Adsorption and separation of water-soluble aromatic molecules by cyclodextrin-functionalized mesoporous silica†
A. Bibby and L. Mercier*
Department of Chemistry and Biochemistry, Laurentian University, Sudbury, Ontario, Canada P3E 2C6. E-mail: lmercier@nickel.laurentian.ca
First published on 5th December 2002
Abstract
The adsorption properties of various water-soluble aromatic molecules (p-nitrophenol, p-nitroaniline, m-nitrophenol, p-chlorophenol and phenol), with CD-HMS, a new class of cyclodextrin-containing materials with uniform framework mesoporosity, has been investigated. The adsorption isotherms, uptake kinetics and distribution coefficients of the various solutes with the adsorbents were measured and the results interpreted based on the physicochemical properties of the adsorbents, the chemical structure of the adsorbates, and the inclusion thermodynamics between the adsorbates and the cyclodextrin binding sites. The CD-HMS adsorbents were thus shown to be promising materials for the selective separation of aromatic molecules from water.
Green ContextGreen chemistry encompasses reductions in the environmental impact of all chemical processes including remediation processes. The separation and isolation of organic compounds from aqueous process streams in a way that does not add to the environmental footprint of the whole process is an important goal in this context. Here we see how by utilising the excellent host–guest chemistry of cyclodextrins within easily accessible mesoporous silica structure, we can achieve efficient adsorption of organic molecules from water. The supported reagents can be prepared in a quite environmentally friendly way and they are effective under the typically harsh conditions (above ambient temperature, low pH, presence of bacteria, etc) found in industrial and waste water treatment plants.JHC |
Introduction
Cyclodextrins are cyclic oligosaccharides consisting of six or more linked D-glucopyranose units. The most widely studied cyclodextrins are those composed of six, seven and eight of these units, denoted α-, β- and γ-cyclodextrin, respectively. The conical shape of these molecules results in well-defined hydrophobic central cavities (with top and bottom diameters of 5.3 and 4.7 Å for α-cyclodextrin, 6.5 and 6.0 Å for β-cyclodextrin, and 8.3 and 7.5 Å for γ-cyclodextrin) that can accommodate the inclusion of various organic molecules with suitable geometry and function.1Fig. 1 shows the chemical structure of β-cyclodextrin and the depicts the formation of an inclusion complex with the cavity of the molecule. Cyclodextrins therefore behave as receptors for the selective binding of organic molecules, forming weak inclusion complexes with bound guest species.1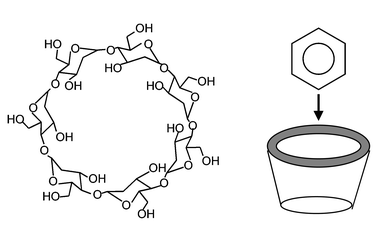 | ||
| Fig. 1 (left) Molecular structure of β-cyclodextrin and (right) depiction of the macromolecule’s inclusion cavity into which organic molecules can bind. | ||
The unique host–guest chemistry of cyclodextrins is highly conducive to the selective uptake of organic molecules from aqueous systems.2–5 Moreover, the chiral nature of the cavity in these molecules makes them useful for enantiomeric separation applications.1 These macromolecules thus demonstrate potential usefulness in a wide variety of applications, such as for chromatography, for the remediation of contaminated water, and for the purification of organic products. Because product/reagent separation, waste minimization and trace component analysis are important objectives of green chemistry and clean technology, cyclodextrins may be useful towards the achievement of these goals.
Cyclodextrins themselves, however, are highly water soluble, and must therefore be processed into solid forms before they can be implemented into usable separation technology. Thus, three different approaches for the preparation of cyclodextrin-functionalized solid materials have up until now been developed. The first of these involve the incorporation of cyclodextrins into polymers by attaching the moieties via chemical linkers to the backbone of polymer chains (Fig. 2(a)).2–8 The second approach involves the coating or grafting of cyclodextrin moieties onto stationary phases such as organic polymers9,10 or silica gel11–18 (Fig. 2(b)). Most recently, a third type of cyclodextrin-modified materials, denoted CD-HMS, was developed in which the cyclodextrin groups are located within the framework of nanoporous oxides (Fig. 2(c)).19
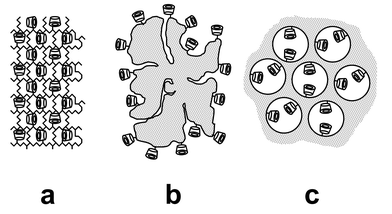 | ||
| Fig. 2 Schematic representations of diverse cyclodextrin-functionalized materials: (a) polymer matrices, (b) coated substrates and (c) nanoporous frameworks. | ||
CD-containing polymers are useful materials for organic molecule separation because they possess high CD group content and can easily be processed into various forms for practical applications (i.e., resin beads, membranes, etc.). These polymers, however, are plagued by their very low specific surface areas and absence of framework nanoporosity,2–8 drawbacks that impede the access of adsorbate molecules to the crowded cyclodextrin sites within the network of polymer chains (Fig. 2(a)), and subsequently result in incomplete sorption to the binding sites and slow diffusion kinetics. In addition, the lack of thermal and chemical stability of polymers often make them incompatible with the harsh conditions used in many industrial processes. In contrast, CD-coated (or grafted) materials such as silica offer improved access to the binding sites because the moieties are located on the external surface of the support, and the structure has improved stability owing to the robustness of the substrate (Fig. 2(a)). Unfortunately, the low CD loading of these materials impart them with greatly limited adsorption capacities. Our recently reported success in incorporating CD groups inside the pore channels of mesoporous silica with uniform channel structure (denoted CD-HMS) has resulted in a new class of CD-functionalized materials with both high CD group loadings and chemically robust structures (Fig. 2(c)).19 Preliminary adsorption studies on CD-HMS materials have shown that their open-framework structures promote unhindered interactions of organic guests with their CD binding sites because they easily permeate the material and reach the abundant CD groups inside the adsorbent (Fig. 2(c)).19
CD-HMS materials are prepared using an entirely aqueous/ethanolic synthesis procedure involving surfactant structure-directing agents (alkylamines), pore expanding additives (trimethylbenzene, TMB) and ethoxysilane precursors (for an extensive description of the CD-HMS synthesis process, see ref. 19). Once the materials are formed, the surfactant and TMS are removed from their framework by ethanol extraction, and can therefore be recycled for further use. The environmentally friendly aspects of CD-HMS preparation are therefore compatible with the principles of ‘green synthesis’, avoiding the use and disposal of toxic solvents and precursors which are often necessitated in the preparation of organic polymers and coated silicas.
Mesoporous silicas related to CD-HMS but functionalized with ligand groups have in recent years been extensively investigated for their ability to bind toxic inorganic species such as heavy metal ions from solution.20–28 In contrast, similar efforts to apply ordered mesoporous silicas for the selective adsorption of organic molecules have up until now remained virtually non-existant. This report investigates and compares the sorption properties of various soluble aromatic compounds by a series of mesoporous CD-HMS adsorbents having varying loadings of cyclodextrin groups. To the best of our knowledge, CD-HMS represents the first class of mesoporous materials which can selectively separate organic molecules by using the shape- and function-specific inclusion properties of receptor binding sites such as those of cyclodextrin. Because the designed of tailored solid adsorbents (silica-based materials in particular) applied towards the separation and isolation of reagents, products and wastes from process streams constitutes one of the primary objectives of green chemistry,29,30 CD-HMS materials represent an important advancement in this area.
Experimental
Adsorbents and solutions
Nanoporous CD-HMS materials containing various loadings of CD groups were synthesized by the crosslinking of a silylated derivative of β-cyclodextrin with tetraethoxysilane (TEOS) in the presence of a structure-directing surfactant, following previously described processes.19 The CD-containing nanoporous materials thus formed were designated as CD-HMS-X%, where X refers to the molar percentage of CD groups in the materials with respect to their Si content. The adsorbents were structurally and chemically characterized according to the methods described in ref. 19. Aqueous solutions with varying concentrations (in the range 0–0.4 mM) of the following aromatic compounds (Aldrich) were prepared using deionized water: p-nitrophenol (O2NC6H4OH, pNP), p-nitroaniline (O2NC6H4NH2, pNA), m-nitrophenol (O2NC6H4OH, mNP), p-chlorophenol (ClC6H4OH, pCP) and phenol (C6H5OH, PhOH).Aromatic molecule adsorption
For each CD-HMS adsorbent, 5 mg samples were stirred with 25 ml of the adsorbate solutions for 24 h. The suspension was then filtered and the residual solute concentration in the filtrate was measured using UV–vis spectroscopy (using the intensity of the λmax peak for each aromatic solute: pNP = 405 nm, pNA = 405 nm, mNP = 288 nm, pCP = 281 nm and PhOH = 400 nm). The amount of aromatic solute adsorbed by the CD-HMS was then determined by subtracting the concentration in the supernatent from that of the initial untreated solution.Adsorption kinetics
5 mg samples of each adsorbent were slurried in 20 ml water. 20 ml of aromatic molecule solution (0.22 mM) were then rapidly added to the slurry, after which the suspensions were stirred for specific time periods (up to 600 s), then rapidly vacuum filtered through a Whatmann paper filter. The residual solute concentrations in the filtrates was measured by UV–vis spectroscopy. The amount of aromatic solute adsorbed by the CD-HMS was then determined by subtracting the concentration in the supernatent from that of the initial untreated solution.Results and discussion
Adsorbent characteristics
The nomenclature and physico-chemical properties of the CD-HMS materials are shown in Table 1. The adsorbents had high surface areas (200–550 m2 g−1) and pore volumes (0.21–0.51 cm3 g−1), and uniform pore channels with diameters in the range 38–42 Å (Table 1).19 The CD contents in the materials (up to 0.39 mmol g−1, or about 30% by weight) were comparable to those of typical CD-containing polymers,2–8 and clearly superior to those of CD-coated adsorbents.19| Material | Surface area/ m2 g−1 | Pore volume/ cm3 g−1 | Pore diameter/Å | CD content/mmol g−1 |
|---|---|---|---|---|
| CD-HMS-2% | 547 | 0.51 | 38 | 0.14 |
| CD-HMS-4% | 319 | 0.26 | 39 | 0.32 |
| CD-HMS-6% | 197 | 0.21 | 40 | 0.33 |
| CD-HMS-8% | 235 | 0.27 | 42 | 0.39 |
Adsorption isotherms
Representative adsorption isotherms for CD-HMS-8% with each of the aromatic guests are shown in Fig. 3. The aromatic solutes were taken up by the adsorbents until a maximum saturation capacity was reached, the magnitude of which being related to both the amount of cyclodextrin groups incorporated within the CD-HMS materials and to the type of aromatic solute, as shown in Table 2. Control adsorption experiment in which non-functionalized HMS silica was used under otherwise identical experimental conditions revealed negligible uptake of the aromatic solutes. This shows that the uptake of the aromatic molecules results exclusively from their inclusion within the CD groups of the materials, and not through non-specific binding interactions with the silica support.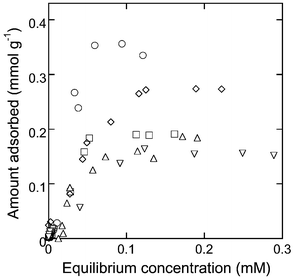 | ||
| Fig. 3 Aromatic molecule adsorption isotherms for CD-HMS-8% (pNP = ○, pNA = □, mNP = ◇, pCP = △, PhOH = ▽). Expressed in ppm concentration units, 0.1 mM corresponds to 13.9 ppm for pNP and mNP, 13.8 ppm for pNA, 12.9 ppm for pCP, and 9.4 ppm for PhOH. | ||
| Adsorption capacities/mmol g−1 | ||||
|---|---|---|---|---|
| Adsorbate | CD-HMS-2% | CD-HMS-4% | CD-HMS-6% | CD-HMS-8% |
| a Amount of CD groups in adsorbent (mmol g−1). | ||||
| (theoretical maxima)a | 0.14 | 0.32 | 0.33 | 0.39 |
| pNP | 0.13 (93%) | 0.24 (75%) | 0.30 (91%) | 0.36 (92%) |
| mNP | 0.13 (93%) | 0.17 (53%) | 0.23 (70%) | 0.28 (72%) |
| pCP | 0.10 (71%) | 0.14 (44%) | 0.16 (48%) | 0.18 (46%) |
| pNA | 0.10 (71%) | 0.13 (41%) | 0.15 (45%) | 0.19 (49%) |
| PhOH | 0.06 (43%) | 0.10 (31%) | 0.12 (36%) | 0.15 (38%) |
Interestingly, the maximum adsorption capacity for the different solutes, expressed in mmol g−1, were very dissimilar for each CD-HMS adsorbent investigated. For instance, the maximum adsorption capacity for pNP was found to mirror that of the cyclodextrin content in each of the CD-HMS materials (Table 2), suggesting that 1∶1 complexation of the solute is occurring with virtually every cyclodextrin group present. The decreasing adsorption capacity of the adsorbents towards other aromatic solutes, however (Table 2), denoted weakened binding affinity of the materials for aromatic molecules with different chemical structures. The affinity of the CD-HMS adsorbents for the aromatic molecules showed the following trend: pNP > mNP > pCP ≈ pNA > PhOH (Table 2), which is found to correlate with the magnitude of the free energies measured for solution-phase complexation of the solutes with β-cyclodextrin (pNP: −14.5 kJ mol−1, pNA: −14.3 kJ mol−1, mNP: −13.9 kJ mol−1, pCP: −13.7 kJ mol−1, PhOH: −11.3 kJ mol−1).1 The degree of access of the CD binding sites within the adsorbents is thus related to the complexation affinity of these sites towards the different aromatic solutes. The ability of the CD sites to bind with certain aromatic molecules while completely exclude others demonstrates the ability of CD-HMS to selectively adsorb and separate different water-soluble aromatic molecules.
A close inspection of the adsorption isotherms at very low equilibrium solute concentrations reveals that the uptake of the aromatic molecules is efficient, but the amount adsorbed levels off at values well below that of the maximum adsorption capacity of the materials (up to about 0.03 mmol g−1). This signifies that, although the materials can act as effective adsorbents for low levels of aromatic molecules, significant uptake of the solutes only occurs at comparatively high concentrations (greater that about 3 × 10−4 M). Such adsorption behaviour attests the inherently weak interactions which exist between the cyclodextrin binding sites and the targeted aromatic guests. Previous studies have also found that, as in the case of CD-HMS, the adsorption capacity of CD-functionalized polymers is highly dependent on the solute concentration of the treated solutions.2,3
Adsorption kinetics
Representative kinetic uptake curves for each solute with CD-HMS-8% are shown in Fig. 4. The uptake of aromatic molecules by the CD-HMS materials were generally very rapid, reaching the maximum adsorption capacity of the adsorbents after about 5 min of solution exposure. In particular, pNP demonstrated exceptionally rapid uptake at the early stages of the adsorption process, with almost half of the CD sites being accessed within the first minute of exposure (Fig. 4). The sorption kinetics of the CD-HMS materials were vastly improved compared to those reported for CD-containing polymers, which under similar treatment conditions reached saturation only after 30 min of exposure.2,3 The fast adsorptivity exhibited by these materials can be attributed to the open-framework nature of the mesoporous adsorbents, resulting in greatly improved access of the targeted adsorbate molecules to the cyclodextrin binding sites.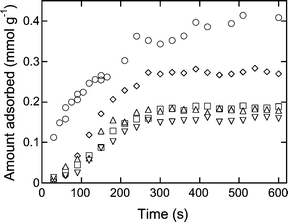 | ||
| Fig. 4 Kinetic uptake plots of aromatic molecules (pNP = ○, pNA = □, mNP = ◇, pCP = △, PhOH = ▽) for CD-HMS-8%. | ||
Uptake effectiveness
The effectiveness of the CD-HMS materials towards the removal and separation of aromatic molecules from water was gauged by comparing the distribution coefficients, Kd, of the adsorbents at different solute concentrations with respect to the various solutes investigated. The value of Kd for each adsorbent–adsorbate system were obtained from eqn. (1)27,28 using their respective equilibrium adsorption data:| Kd = (Ci − Cf)Vsol/(Cfmads) | (1) |
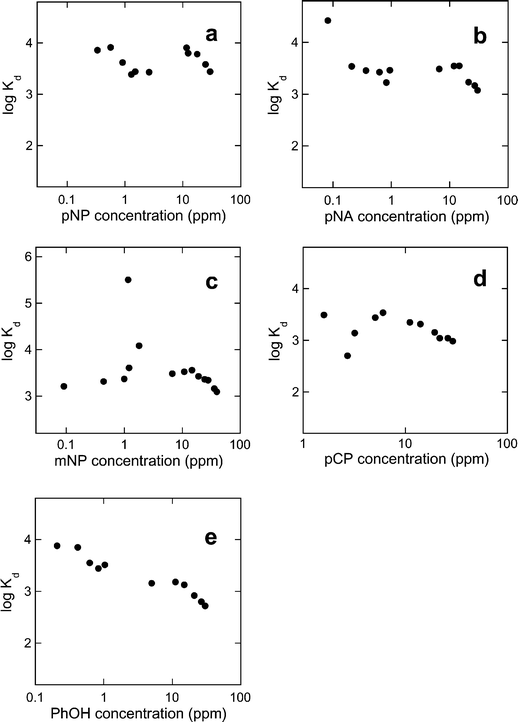 | ||
| Fig. 5 Distribution coefficient (Kd) profiles of CD-HMS-8% towards (a) pNP, (b) pNA, (c) mNP, (d) pCP and (e) PhOH.31 | ||
The Kd values for pNP and pNA generally followed a decreasing trend as a function of increasing solute concentrations (Fig. 5). This type of separation behaviour is typical for materials that have appreciable binding interactions with targeted guest compounds (especially in the case of heavy metal ions)27,28 because adsorbents with adsorbate-specific binding sites (such as in CD-HMS) are usually most effective at binding when these sites are largely unoccupied (or uncomplexed) by the adsorbate. As saturation of these sites is approached (i.e, at higher adsorbate concentration), the binding affinity of the materials decrease.
The decreasing values of Kd , however, is not systematic for most solutes. For instance, the Kd values for pNP and pNA adsorption with CD-HMS decreased in the low adsorbate concentrations range (below 1 ppm) (Fig. 5(a) and (b)), but the values subsequently increased thereafter, reaching a second maximum values at adsorbate concentrations near 10 ppm (beyond which the values once again resume their decreasing trend).31 The presence of this second Kd maximum suggests the presence of two distinct types of CD binding sites within the adsorbents. The first of these respond well to the adsorption of low concentrations of the adsorbates, and likely represents the complexation of the aromatic molecules with the most readily accessible CD groups found either on the perifery of the adsorbate particles, or near the openings of the pore channels. The second type of site, which only interact with the adsorbate molecules at higher concentrations (ca. 10 ppm) can be assigned to the interaction of the adsorbates with CD groups located deeper within the pore channels of the materials (requiring a higher adsorbate concentration so that these can be driven to interact with these more sterically impeded sites). This interpretation is consistent with the shape of the adsorption isotherms (Fig. 3,vide supra), in which two distinct adsorption inflexions were also reported (denoting two distinct types of binding environments).
Unlike those of pNP and pNA, which showed optimal adsorption effectiveness at low concentrations (below 0.3 ppm), the Kd values for mNP, pCP and PhOH were in general significantly reduced in this concentration range. This discrepancy can be rationalized by comparing the free energies for the solution-phase complexation for pNP and pNA with β-cyclodextrin (measured at −14.5 and −14.3 kJ mol−1, respectively)1 with those of mNP, pCP and PhOH (−13.9, −13.7 and −11.3 kJ mol−1, respectively).1 The ability of the CD-HMS materials to effectively adsorb pNP and pNA at low concentrations can therefore be attributed to the inherently higher free energies of complexation of these solutes with the most accessible (peripheral) binding sites compared to that of the other aromatic species. The Kd values for mNP, pCP and PhOH reach maximum values at higher adsorbate concentrations (between 1 and 10 ppm), where the adsorbate molecules interact with the CD groups located inside the pore channels of the adsorbent framework.
Conclusions
Mesoporous CD-HMS materials have been shown to be an effective new class of adsorbents for the selective adsorption of organic molecules from water. The open-framework structure of the adsorbents and their high CD content allowed for the rapid and unimpeded access of the targeted adsorbate molecules to potentially every binding site within the structure. The chemical and biological robustness of the silica framework encapsulating the cyclodextrin groups make CD-HMS materials particularly promising for use under the harsh conditions often encountered in industrial and wastewater treatment applications (i.e., temperature, pH, presence of bacteria or algae, etc.).19 Finally, that CD-HMS can be prepared in an environmentally-friendly manner makes these materials of particular interest for green chemistry applications. All of these beneficial characteristics therefore make CD-HMS materials clearly superior to other related adsorbents materials (such as polymers and coated silicas) from both industrial process and green chemistry points of view.The results of the experiments presented in this work demonstrate that mesoporous CD-containing materials such as CD-HMS represent a promising emerging technology for a wide variety of environmental and separation applications, including pesticide remediation, VOC capture, reagent/product isolation, chiral drug separations and liquid chromatography.
Acknowledgements
The authors would like to thank the Natural Science and Engineering Research Council of Canada (NSERC) and Laurentian University for financial support.References
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875 CrossRef CAS
.
- G. Crini, S. Bertini, G. Torri, A. Naggi, D. Sforzini, C. Vecchi, L. Janus, Y. Lekchiri and M. Morcellet, J. Appl. Polym. Sci., 1998, 68, 1973 CrossRef CAS
.
