cost saving. Scale inhibitors are often added to cooling water to prevent deposition of calcium carbonate. Application of pulsed ultrasound or electric fields outside the pipe causes the calcium carbonate to form in fine crystals that sweep through with the water.32 Machining can be done without cutting oils when the tool is coated with a nanocomposite film of Ti1–xAlx/a–Si3N4 put on by plasma deposition.33 Vacuum pumps that use no oil are now in use by the semiconductor industry.34 They are also suitable for mass spectrometry. It has been possible to produce 140 nm features by irradiating a silicon chip through a quartz mold with a laser pulse of 20 nanoseconds.35 The mold sinks into the molten surface of the chip and is then removed. This may eliminate the need for many photoresists and the cleaning needed with them.
Refrigeration can be done without refrigerants using thermoelectric, magnetocaloric or thermoacoustic systems. The solid state devices have no moving parts, no emission of toxic gases, low maintenance, high reliability and long life. The energy consumption of the thermoelectric devices is too high for general use, but efforts are underway to increase ZT, the figure of merit, by choice of compounds other than the conventional ones such as Bi2Te3.36 Thermoacoustic systems are being scaled up by Praxair and Los Alamos labs.37
Drinking water can be sterilized by heat, high pressure, ultraviolet light, membrane microfiltration, electron beam or pulsed electric fields without the need for chemicals.
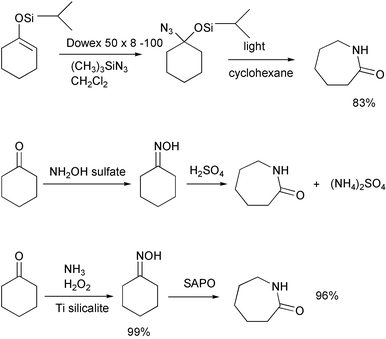
Not all syntheses are as green as the authors say they are. A recent synthesis of caprolactam is said to be convenient and environmentally benign.38 The starting silylenol ether is made from cyclohexanone.
Methylene chloride is cancer-suspect. Azidotrimethylsilane is highly toxic. The first step requires 2–5 days. Both intermediate and product are purified by chromatography, the first in hexane and the second in ethylacetate–hexane. In contrast, the usual commercial process converts cyclohexanone to its oxime and rearranges it to caprolactam with sulfuric acid. The principal disadvantage is that 4.4 pounds of ammonium sulfate are produced for each pound of caprolactam. The new Enichem–Sumitomo process gives high yields and produces no ammonium sulfate. Caprolactam is availabe commercially for $0.77 per pound.
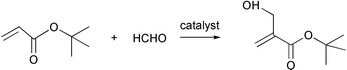
A Bayliss–Hillman Reaction has been described as green and atom-economical with no aqueous or organic waste streams in an environmentally friendly drug synthesis.39 The catalyst can be recycled.
However, formaldehyde is a carcinogen and while this one step in the synthesis of the drug is atom-economical, the reaction sequence is not since the tert-butyl blocking group is removed in a later step in the synthesis.
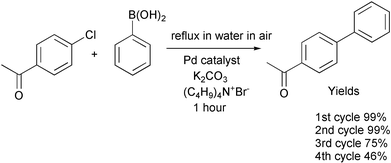
An aqueous Suzuki Reaction gives 99% yields in the first and second cycles, but the yield drops to 75% in the third cycle and to 46% in the fourth cycle, indicating deactivation or loss of the catalyst.40 This is a common problem in many reactions, especially those with supported catalysts. The reaction also produces waste salts. Elimination of the usual organic solvent is a step forward.
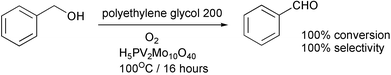
An oxidation of benzyl alcohol to benzaldehyde by oxygen uses polyethylene glycol 200 as a non-toxic, cheap, non-volatile solvent.41 The product can be recovered by vacuum distillation. There is no organic ligand in the catalyst to oxidize. The solvent-catalyst phase can be reused in the next run. The problem is that 5% of the solvent is lost in each cycle. Could it be run without a solvent?
1,3-Butadiene can be polymerized to a stereoregular polymer by a combination of light and pressure.42 This is ″potentially leading to practical green chemistry without the use of solvents, catalysts and radical initiators″. The problem is that the pressure used is too high for most commercial processes.
