News &Views
Greening the manufacture of pesticides for developing countries
Highlights
Duncan Macquarrie highlights some of the recent literature in green chemistry
Lewis acids
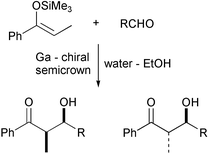
Lewis acids capable of functioning in aqueous environments are interesting as potentially recoverable and reusable Lewis acids, in contrast to the more traditional metal halides which are very prone to hydrolysis. Thus lanthanides have been widely researched in the last few years, since they function well in aqueous and protic environments. Chao-Jun Li from Tulane University, USA, and colleagues at the Chinese Academy of Science in Beijing have now shown that a chiral gallium complex can function as an efficient enantioselective catalyst in aqueous ethanol (Chem. Commun., 2002, 2994).They found that the complex shown was an efficient catalyst for the Mukaiyama aldol reaction, giving high yields, good syn/anti selectivities and very good enantioselectivities under mild conditions and reasonable times. While several metal centres were of similar activity, gallium was the only one which afforded enantioselectivity.
Aldol reactions
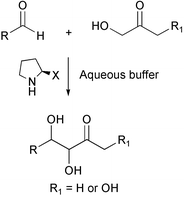
A further aqueous system for the aldol reaction has been uncovered by Carlos F Barbar III and his groups at the Scripps Institute, USA (Chem. Commun., 2002, 3024). They have found that cyclic amines such as L-proline are excellent catalysts for the aldol condensation of acetone and several aldehydes, especially in a phosphate buffered system. In aqueous systems no diastereoselectivity or enantioselectivity were noted; diastereoselectivity was found in DMSO as solvent. In many cases yields were very high and conditions were mild. Interestingly, the authors propose that this system might represent a prebiotic route to sugars.Enzyme-catalysed reactions
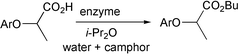
Enzyme-catalysed reactions are attractive options for many reactions. However, enantioselectivity is often disappointingly low, and strategies to improve this are being investigated. One approach is the use of a chiral additive in small amounts—this can sometimes have a substantial impact on selectivity. A paper from Shin-ichi Ueji and colleagues at Kobe University, Japan, now shows that two additives can be much better than one (Bull. Chem. Soc. Japan., 2002, 75, 2239). They found that the enantioselectivity obtained in the esterification of phenoxypropanoic acids with 1-butanol could be improved to some extent (85–90%) by the addition of camphor; water had a similar effect (85–91%). By adding both water and camphor, the enantioselectivity jumped to 96%.Supported aqueous phase catalysis
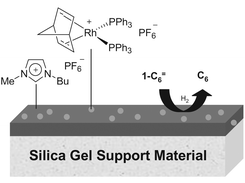
Supported aqueous phase catalysis (SAP) was put forward as a novel mode of heterogeneous catalysis by Mark Davis over a decade ago. Now Christian Mehnert and colleagues from ExxonMobil in Annandale, USA, have extended the concept to supported ionic liquid phases on a silica gel support (Chem. Commun., 2002, 3010). They have immobilised a layer of an ionic liquid onto a silica and used it as a host for a Rh catalyst. This approach led to a catalyst which was significantly more active that an equivalent homogeneous system, and which could also be used in fixed bed reactors. Catalyst stability was excellent, with no loss of Rh being found, and 18 runs with the same catalyst proved consistency over several cycles.Nylon
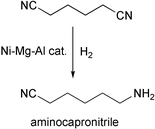
Alternative routes for the production of nylon include the use of aminocapronitrile as a precursor to caprolactam. There is considerable industrial effort in this area, and catalysts for the tricky partial hydrogenation of adiponitrile to aminocapronitrile are sought. Bernard Coq and his team at the CNRS in Montpellier, France, have recently published details of a Ni–Mg–Al hydrotalcite catalyst which displays very good selectivity at relatively high conversions (e.g. 66% selectivity at 70% conversion) (J. Catal., 2002, 211, 511). This is attributed to the combination of the faster desorption from the catalyst surface which reduces over-hydrogenation and the catalysts ability to maintain intact the very small Ni assemblies found—the formation over time of larger Ni assemblies is known to lead to deleterious side reactions in many systems.Carbamates
