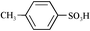
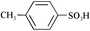
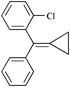
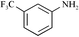
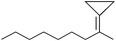
Heptadecafluorooctanesulfonic acid catalyzed ring opening reactions of methylenecyclopropanes with aromatic amines, sulfonamides and alcohols in supercritical carbon dioxide
Min Shi*a, Yu Chenb, Bo Xua and Jie Tangb
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Science, 354 Fenglin Lu, Shanghai, 200032, China
bDepartment of Chemistry, East China Normal University, 3663 Zhong Shan Bei Lu, Shanghai, 200062, China. E-mail: Mshi@pub.sioc.ac.cn; Fax: 86-21-64166128
First published on 13th January 2003
Abstract
The ring opening reactions of methylenecyclopropanes (MCPs) with ArNH2, RSO2NH2 and alcohols can be carried out in scCO2 in the presence of heptadecafluorooctanesulfonic acid which offer a way to synthesize homoallylic amines, sulfonamides and ethers in environmentally benign conditions.
Green ContextSupercritical carbon dixide is now widely recognised as an environmentally benign solvent for chemical transformations and has now been proven in this context on an industrial scale. Many organic reactions require catalysis to enable the reaction to be carried out under moderate conditions or to give good product selectivity. Here a new catalyst–scCO2 combination is described. Perfluorooctylsulfonic acid in scCO2 is shown to be a good catalyst for the ring opening reactions of methyl cyclopropanes with various nucleophites.JHC |
Introduction
Chemistry has made a great contribution to the development of human society. The pollution and waste from chemical industries and laboratories, however, have deteriorated our environment. In order to achieve mutual societal, economic and environmental benefits, scientists have developed ‘green chemistry’ which is providing challenges and opportunities to those who practice chemistry in industry, education and research.1–5 Presently the area of employing ‘novel’ solvent alternatives is receiving increasing attention. Among suitable alternatives such as ionic liquids,6 perfluorinated hydrocarbons7and water,8 supercritical carbon dioxide has been widely investigated and has been most attractive in green chemistry. Several of the advantages include that CO2 is inexpensive, nonflammable, environmentally benign and readily separated from products.9–11 Above the critical temperature and pressure, (Tc = 31 °C, Pc = 7.4 MPa) CO2 has a gas-like viscosity and a liquid-like density. These moderate critical conditions allow for safe commercial and laboratory operating conditions. For example, in 1994 Noyori and coworkers,12 reported one of the first synthetically useful homogeneous catalytic hydrogenetions catalyzed by an Ru(II) catalyst involving scCO2 as both solvent and substrate. Tanko and coworkers,10,11Wells and DeSimone,12 Jessop et al.,13 and Beckman and coworkers14 reported radical reactions in scCO2. In addition, many applications such as oxidation,15 hydroformylation,16 Diels–Alder cycloaddition,17 Mukaiyama aldol condensation18 and various metal-catalyzed19 reactions have been carried out in the presence of scCO2.Recently, we have reported that metal Lewis acid such as Sn(OTf)2 or Yb(OTf)3 catalyzed reactions of MCPs with ArNH2 and alcohols in 1,2-dichloroethane (DCE).20 However, is well known that halogenated solvents are environmentally hazardous materials. In addition, many metal catalysts are also pollution sources for the environment. In order to explore an environmentally benign condition for this process, we attempted to carry out this reaction in scCO2 in the absence of metal catalyst. Herein, we wish to report the ring opening reactions of MCPs with aromatic amines, sulfonamides or alcohols catalyzed by the Brønsted acid RfSO3H in scCO2 under mild conditions.
First of all, we chose various RSO3H as Brønsted acid catalysts (10 mol%) to promote the reaction of diphenylmethylenecyclopropane 1a (0.45 mmol) with 3-(trifluoromethyl)aniline 2a (0.15 mmol) at 85 °C and 10 MPa in scCO2. The results are listed in Table 1 (entry 1). The reaction proceeded smoothly to give two adducts: dialkylated product 3a (39%) and monoalkylated 4a (11%) in moderate yields in the presence of CF3SO3H (Table 1, entry 1). Using C8F17SO3H as the catalyst under the same conditions the two adducts were obtained in very high yields (Table 1, entry 2).21 It should be noted that no reaction occurred when using p-toluenesulfonic acid as the Brønsted acid catalyst even with the addition of perfluorotoluene as a CO2-philic additive (Table 1, entries 3 and 4). A long chain perfluorinated sulfonic acid should be used as a promoter for this novel ring opening reaction. This is because a long perfluorinated alkyl chain can allow the scCO2 reaction system to become an effective homogenous phase. Viewing through the high pressure glass window placed in the scCO2 reaction vessel, we confirmed that the C8F17SO3H, 1a, and aniline are completely dissolved in scCO2 to form a homogeneous system.
Further investigations were performed using various MCPs and ArNH2 or RSO2NH2 to examine this reaction (Table 2). We chose different MCPs and RNH2 or RSO2NH2 as substrates. As can be seen from Table 2, many of the reactions take place to give two adducts 3 and 4 in moderate to high yields catalyzed by C8F17SO3H (10 mol%) in scCO2. The ring opened products are obtained in higher yields when using aromatic amines which bear an electron-withdrawing group on the benzene ring.20 For example, the reaction of 1a with 2b or 2c affords the corresponding adducts in high yields, respectively (Table 2, entries 1 and 2). For aniline 2d and aromatic amine 2e having an electron-donating group, the reactions also proceeded smoothly to give the two products in good yields (Table 2, entries 3 and 4). For MCP 1b having an electron-withdrawing group on the benzene ring and aliphatic MCP 1c, the reactions can take place as well. However, the ring opened products were obtained in lower yields (Table 2, entries 5 and 6). On the other hand, the yields can be improved dramatically using MCP 1d having an electron-donating group on the phenyl ring instead of aliphatic MCP 1c (Table 2, entry 7). Apart from aromatic amines, the reaction of 1a with various sulfonamides also can proceed smoothly to give 3 and 4 in the presence of C8F17SO3H (10 mol%) in scCO2. For example, the reactions of 1a with 2f and 2g give the two corresponding adducts 3i or 3j and 4i or 4j in high yields, respectively (Table 2, entries 8 and 9). However, using p-nitrobenzenesulfonamide 2h as a substrate to react with 1a under the same conditions led to no reaction owing to the insolubility of p-nitrobenzenesulfonamide 2h in scCO2. Based on these results, we can conclude that C8F17SO3H is an excellent Brønsted acid catalyst in scCO2 and it is applicable to extensive ring opening reactions of MCPs with ArNH2 or RSO2NH2.
The ring opening reaction of MCPs (0.5 mmol) with ethanol (200 μL, 3.4 mmol) was also carried out in scCO2 in the presence of C8H17SO3H (15 mg, 10 mol%) to give the ring opened product 5a in very high yield (Table 3, entry 1). In general, it was found that the reactions proceed very well and the corresponding ring opened products 5 were obtained in excellent yields with complete conversions for various MCPs under the same conditions (Table 3). We believe that this is because the alcohols themselves can modify the physical properties of scCO2 and subsequently enhance the solubilities of the reactants in scCO2 and improve the reaction efficiency.
It should be emphasized here that the CO2-philic catalyst C8F17SO3H can be recovered from the reaction mixture by extraction with toluene. The catalyst C8F17SO3H is insoluble in toluene whereas the products are soluble in toluene. The extracts can be subjected to column chromatography and the catalyst residue can be reused for the next reaction as a catalyst.
A plausible mechanism for the ring-opening reactions of MCPs with ArNH2, NH2SO2R and ROH is shown in Scheme 1. The MCPs first give cation 6 which immediately rearranges to the ring-opened cation 721 in the presence of Brønsted acid C8F17SO3H. The subsequent nucleophilic attack of RNH2, NH2SO2R or ROH to 7 affords the adduct 8. The final product is formed after proton elimination.
 | ||
| Scheme 1 | ||
In conclusion, serious environmental problems necessitate our rethinking of strategies toward organic synthesis. The ideal reaction would incorporate all of the atoms of the reactions. Major benefits that derive from atom-economic processes include more effective use of limited raw materials and decreased emissions and waste disposal. In this article, we have disclosed an atom-economical reaction of MCPs applied to scCO2 catalyzed by the Brønsted acid C8F17SO3H.22 This reaction offers an access to the formation of homoallylic amines, sulfonamides and ethers in environmentally benign conditions. By comparison of the reaction procedure in scCO2 reported in this paper with those reported in previous papers,20a it is very clear that when the reaction is carried out in scCO2 by means of a CO2-philic catalyst C8F17SO3H, the use of the hazardous solvent 1,2-dichloroethane can be avoided and the very similar yields can be achieved. Especially, for the reactions of MCPs with alcohols in scCO2, the reactions proceed completely and no chromatography is required. We also hope that this type of reaction is conducive to the development of green chemistry as well as new organic syntheses.
Experimental
Typical reaction procedure: 1a (93 mg, 0.45 mmol), 2a (24 mg, 0.15 mmol) and C8F17SO3H (7.5 mg, 10 mol%)22 were placed in the scCO2 reactor. The reaction proceeded at 85 °C at 10 MPa for 24 h. The residue was purified by flash chromatography (SiO2) using EtOAc–hexane (1∶100) or CH2Cl2–hexane (1∶8) as the eluent to yield 3a (45 mg, 52%) as a colorless solid and 4a (26 mg, 47%) as an oil.3a: mp 118–120 °C, IR (neat): ν 3052, 3028, 2925, 1609, 1495, 1454, 1322 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.30–2.38 (m, 4H, CH2), 3.29 (t, 4H, J = 7.6 Hz, CH2), 6.05 (t, 2H, J = 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.40–6.43 (m, 1H, Ar), 6.69 (s, 1H, Ar), 6.81 (d, 1H, J = 8.0 Hz, Ar), 7.07–7.39 (m, 21H, Ar); MS (EI): m/z 573 (M+, 0.3), 380 (54), 167 (100), 129 (61), 91 (53%); Anal. Calc. for C39H34F3N (%): C, 81.68; H, 5.93; N, 2.44. Found: C, 81.72; H, 6.09; N, 2.41.
CH), 6.40–6.43 (m, 1H, Ar), 6.69 (s, 1H, Ar), 6.81 (d, 1H, J = 8.0 Hz, Ar), 7.07–7.39 (m, 21H, Ar); MS (EI): m/z 573 (M+, 0.3), 380 (54), 167 (100), 129 (61), 91 (53%); Anal. Calc. for C39H34F3N (%): C, 81.68; H, 5.93; N, 2.44. Found: C, 81.72; H, 6.09; N, 2.41.
4a: IR (neat): ν 3421, 3054, 2986, 1614, 1495, 1449, 1421, 1265 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.43–2.50 (m, 2H, CH2), 3.24 (t, 2H, J = 6.8 Hz, CH2), 3.81 (s, 1H, NH), 6.12 (t, 1H, J = 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.64–6.67 (m, 1H, Ar), 6.74 (s, 1H, Ar), 6.90–6.92 (d, 1H, J = 7.5 Hz, Ar), 7.10–7.41 (m, 11H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 29.81, 43.89, 109.79, 111.49, 113.88, 123.75 (q, J = 271.4 Hz), 125.80, 127.27, 127.39, 127.45, 128.37, 128.58, 129.80, 129.94, 130.060 (q, J = 31.6 Hz), 139.87, 143.54, 145.98, 149.83; MS (EI): m/z 367 (M+, 1.8), 174 (100), 145 (9), 91 (6), 77 (3%); HRMS (EI): Calc. for C23H20F3N 367.1548 (M+), Found: 367.1526.
CH), 6.64–6.67 (m, 1H, Ar), 6.74 (s, 1H, Ar), 6.90–6.92 (d, 1H, J = 7.5 Hz, Ar), 7.10–7.41 (m, 11H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 29.81, 43.89, 109.79, 111.49, 113.88, 123.75 (q, J = 271.4 Hz), 125.80, 127.27, 127.39, 127.45, 128.37, 128.58, 129.80, 129.94, 130.060 (q, J = 31.6 Hz), 139.87, 143.54, 145.98, 149.83; MS (EI): m/z 367 (M+, 1.8), 174 (100), 145 (9), 91 (6), 77 (3%); HRMS (EI): Calc. for C23H20F3N 367.1548 (M+), Found: 367.1526.
The products 3i and 4i were isolated by column chromatography as colorless oily compounds (eluent: EtOAc–hexane (1∶10)).
3i: IR (neat): ν 3055, 2986, 1653, 1598, 1494, 1266 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.16–2.24 (m, 4H, CH2), 2.35 (s, 3H, CH3), 3.13 (t, 4H, J = 7.5 Hz, CH2), 5.92 (t, 2H, J = 7.3 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.03–7.59 (m, 24H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 21.78, 28.75, 47.38, 125.15, 127.18, 127.59, 128.36, 128.42, 128.58, 128.72, 129.87, 129.97, 137.38, 139.89, 142.33, 143.21, 144.18; MS (EI): m/z 583 (M+, 2.0), 390 (47), 193 (17), 155 (8), 167 (100), 91 (41%); HRMS (EI): Calc. for C24H24NSO2 391.1606 (M+
CH), 7.03–7.59 (m, 24H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 21.78, 28.75, 47.38, 125.15, 127.18, 127.59, 128.36, 128.42, 128.58, 128.72, 129.87, 129.97, 137.38, 139.89, 142.33, 143.21, 144.18; MS (EI): m/z 583 (M+, 2.0), 390 (47), 193 (17), 155 (8), 167 (100), 91 (41%); HRMS (EI): Calc. for C24H24NSO2 391.1606 (M+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C15H13+) (M+
= C39H57NSO2). Found: 391.1570.
C15H13+) (M+
= C39H57NSO2). Found: 391.1570.
4i: IR (neat): ν 3283, 3056, 2928, 1638, 1598, 1494 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.24–2.31 (m, 2H, CH2), 2.42 (s, 3H, CH3), 3.02–3.08 (m, 2H, CH2), 4.40 (t, 1H, J = 6.0 Hz, NH), 5.90 (t, 1H, J = 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08–7.71 (m, 14H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 21.79, 30.07, 43.29, 124.81, 127.25, 127.31, 127.32, 127.46, 127.52, 128.37, 128.62, 129.95, 137.02, 139.65, 142.21, 143.61, 144.82; MS (EI): m/z 193 (M+
CH), 7.08–7.71 (m, 14H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 21.79, 30.07, 43.29, 124.81, 127.25, 127.31, 127.32, 127.46, 127.52, 128.37, 128.62, 129.95, 137.02, 139.65, 142.21, 143.61, 144.82; MS (EI): m/z 193 (M+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C8H10NSO2, 44.0), 184 (16), 155 (18), 91 (28), 84 (100%); HRMS (EI): Calc. for C23H23NSO2 377.1449 (M+), Found: 377.1432.
C8H10NSO2, 44.0), 184 (16), 155 (18), 91 (28), 84 (100%); HRMS (EI): Calc. for C23H23NSO2 377.1449 (M+), Found: 377.1432.
The products 3j and 4j were isolated by column chromatography as colorless oily compounds (eluent: EtOAc–hexane (1∶4)).
3j: IR (neat): ν 3038, 2928, 1654, 1599, 1494, 1445 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.27–2.34 (m, 4H, CH2), 2.69 (s, 3H, CH3), 3.18 (t, 4H, J = 7.5 Hz, CH2), 5.99 (t, 2H, J = 7.6 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.11–7.39 (m, 20H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 28.97, 39.16, 47.05, 124.91, 127.44, 127.52, 127.55, 128.44, 128.61, 129.90, 139.81, 142.20, 144.42; MS (EI): m/z 507 (M+, 1.3), 314 (6), 193 (24), 167 (38), 105 (100), 91 (21), 84 (86), 77 (61%); HRMS (EI): Calc. for C33H33NSO2 507.2232 (M+), Found: 507.2214.
CH), 7.11–7.39 (m, 20H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 28.97, 39.16, 47.05, 124.91, 127.44, 127.52, 127.55, 128.44, 128.61, 129.90, 139.81, 142.20, 144.42; MS (EI): m/z 507 (M+, 1.3), 314 (6), 193 (24), 167 (38), 105 (100), 91 (21), 84 (86), 77 (61%); HRMS (EI): Calc. for C33H33NSO2 507.2232 (M+), Found: 507.2214.
4j: IR (neat): ν 3291, 3028, 2939, 1653, 1599, 1494, 1444 cm−1; 1H NMR (300 MHz, CDCl3, TMS), δ 2.35–2.42 (m, 2H, CH2), 2.83 (s, 3H, CH3), 3.17–3.24 (m, 2H, CH2), 4.64 (t, 1H, J = 5.8 Hz, NH), 6.04 (t, 1H, J = 7.3 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.14–7.41 (m, 10H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 30.65, 40.47, 43.34, 124.78, 127.50, 127.55, 127.62, 128.56, 128.67, 130.32, 139.74, 142.19, 145.0; MS (EI): m/z 301 (M+, 13.9), 206 (57), 193 (100), 165 (17), 115 (56), 91 (19%); HRMS (EI): Calc. for C17H19NSO2 301.1136 (M+), Found: 301.1143.
CH), 7.14–7.41 (m, 10H, Ar); 13C NMR (75 MHz, CDCl3, TMS), δ 30.65, 40.47, 43.34, 124.78, 127.50, 127.55, 127.62, 128.56, 128.67, 130.32, 139.74, 142.19, 145.0; MS (EI): m/z 301 (M+, 13.9), 206 (57), 193 (100), 165 (17), 115 (56), 91 (19%); HRMS (EI): Calc. for C17H19NSO2 301.1136 (M+), Found: 301.1143.
Acknowledgements
We thank the State Key Project of Basic Research (Project 973) (No. G2000048007), Shanghai Municipal Committee of Science and Technology, and the National Natural Science Foundation of China (20025206 and 20272069) for financial support.References
- J. H. Clark, Green Chem., 1999, 1, 1 RSC.
- B. M. Trost, Science, 1991, 254, 1471 CAS.
- (a) K. Nagayama, I. Shimizu and A. Yamamoto, Chem. Lett., 1998, 1143 CrossRef CAS; (b) Color Me Green, C & E News, 2000, July 10. p. 49 Search PubMed; (c) Z. W. Xi, N. Zhou, Y. Sun and K. L. Li, Science, 2001, 292, 1139 CrossRef CAS.
- See for example: (a) P. Tundo, F. Trotta and G. Moraglio, J. Org. Chem., 1987, 52, 1300 CrossRef; (b) M. K. Stern, J. Org. Chem., 1993, 58, 6883 CrossRef CAS; (c) D. Delledonne, F. Rivetti and U. Romano, J. Organomet. Chem., 1995, 488, C15 CrossRef CAS.
- (a) L. Shi, W. Wang, Y. Wang and Y. Huang, J. Org. Chem., 1989, 54, 2027 CrossRef CAS; (b) F. J. Waller, A. G. M. Barrett, D. C. Braddock and D. Ramprasad, Chem. Commun., 1997, 613 RSC.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS.
- I. T. Horvath, Acc. Chem. Res., 1998, 31, 641 CrossRef CAS.
- (a) C. J. Li and T. H. Chan, Organic Reactions in Aqueous Media, New York, John Wiley & Sons, 1997 Search PubMed; (b) J. P. Genet and M. Savignac, J. Organomet. Chem., 1999, 576, 305 CrossRef.
- M. Mchugh and V. Krukonis, Supercritical Fluid Extraction: Principles and Practice, Butterworths, Boston, 1986 Search PubMed.
- J. M. Tanko and J. F. Blackert, Science, 1994, 263, 203 CAS.
- B. Fletcher, N. K. Suleman and J. M. Tanko, J. Am. Chem. Soc., 1998, 120, 11839 CrossRef CAS.
- S. L. Wells and J. DeSimone, Angew. Chem., Int. Ed., 2001, 40, 518 CrossRef CAS.
- P. G. Jessop, T. Ikariya and R. Noyori, Nature, 1994, 368, 231 CrossRef CAS.
- S. Hadida, M. S. Super, E. J. Beckman and D. P. Curran, J. Am. Chem. Soc., 1997, 119, 7406 CrossRef CAS.
- (a) R. S. Oakes, A. A. Clifford, K. D. Bartle, M. Thornton-Pett and C. M. Rayner, Chem. Commun., 1999, 247 RSC; (b) G. R. Haas and J. W. Kolis, Organometallics, 1998, 17, 4454 CrossRef CAS.
- (a) J. W. Rathke, R. J. Klinger and T. R. Krause, Organometallics, 1991, 10, 1350 CrossRef CAS; (b) D. Koch and W. Leitner, J. Am. Chem. Soc., 1998, 120, 13398 CrossRef CAS.
- (a) M. E. Paulaitis and G. C. Alexander, Pure Appl. Chem., 1987, 59, 61 CAS; (b) C. Chapuis, A. Kucharska, P. Rzepecki and J. Jurczak, Helv. Chim. Acta., 1998, 81, 2314 CrossRef CAS; (c) R. S. Oakes, T. J. Heppenstall, N. Shezad, A. A. Clifford and C. M. Rayner, Chem. Commun., 1999, 1459 RSC; (d) J. Matsui, T. Tsuchiya, K. Odashima and S. Kobayashi, Chem. Lett., 2000, 178 CrossRef CAS.
- I. Komoto and S. Kobayashi, Org. Lett., 2002, 4, 1115 CrossRef CAS.
- (a) M. A. Carroll and A. B. Holmes, Chem. Commun., 1998, 1395 RSC; (b) M. T. Reetz, W. Konen and T. Strack, Chimia, 1993, 47, 493 Search PubMed; (c) N. Jeong, S. H. Hwang, Y. W. Lee and J. S. Lim, J. Am. Chem. Soc., 1997, 119, 10549 CrossRef CAS; (d) A. Furstner, D. Koch, K. Langemann, W. Leitner and C. Six, Angew. Chem., Int. Ed., 1997, 36, 2466 CrossRef CAS.
- (a) M. Shi, Y. Chen, B. Xu and J. Tang, Tetrahedron Lett., 2002, 43, 8019 CrossRef CAS; (b) M. Shi and B. Xu, Org. Lett., 2002, 4, 2145 CrossRef CAS; (c) For a recent review: I. Nakamura and Y. Yamamoto, Adv. Synth. Catal., 2002, 2, 111 Search PubMed.
- F. A. Carey and H. S. Tremper, J. Am. Chem. Soc., 1969, 91, 2967 CrossRef CAS.
- C8F17SO3H was purchased from TCI (Tokyo Kasei, H0781).
| This journal is © The Royal Society of Chemistry 2003 |