It is better to green some than not to green at all. Some of these syntheses do represent steps forward. The caprolactam synthesis reminds one of the comment of one reviewer of a book on green chemistry who said that he didn′t want ″green chemistry″ to be a synonym for more complicated chemistry.43
The biggest problem with green chemistry is the slowness with which it is being adopted. We need to move toward a sustainable future.44 History is replete with predictions of the future that failed. However the future in the area of green chemistry may bring:
• Smaller chemical plants
• Less storage of toxic chemicals and more made on demand
• More in line monitoring of reactions
• More leakless valves
• Liquid acid catalysts replaced by solid ones
• More reactions run without organic solvents
• A shift of emphasis from
oxidation
of hydrocarbons to deoxygenation of sugars
• Novel reactions catalyzed by antibodies
• Hydrogen peroxide made commercially from hydrogen and oxygen in microreactors
• More organic electrochemistry
• More reactions catalyzed by sunlight
• More surfactants made from renewable raw materials
• Washing clothes at room temperature
• Useful plastics from lignin.
• Useful plastics from proteins plus fatty acids
• Cheap cellulose derivatives made in extruders
• More genetically-engineered organisms
• More chemicals and vaccines produced in crop plants
• More chemicals from plant and animal tissure culture
• More biocatalysis using wood, biomass and agricultural wastes
• More physical methods to replace chemicals—cutting oils, scale
inhibitors
, refrigerants, electroplating and photoresists
• Coloring fabrics without water and cars without organic solvents
• The demise of the cathode ray tube—replaced by flat screens that contain no mercury or lead
• The demise of the fluorescent lamp containing mercury—replaced by light emitting diodes or filament lamps more energy-efficient than the fluorescent ones
• The taxing of waste
• The outlawing or taxing of some plastic items e.g. poly(vinyl chloride) packaging
• More regulations
• More leasing, less buying
• More interdisciplinary courses?
• Green chemistry in all chemistry, physics and biology courses?
• Broader techniques shown in chemistry laboratory courses?
| Albert Matlack is an adjunct Professor at the University of Delaware, Newark, USA. He can be contacted at 3751 Mill Creek Road, Hockessin, Delaware, 19707-9725, USA (email: amatlack@udel.edu) |
References
- (a) P. T. Anastas and M. M. Kirchoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS; (b) S. Morrissey, Chem. Eng. News, 25 November 2002, 46 Search PubMed; (c) Handbook of Green Chemistry and Technology, eds. J. H. Clark and D. Macquarrie, Blackwell Science Publishers, Oxford, 2002 Search PubMed; (d) D. T. Allen and D. R. Shonnard, Green Engineering: Environmentally Conscious Design of Chemical Processes, Prentice-Hall, UpperSaddle River, New Jersey, 2001 Search PubMed; (e) M. Rouhi, Chem. Eng. News, 22 April 2002, 42 Search PubMed; (f) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (g) A. S. Matlack, Introduction to Green Chemistry, Marcel Dekker, New York, 2001 Search PubMed.
- J. Johnson, Chem. Eng. News, 26 November 2001, 19 Search PubMed; Chem. Eng. News, 7 October 2002, 6 Search PubMed.
- J. M. DeSimone, Science, 2002, 297, 799 CrossRef CAS.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746 CrossRef CAS.
- Recent references to the literature are given in this review. Most earlier ones can be found in A. S. Matlack, Introduction to Green Chemistry, Dekker, New York, 2001. Search PubMed.
- (a) S. K. Ritter, Chem. Eng. News, 1 July 2002, 26 Search PubMed; (b) R. Dagani, Chem. Eng. News, 15 May 2000, 11 Search PubMed.
- M. Poliakoff, N. J. Meehan and S. K. Ross, Chem. Ind. (London), 1999, 750 Search PubMed.
- S. van den Hark and M. Harrod, Ind. Eng. Chem. Res., 2001, 40, 5052 CrossRef CAS.
- T. Sato, G. Sekiguchi, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2002, 41, 3064 CrossRef.
- P. A. Hamley, T. Ilkenhans, J. M. Webster, E. Garcia-Verdugo, E. Venasrdou, M. J. Clarke, R. Auerbach, W. B. Thomas, K. Whiston and M. Poliakoff, Green Chem., 2002, 4, 235 RSC.
- Y. Ikushima, K. Hatakeda, M. Sato, O. Sato and M. Arai, Chem. Commun., 2002, 2208 RSC.
- R. Dagani, Chem. Eng. News, 2002, 38 Search PubMed.
- D. Amantini, F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, Green Chem., 2002, 3, 229 Search PubMed.
- (a) J. S. Wilkes, Green Chem., 2002, 4, 73 RSC; (b) H. Olivier-Bourbigou and L. Manga, J. Mol. Catal. A, 2002, 182–183, 419 CrossRef CAS; (c) Ionic Liquids—Industrial Applications for Green Chemistry, eds. R. D. Rogers and K. R. Seddon, A.C.S. Symp. 818, Washington, D.C., 2002 Search PubMed; (d) P. Wasserscheid, Ionic Liquids in Synthesis, Wiley, New York, 2002 Search PubMed.