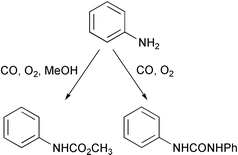
Carbamates are very important intermediates in many reactions systems, and a clean synthesis of this class of compounds would obviate the use of dangerous reagents such as phosgene in many processes. Feng Shi and Youquan Deng from the Centre for Green Chemistry at the Chinese Academy of Sciences in Langzhou have now demonstrated that the oxidative carbonylation of anilines is indeed possible and can be applied to the synthesis of carbamates from anilines, CO, oxygen and methanol in high yields and excellent selectivity (J. Catal., 2002, 211, 548). Their catalyst is a gold species immobilised on an ion-exchange polymer. The system can also be utilised in the related conversion of an aniline to a symmetrical urea.Aromatic halide couplings
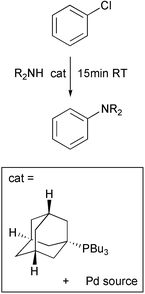
The coupling of aromatic halides with amines and aryl boronic acids is a highly active field. The search for an effective catalyst which will work under mild conditions with aryl chlorides is still ongoing. A remarkably active system has just been disclosed by John Hartwig and his group at Yale University, USA (Angew. Chem. Int. Ed., 2002, 41, 4746). They have found that the air stable complexes of Pd with bulky phosphines such as alkyl di-t-butyl phosphane are capable of the coupling of a range of aryl chlorides with amines in 15 min at room temperature and catalyst levels of 0.5mol%. Excellent yields were obtained. The system was also extremely active for the Suzuki coupling of o,o-disubstituted aryl bromides.Asymmetric allylic alkylation
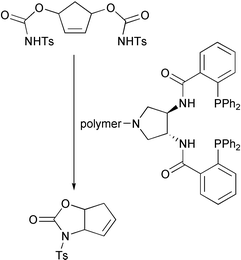
Barry Trost and his team at Stanford, USA, have published details of a study on the development of a highly efficient polymer-bound palladium complex for the asymmetric allylic alkylation reaction (Angew. Chem., Int. Ed., 2002, 41, 4691). They have supported the ligand system shown above on a variety of polymeric backbones using different linkers, and found that a combination of ArgoGel and an amide-linked ligand system. With this catalyst, excellent ee’s were obtained in THF at high conversions. Recycling was also effective with the catalyst performing constantly over four cycles.Friedel–Crafts reactions
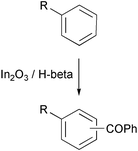
The Friedel–Crafts reaction of unactivated aromatics with alkylating agents and, less commonly, with acylating agents is an area of intense activity. While the alkylation is relatively facile (with selectivity being the main issue) the acylation of unactivated aromatics remains a major challenge. A contribution to this field has been made by researchers at the National Chemical Laboratory in Pune, India, and at RMIT University in Melbourne, Australia, led by V. R. Choudary (Microp. Mesop. Mater., 2003, 57, 21). They have found that indium oxide-doped H-beta is a very active catalyst for the acylation of toluene and similar aromatics with benzoyl chloride. While the amount of catalyst was relatively high (ca. 35 wt%), the activity was impressive (80% conversion of benzoyl chloride in 1 h at 80 °C in the reaction with toluene) and the alkyl aromatics reacted almost as rapidly as anisole. A trace of moisture was found to be beneficial.Aromatic ketones
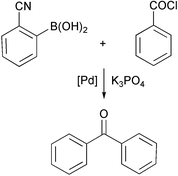
An alternative route to aromatic ketones (which is applicable to electron-poor aromatics) has been investigated by Yoshio Urawa and Katsuyuki Ogura (Tetrahedron Lett., 2003, 44, 271). They have shown that Pd-catalysed coupling of acyl halides with aryl boronic acids can be readily achieved in excellent yield using toluene and hydrated potassium phosphate as base. Reaction times are relatively short and yields are high.Oxidations
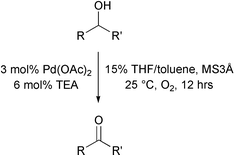
Oxidation of molecules using clean oxidants is still a challenge. A contribution to this field has been published by Matthew Sigman and co-workers from the University of Utah, USA (Chem. Commun., 2002, 3034). They have shown that oxygen will oxidise primary and secondary alcohols to aldehydes or ketones at room temperature with palladium acetate and a small amount of a base such as triethylamine. They show that a range of alcohols can be easily oxidised under these very mild conditions to the ketone in very good yield. The mixed solvent system of THF and toluene is perhaps not ideal from a green perspective, but the implication is that THF could be used, giving excellent yields but taking longer to complete.Ozonolysis
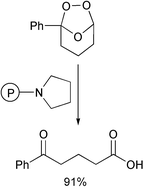
Ozonolysis is a potentially clean route to carbonyl compounds, especially from symmetrical or cyclic alkenes. However, the usual drawback is the reduction of the ozonide formed, often requiring O-acceptors such as phosphines and sulfides in stoichiometric amounts (although Pd/H2 is a very attractive option giving very low residual peroxide levels). An alternative method has been published by Yung-Son Hon from the National Chung Cheng University and Kun-Chan Wu of the Academia Sinica, China (Tetrahedron, 2003, 59, 493), they have shown that treatment of the ozonide with a polymer supported tertiary amine can give very good yields of the carbonyl compound. The polymer can be successfully regenerated and reused at least four times.Greening the manufacture of pesticides for developing countries
R. J. Krupadam, a scientist based at the National Environmental Engineering Research Institute in Nagpur in India (email rkjoshua@rediffmail.com) and Y. Anjaneyulu and K. Babu Rao, based at the Centre for Environment at the Jawaharlal Nehru Technological University in Hyderabad, also in India, discuss
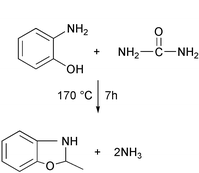
The manufacture of the pesticide phosalone for use in developing countries involves the reaction of o-aminophenol with urea to form benzoxazolone and ammonia as a key step in the process:In a typical factory manufacturing 10 tons per day, 7000 l of effluent are produced which contain ca. 500 kg of ammonia.
Aeration is used as a common method for removal of dissolved gases in wastewater. Ammonium ions in wastewater exist in equilibrium with ammonia. As the pH of wastewater is increased above 7, the equilibrium is shifted to the left and the ammonium ion is converted to ammonia, which may be removed as a gas by agitating the wastewater in the presence of air. With a view to utilizing the ammonia present in the liquid effluents of the phosalone process, it has been shown that it is possible to recover it as a value-added byproduct. It was observed that the recovery of ammonia is accomplished in a steam stripper column. It is thus possible to make 25% solutions of aqueous ammonia, which is used for indigenous purposes and also offered on the open market.
The process (Fig. 1) is performed in the temperature range of 80–120 °C. The liquid effluent containing ammonia after pH adjustment by sodium hydroxide solution (25%) is transported to a steam stripper column. The top vapour consisting of ammonia, water and organic impurities is cooled down by a partial condenser. In this condenser most of the water and organic impurities are condensed while crude ammonia vapour leaves the condenser. The condensate passes through a separator and the top layer, comprising organic fluid is sent to a destructor (incinerator). The bottom layer is a mixture of water and ammonia, and is recirculated into the steam stripped column.
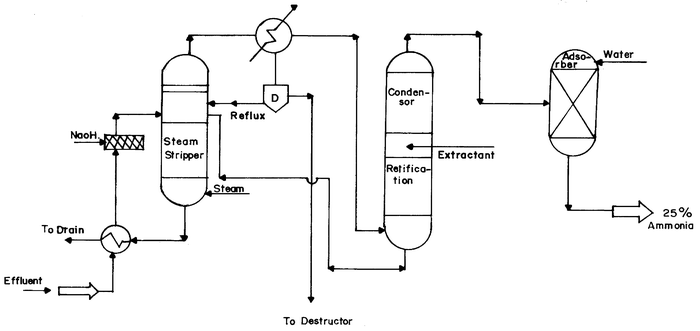 | ||
| Fig. 1 Set-up for ammonia recovery. | ||
The crude ammonia vapour leaving the condenser is sent to a rectification column containing a condenser and is cooled to below 0 °C. The ammonia vapour condenses in the top condenser and is then re-circulated in the column in order to remove impurities. When pure ammonia is needed, a small amount of a suitable extractant such as toluene is dosed into the rectification column. This helps in removing impurities which tend to form azeotropes with ammonia. It also helps in extracting organic impurities from the ammonia vapour.