- G. Crini, L. Janus, M. Morcellet, G. Torri, A. Naggi, S. Bertini and C. Vecchi, J. Appl. Polym. Sci., 1998, 69, 1419 CrossRef CAS
.
- D. Li and M. Ma, CHEMTECH, 1999, 29, 31 Search PubMed
.
- M. Ma and D. Li, Chem. Mater., 1999, 11, 872 CrossRef CAS
.
- A. Harada, J. Li and M. Kamachi, Nature, 1993, 364, 516 CrossRef CAS
.
- X. X. Zhu, F. Brizard, C. C. Wen and G. R. Brown, J. Macromol. Sci. Pure Appl. Chem., 1997, A34, 335 Search PubMed
.
- G. Crini, C. Cosentino, S. Bertini, A. Naggi, G. Torri, C. Vecchi, L. Janus and M. Morcellet, Carbohydr. Res., 1998, 308, 37 CrossRef CAS
.
- X.-B. Zhao and B.-L. He, React. Polym., 1994, 24, 9 Search PubMed
.
- K. Sreenivasan, J. Appl. Polym. Sci., 1996, 60, 2245 CrossRef CAS
.
- M. Tanaka, M. Yoshinaga, M. Ito and H. Ueda, Anal. Sci., 1995, 11, 227 Search PubMed
.
- I. Ciucanu and W. A. König, J. Chromatogr. A, 1994, 685, 166 CrossRef CAS
.
- I. Ciucanu, J. Chromatogr. A, 1996, 727, 195 CrossRef CAS
.
- G. Crini, M. Morcellet and G. Torri, J. Chromatogr. Sci., 1996, 34, 477 Search PubMed
.
- B. Sébille, M. Guillaume, C. Vidal-Madjar and N. Thuaud, Chromatographia, 1997, 45, 383 CAS
.
- L. Zhang, Y. Wong, L. Chen, C. B. Ching and S. Ng, Tetrahedron Lett., 1999, 40, 1815 CrossRef CAS
.
- T. N. T. Phan, M. Bacquet, J. Laureyns and M. Morcellet, Phys. Chem. Chem. Phys., 1999, 1, 5189 RSC
.
- G. Crini, Y. Lekchiri, L. Janus, M. Morcellet and N. Morin, Chromatographia, 1999, 50, 661 CAS
.
- R. Huq, L. Mercier and P. J. Kooyman, Chem. Mater., 2001, 13, 4512 CrossRef CAS
.
- L. Mercier and T. J. Pinnavaia, Adv. Mater., 1997, 9, 500 CrossRef CAS
.
- X. Feng, G. E. Fryxell, L.-Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS
.
- L. Mercier and T. J. Pinnavaia, Microporous Mesoporous Mater., 1998, 20, 101 CrossRef CAS
.
- M. H. Lim, C. F. Blanford and A. Stein, Chem. Mater., 1998, 10, 467 CrossRef CAS
.
- L. Mercier and T. J. Pinnavaia, Environ. Sci. Technol., 1998, 32, 2749 CrossRef CAS
.
- J. Brown, L. Mercier and T. J. Pinnavaia, Chem. Commun., 1999, 69 RSC
.
- J. Brown, R. Richer and L. Mercier, Microporous Mesoporous Mater., 2000, 37, 41 CrossRef
.
- M. C. Burleigh, S. Dai, E. W. Hagaman and J. S. Lin, Chem. Mater., 2001, 13, 2537 CrossRef CAS
.
- K. Z. Hossain and L. Mercier, Adv. Mater., 2002, 14, 1053 CrossRef CAS
.
- P. M. Price, J. H. Clark and D. J. Macquarrie, J. Chem. Soc., Dalton Trans., 2000, 101 RSC
.
- A. G. S. Prado, L. N. H. Arakaki and C. Airoldi, Green Chem., 2002, 4, 42 RSC
.
- The distribution coefficient profiles of the other adsorbents (viz., CD-HMS-2%, CD-HMS-4% and CD-HMS-6%) for each solute were virtually identical to each other, attesting to the reproducibility of the results. The complete distribution coefficient profiles for all CD-HMS materials has been provided as ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Figs. S1–5: distribution coefficient profiles. See http://www.rsc.org/suppdata/gc/b2/b209251b/ |
| This journal is © The Royal Society of Chemistry 2003 |