- A. M. Scurto, N. V. K. Aki and J. F. Brennecke, J. Am. Chem. Soc., 2002, 124, 10276 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS.
- P. Lozano, T. deDiego, D. Carrie, M. Vaultier and J. L. Iborra, Chem. Commun., 2002, 692 RSC.
- M. Dery, Kirk-Othmer Encyclopedia of. Chemical Technology, 4th edn. 1996, 20, 739–767 Search PubMed.
- V. P. Balema, J. W. Wiench, M. Pruski and V. K. Pecharsky, J. Am. Chem. Soc., 2002, 124, 6244 CrossRef CAS; V. P. Balema, J. W. Wiench, M. Pruski and V. K. Pecharsky, Chem. Commun., 2002, 724 RSC.
- M. Suzuki and Y. Yatsugi, Chem. Commun., 2002, 162 RSC.
- (a) F. F. Huerta, A. B. E. Minidis and J.-E. Backvall, Chem. Soc. Rev., 2001, 30, 321 RSC; (b) J. H. Choi, Y. H. Kim, S. H. Nam, S. T. Shin, M.-J. Kim and J. Park, Angew. Chem. Int. Ed., 2002, 41, 2373 CrossRef CAS.
- Org. Process Res. Dev., 2001, 5, 612–664 Search PubMed.
- H. Kestenbaum, A. L. deOliveira, W. Schmidt, F. Schuth, W. Ehrfeld, K. Gebauer, H. Lowe, T. Richter, D. Lebiedz, I. Untiedt and H. Zuchner, Ind. Eng. Chem. Res., 2002, 41, 710 CrossRef CAS.
- B. Dunk and R. Jachuck, Green Chem., 2000, 2, G13 RSC.
- P. Oxley, C. Brechtelsbauer, F. Ricard, N. Lewis and C. Ramshaw, Ind. Eng. Chem. Res., 2000, 39, 2175 CrossRef CAS.
- Microwave-assisted continuous flow reactors for laboratory use are available from (a) CEM Corporation of Matthews, North Carolins, www.cemsynthesis.com; (b) CPC—Cellular Process Chemistry Systems GmbH, Hanauer Landstrasse 526, G58 lll, D-60343, Frankfurt, Germany, www.cpc-net.com and (c) Personal Chemistry, www.personalchemistry.com.
- A. K. Bose, M. S. Manhas, S. N. Ganguly, A. Sharma and K. V. N. Rao, A.C.S. Meeting, April 2002, IEC 36 Search PubMed.
- M. Jeselnik, R. S. Varma, S. Polanc and M. Kocevar, Chem. Commun., 2001, 1716 RSC.
- L. Si, A. Zheng, H. Yang, R. Guo, Z. Zhu and Y. Zhang, J. Appl. Polym. Sci., 2002, 85, 2130 CrossRef CAS.
- W. Mormann and D. Spitzer, Macromol. Symp., 2001, 176, 279 Search PubMed.
- S. Lamare, B. Caillaud, K. Roule, I. Goubet and M. D. Legoy, Biocatal. Biotransformation, 2001, 19, 361 Search PubMed.
- (a) Anon., Chem. Eng. Prog., 2002, 98(9), 10 Search PubMed; (b) Clear Water Enviro Technologies, Inc., Clearwater, Florida..
- S. Veprek and M. Jilek, Pure Appl. Chem., 2002, 74, 475 Search PubMed.
- Pfeiffer Vacuum Technolgies, Nashua, New Hampshire.
- S. Y. Chou, C. Keimel and J. Gu, Nature, 2002, 417, 835 CrossRef CAS.
- T. C. Harman, P. J. Taylor, M. P. Walsh and B. E. LaForge, Science, 2002, 297, 2229 CrossRef CAS.
- Anon., Chem. Eng. Prog., 2002, 98(8), 14 Search PubMed.
- J. D. Nelson, D. P. Modi and P. A. Evans, Org. Syn., 2002, 79, 165 CAS.
- P. J. Dunn, A.C.S. Meeting, April 2002, IEC 252 Search PubMed.
- L. Botella and C. Najera, Angew. Chem. Int. Ed., 2002, 41, 179 CrossRef CAS.
- A. Haimov and R. Neumann, Chem. Commun., 2002, 876 RSC.
- M. Citroni, M. Ceppatelli, R. Bini and V. Schettino, Science, 2002, 295, 2058 CrossRef CAS.
- G. Kaupp, Angew. Chem. Int. Ed., 2001, 40, 4506 CrossRef CAS.
- K. Geiser, Materials Matter.: Toward a Sustainable Materials Policy, MIT Press, Cambridge, Mass., 2001 Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |