Solid base catalysts for the synthesis of phytosterol esters
Y. Pouilloux*a, G. Courtoisa, M. Boisseaua, A. Piccirillib and J. Barraulta
aLaboratoire de Catalyse en Chimie Organique, UMR 6503, ESIP 40, Avenue du Recteur Pineau, 86022, Poitiers Cedex, France. E-mail: yannick.pouilloux@univ-poitiers.fr
bLaboratoires Pharmascience, 51, rue Saint Denis, 28230, Epernon cedex
First published on 20th January 2003
Abstract
The main objective of this study is the synthesis of phytosterol esters from natural sterols and methyl esters in the presence of basic solid catalysts which are less corrosive and more selective than homogeneous catalysts such as alkaline hydroxides and carbonates. Phytosterol esters are effective in reducing both blood cholesterol levels and triglycerides and can be used as biological compounds in pharmaceutics and cosmetics. The highest yields of phytosterol esters are obtained in the presence of magnesium oxide or zinc oxide. However, magnesium oxide which is more basic basic favored the side reaction of sitosterol dehydration. The phytosterol ester yields can reach 80% and the formation of dienes is strongly inhibited when the reaction is carried out under a nitrogen flow.
Green ContextPhytosterol esters are useful in food applications and in other areas such as cosmetics. Their synthesis is based on classic esterification methodology involving strong acids or transesterification methods based on strong bases. Here a greener chemical method based on solid reusable catalysts is described. Basic solid catalysts such as magnesium or zinc oxide are shown to be effective for the transesterification of β-sitosterol with methyl dodecanoate. Reuse characteristics of the solid catalysts are excellent.JHC |
Introduction
The aim of this work consists in the synthesis of phytosterol esters from sterols and fatty acids or fatty methyl esters in an esterification or transesterification reaction performed in the presence of solid catalysts (Scheme 1). In our laboratory, a general program on the selective transformation of fatty acids or esters and polyols originating from vegetable oils was performed to replace homogeneous catalysts by new solid catalysts which are more easily recycled and environmentally friendly.1–4 Besides triglycerides, plant sterols (sitosterol, stigmasterol, campesterol,…) are present in the unsaponifiable fraction of vegetable oils. In food applications phytosterol esters are effective in reducing levels of both serum cholesterol and triglycerides. As the free sterols (unesterified) are poorly soluble in fats and oils, improvement of solubility for incorporation in foods could result from sterol esterification with fatty acids. On the other hand, phytosterol esters can be used in cosmetics as emulsifiers (significant solubility in water) or as anti-inflammatory and antioxidant compounds.5,6 | ||
| Scheme 1 Synthesis of phytosterol esters. | ||
Phytosterol esters can be prepared by esterification or transesterification reactions but the usual esterification reaction requires acid catalysts (H2SO4, H3PO4 or p-TSA) which may also favor the dehydration of sterols to stigmastadienes. Sodium alkoxides (NaOMe, NaOEt,…) are mainly used in transesterification reactions7–9 as well as hydroxides (NaOH, KOH, Ca(OH)2…)10 or alkaline sulfates or carbonates.11,12 Unfortunately, these bases are well known to favor the formation of soaps. Moreover, homogeneous catalysts are corrosive, difficult to separate from the products and lead to excessive waste.
The ability of oxide catalysts such as magnesium or zinc oxide to prepare phytosterol esters from fatty methyl esters and sitosterols without solvent13 are investigated and presented in this paper.
Experimental
Catalytic test
The transesterification of β-sitosterol with methyl dodecanoate was chosen in this study as a model reaction. This reaction was carried out at atmospheric pressure under a nitrogen flow in a Pyrex reactor equipped with a mechanical stirrer. 0.07 mol of sterol (15 g) and 0.07 mol of methyl dodecanoate (29 g) were heated to 240 °C. Then 2 g of catalyst (5 wt%) was added to the mixture (starting time of the reaction). Samples of the reaction medium were analyzed with a GPC equipped with a FID detector and an on-column injector. The percentage of each compound was determined by using standardization methods with hexadecane as an internal standard. The conversion was estimated with respect to the initial and the final content of fatty methyl ester and free sterol in the solution.Catalysts
The usual catalyst Na2CO3 was dried at 100 °C for 24 h before use. Zinc oxide and magnesium oxide were supplied by Union Minière and by Prolabo, respectively. The oxide catalysts were used after a pretreatment with nitrogen at 450 °C for 4 h.Results and discussion
Transesterification of methyl dodecanoate with β-sitosterol in the presence of usual basic catalysts
First, the transesterification of the methyl dodecanoate with β-sitosterol was carried out in the presence of Na2CO3. Table 1 shows that the conversion of the sterol is three times higher than the conversion of methyl dodecanoate when the reaction is performed without catalyst but the yield of the phytosterol ester is low (12%). The main products of the reaction are the phytosterol ester and stigmasta-3,5-diene which is the side-product of the dehydration of the β-sitosterol (Scheme 2). Due to the high temperature and the free residual acidity, there is a competition between the transesterification reaction and the dehydration of the sterol since the selectivity to the phytosterol ester and diene are close to 50% (Fig. 1).| Conversion (%) | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|
| Catalyst | Ester | Sterol | Sterol ester | Diene | Other | Yield (%) | Activity/mmol g−1 h−1 |
| Temperature = 240 °C, reaction time = 7 h, DN2 = 5 L h−1, ester/sterol ratio = 1, catalyst = 5 wt%.a Soap formation. | |||||||
| None | 25 | 74 | 49 | 46 | 5 | 12 | — |
| Na2CO3 | 69 | 62 | 92 | 4 | 4 | 63a | — |
| MgO | 98 | 95.1 | 80 | 10 | 10 | 78 | 21 |
| LiMgO | 100 | 98 | 38 | 40 | 22 | 38 | 40 |
| ZnO | 82 | 97 | 93 | 3 | 4 | 76 | 9 |
 | ||
| Scheme 2 Dehydration of β-sitosterol to stigmasta-3,5-diene. | ||
 | ||
| Fig. 1 Preparation of phytosterol ester from methyl dodecanoate and β-sitosterol in the absence of catalyst. Temperature = 240 °C, reaction time = 7 h, DN2 = 5 L h−1, ester/sterol ratio = 1, catalyst = 5 wt%. | ||
When sodium hydroxide was used at 240 °C, the main reaction was the saponification of the methyl ester leading to the formation of soaps. However, with sodium carbonate, the side reaction of dehydration of β-sitosterol was lowered and the yield of the phytosterol ester increased from 12 to 63%. Similarly to NaOH, sodium carbonate totally solubilized in the reagents, and reacted with methyl dodecanoate with formation of soaps (30%).
Transesterification of methyl dodecanoate with β-sitosterol in the presence of basic oxide catalysts
In order to replace homogeneous catalysts with solid and more easily recycle catalysts, some basic solids were studied in the reaction of the sterol with methyl dodecanoate. As reported in Table 1, the activity of catalysts varied with the acido-basicity of the oxides. The most active oxide was magnesium oxide whereas zinc oxide was less active. The strong basicity of MgO influenced the selectivity to the phytosterol ester. Owing to strong basic sites, the dehydration of the sterol was favored over MgO.Nitrogen flow. As the transesterification reaction was a reversible reaction, the effect of nitrogen flow (to remove methanol) was studied under standard conditions in the presence of oxides (not calcined). The phytosterol ester yield reached a maximum for a nitrogen flow between 5 and 12 L h−1 (Fig. 2). At high nitrogen flow, a fraction of methyl dodecanoate was also removed so that all experiments were carried out with a nitrogen flow of 5 L h−1.
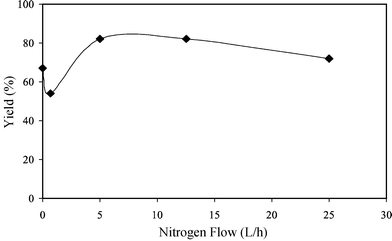 | ||
| Fig. 2 Preparation of phytosterol ester from methyl dodecanoate and β-sitosterol in the presence of zinc oxide. Influence of the nitrogen flow. Temperature = 240 °C, reaction time = 7 h, ester/sterol ratio = 1, catalyst = 5 wt%. | ||
Reaction temperature. In order to evaluate the influence of the temperature, a series of experiments was performed at 230, 240 and 250 °C in the presence of oxide catalysts. As expected, the conversion of ester and sterol increased with the temperature (Table 2). However, increasing temperature favored the side reaction of degradation of reactants with the diene selectivity increasing from 3.5 to 6.8%. The activation energy calculated from experimental data was 128 kJ mol−1, a value similar to that obtained in the reaction of fatty acid methyl esters and glycerol.14
| Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|
| Temperature/°C | Ester | Sterol | Sterol ester | Diene | Other | Yield (%) |
| Reaction time = 7 h, DN2 =5 L h−1, ester/sterol ratio = 1, catalyst = 5 wt%. | ||||||
| 230 | 72.9 | 75.1 | 88 | 3.5 | 8.5 | 64 |
| 240 | 85.1 | 89.6 | 92.6 | 3.8 | 3.6 | 79 |
| 250 | 88.4 | 99 | 89.1 | 6.3 | 4.6 | 79 |
Catalyst recycling. After the reaction, the catalyst can be filtered and reused. It is thus possible (i) to reduce the cost of the process and (ii) to improve industrial processes in avoiding wax formation. To prove the efficiency of the catalyst, a series of four tests was performed. At the end of the reaction, the hot reaction mixture was filtered and the removed solid was reintroduced inside the reactor without washing. The results reported in Table 3 showed that, after four cycles of reaction, the catalytic properties were essentially unchanged since the conversion of the reactant was above 95% and the selectivity to the phytosterol esters was above 90%.
| Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|
| Run | Ester | Sterol | Sterol ester | Diene | Other | Yield (%) |
| Temperature = 240 °C, reaction time = 7 h, DN2 =5 L h−1, ester/sterol ratio = 1, catalyst = 5 wt%. | ||||||
| 1 | 82 | 97 | 93 | 3 | 4 | 76 |
| 2 | 81 | 99 | 93 | 4 | 3 | 75 |
| 3 | 81 | 98 | 94 | 2 | 4 | 75 |
| 4 | 80 | 96 | 91 | 3 | 6 | 72 |
Conclusion
The synthesis of phytosterol ester from the transesterification of methyl dodecanoate with β-sitosterol generally performed in the presence of homogeneous catalysts can be carried out in the presence of basic solid catalysts. Indeed, the best yield of phytosterol ester (above 75%) is obtained over magnesium oxide or zinc oxide. However, the strong basic sites of magnesium oxide favored the side-reaction of dehydration of sitosterol to stigmasta-3,5-diene. The reaction must be carried out under nitrogen flow to remove the methanol formed at 240 °C. A series of four experiments showed that the catalytic performances were not affected when the catalyst was reused (without washing).In conclusion, basic oxides represent a new class of solid catalysts, effective for phytosterol ester synthesis, and appear attractive to replace homogeneous and corrosive catalysts such as strong mineral acids or bases in transesterification processes.
References
- S. Abro, C. Vanhove, Y. Pouilloux and J. Barrault, J. Mol. Catal. A: Chemical, 1999, 149, 243–254 CrossRef
.
- J. Barrault, Y. Pouilloux, C. Vanhove, K. Cottin, S. Abro and J.-M. Clacens, Catal. Org. React., 1998, 13 Search PubMed
.
- Y. Pouilloux, F. Autin, C. Guimon and J. Barrault, J. Catal., 1998, 176, 215–224 CrossRef CAS
.
- J. Barrault and Y. Pouilloux, Catal. Today, 1997, 37, 137–153 CrossRef CAS
.
- D. Burdick, G. Moine, D. Raederstorff and P. Weber, Phytosterols and/or Phytostanol Derivatives, Eur. Pat., EP 1004594A1, 31.05.2000, Hoffmann-La Roche AG Search PubMed
.
- A. Takada and Y. Higaki, Steroid Esters and Cosmetics and Ointments Containing the Same, US Pat., 4 393 044, 12.07.93, to The Nisshin Oil Mills Lim Search PubMed
.
- M. P. Van Amerogen, L. C. Lievense and C. Van Hoosten, Method of Manufacturing an Ester Mixture, World Pat., WO 98/01126, 15.01.98, to Unilever Search PubMed
.
- I. Wester and J. Ekblom, World Pat., WO 99/56558, 11.11.99, to Raisio Benecol Oy
.
- T. Miettinen, H. Vanhanen and I. Wester, Phytosterols Composition, US Pat., 5,502,045, 26.03.96, to Raision Tehtaat Oy Search PubMed
.
- N. Milstein, M. Biermann, P. Leidl and R. Von Kreis, Sterols Esters as Food Additives, World Pat., WO 99/30569, 24.06.99, to Henkel Corp Search PubMed
.
- A. Roden, J. L. Williams, R. Bruce, F. Detraino, M. H. Boyer and J. D. Higgins, Preparation of Sterol and Stanol-esters, Eur. Pat., EP 0982316 A2, 01.03.2000, to McNeil-PPC, Inc Search PubMed
.
- J. D. Higgins, Preparation of Sterols and Stanol Esters, Eur. Pat., EP 0982315 A2, 01.03.2000, to McNeil-PPC, Inc Search PubMed
.
- Patented results, Laboratoires Pharmascience
.
- S. Abro, Réactions d′estérification et de transestérification sélectives du glycérol en présence de catalyseurs solides, Thesis, 22.10.96, University of Poitiers Search PubMed
.
| This journal is © The Royal Society of Chemistry 2003 |