The top vapour leaving the column is purified ammonia and this is absorbed to make 25% aqueous ammonia solution. The bottom product emerging from the rectification column consists of extractant organic impurities and traces of ammonia which is recirculated into the top of the steam stripper.
The recovered aqueous ammonia solution (25%) is utilized for indigenous purposes. It is used in making the ammonium salt of O,O-diethyl phosphoric acid which is an important intermediate in producing phosalone and ethion products. Highly satisfactory results are achieved by using the ammonia salt of such pesticides in place of sodium salts.
The ammonium output is analyzed by both chemical and ion-selective electrode methods and reproducible results are obtained by using both methods.
The process enables the production of ammonia with a high degree of purity from effluents contaminated with volatile and non-volatile organic impurities. Typical results are given in the Table 1.
| Final ammonia solution | ||||||||
|---|---|---|---|---|---|---|---|---|
| S.No | Qty. of Effluent (L) | Initial Assay (%) | Initial COD (mg/L) | Qty. (L) | Density (g/mL) | Assay (%) | COD (mg/L) | Cost of NH3/ ton Effluent Rs. |
| 1 | 286 | 7.01 | 6000 | 80 | 0.896 | 25.0 | 5000 | 2237 |
| 2 | 1500 | 7.20 | 7500 | 424 | 0.895 | 25.2 | 5200 | 2261 |
| 3 | 1500 | 7.20 | 7600 | 421 | 0.893 | 25.4 | 5100 | 2245 |
| 4 | 1500 | 7.1 | 7800 | 418 | 0.893 | 25.3 | 5200 | 2229 |
Furthermore in the actual manufacturing process by introducing the reflux column ″D″ as shown in Fig. 1, the non-ammonical COD is significantly reduced by introducing an extra partial condensor where the phenolic compounds will be also separated. The recovered ammonia (25%) is suitable for integrated ammonia based pesticides manufacturing in the plant.
References
- L. D. Benefiud, J. F. Judkins and B. L. Weand, Process Chemistry for Water and Wastewater Treatment, 1982, Prentice Hall Inc., USA Search PubMed.
- C. Borra, A. Dicorcia, M. Marchetti and T. Samperi, Anal. Chem., 1986, 73, 22–29 Search PubMed.
- W. Fresenius and W. Schneider, Waterwater—Technology—Origin Collection, Treatment and Analysis of Wastewater, 1992, Springer-Verlag, Berlin Heidelberg, 1989 Search PubMed.
- P. G. Goel, Water Pollution-Causes, Effects and Control, 1997, New Age International Publishers, India Search PubMed.
- A. L. Grenberg, S. C. Lenox and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, 20th edn., 1992, American Public Health Association Search PubMed.
- R. J. Krupadam, D. S. Ramteke and R. Sarin, Green Chemistry—An Approach for Design Clean Chemical Processes. J. Environ. Qual., 2002 Search PubMed (submitted).
- R. J. Mastin and K. O. Iwugo, Recovery of Water and Wastewater by Activated Carbon Adsorption process. Proceedings of III International Conference, Poland, 1981, Elsevier Scientific Publishers, 265–284 Search PubMed.
- L. Pawalowski, Physico-chemical Treatment for Water and Wastewater Treatment. Proceedings of III International Conference, Poland, 1982, Elsevier Scientific Publishers. Search PubMed.
- J. Walter and J. Kieber Jr., Physico-chemical Processes for Water Quality Control, 1972, Wiley InterScience Publishers/John Wiley & Sons Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |