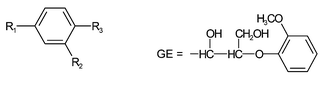Origin of the mediator losses in electrochemical delignification processes: primary and secondary reactions of violuric acid and N,N′-dimethylvioluric acid radicals with lignin model compounds
Markus
Mickel
,
Hee-Cheol
Kim
and
Norbert
Hampp
*
University of Marburg, Institute of Physical Chemistry, Hans-Meerwein-Str. Geb. H, D-35032, Marburg, Germany. E-mail: hampp@mailer.uni-marburg.de
First published on 16th January 2003
Abstract
The use of mediator-based processes for the delignification of wood pulps is of great interest as they promise to be environmentally as well as economically interesting alternatives to the currently dominating chlorine-based processes. Mediator-based processes suffer from the fact that substantial amounts of the mediator molecules are irreversibly lost during the reactions. We have now analyzed in detail the primary and in addition the secondary reactions of the oxime type mediators violuric acid and dimethylvioluric acid with lignin model compounds and softwood pulp in order to find the reasons for the repeatedly reported loss of mediator molecules during enzymatic and electrochemical delignification processes. Using a set of lignin model compounds, representing the various structural subunits in the lignin network, we were able to demonstrate that the loss of mediator is not due to the primary reaction of the mediator radicals with the lignin but occurs in a secondary reaction where the activated lignin subunits react with the mediators in the solution. The primary reaction of the mediator radicals is hydrogen abstraction from phenols and activated aromatic rings which leads to the formation of phenoxyl and phenyl radicals. The secondary reaction is the formation of colored semi-stable covalent adduct molecules from an activated lignin subunit with a mediator molecule in the solution leading to polycyclic N-hydroxy compounds. Only in this secondary reaction mediator molecules are removed from the solution due to covalent attachment to the lignin network. No reaction with the cellulose content in the pulp is observed. The findings reported in this paper point the way to an improved mediator design and appropriately modified processes, where the secondary mediator reactions are suppressed, and removes a major road block towards a technical application of the mediator-based delignification procedures.
Green ContextThe development of more environmentally benign alternatives to chlorine in pulp bleaching processes is an important large scale industrial challenge—Redox mediators and more recently N-hydroxy compounds have been proposed for this purpose. They generate radicals in the presence of enzymes, and these in turn cause pulp delignification. Unfortunately, significant quantities of these ‘mediators’ are lost in the delignification process. This paper analyses this loss, explains its cause and thus opens the door to improved mediator design. Thus electrochemical mediator-based delignification is shown to be an attractive alternative to existing chlorine-based processes.JHC |
Introduction
In the late eighties the principle of mediator-based pulp bleaching processes was proposed, where enzymatically oxidized mediator molecules cause a delignification, i.e. an oxidation of the brown colored lignin in the pulp.1 The redox mediators used first for this purpose were ABTS (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid)) and HBT (1-H-hydroxybenzotriazole). Later N-hydroxy-compounds2,3 and recently also transition metal complexes4–6 were proposed as mediators for pulp delignification. The enzymatic oxidation of these compounds leads to radicals1,3,6–8 or highly oxidized states which are the active agents. The radicals are generated by the enzymes, released and diffuse into the pulp fibers where they react with the lignin. Theoretically there should be no consumption of the mediator at all as it is fully recovered and permanently recycled in the process. However, a significant loss of mediator was observed in cases where radicals are used as oxidizing agent but up to now no analysis of the molecular mechanisms causing this loss has been reported. We found that the mediator loss is not due to the primary reaction of the radicals with the lignin but due to a secondary reaction of the activated lignin network with mediator molecules in the aqueous solution. With the findings reported the use of catalytic amounts of chlorine-, sulfur- and heavy metal-free mediators instead of the stoichiometric use of chlorine in delignification becomes somewhat more realistic. This is a contribution to the environmentally friendly use of sustainable resources.Results and discussion
Electrochemical mediator-based delignification
Many of the mediators proposed in the literature react also with the enzymes required for activation and cause their deactivation. In addition the use of enzymes to produce the required radicals implies a number of restrictions for the processes as pH as well as temperature of the pulp during the enzymatic treatment need to be controlled properly to match the physiological requirements of the enzyme.To overcome these restrictions we introduced an electrochemical process where the enzymatic mediator oxidation is replaced by an electrochemical oxidation at the anode of an electrolyzer cell.5,9 The use of an electrolyzer cell removes the restriction to physiological conditions (pH, temperature) implied by the use of enzymes.
For the electrochemical process described the cyclic oximes violuric acid (Vio) and dimethylvioluric acid (dmVio) are suitable mediators. The molecules are electrochemically as well as enzymatically oxidizable to the corresponding iminoxy radicals5 which are extraordinarily stable in the pH range 2–10 and show a half-life of more than 30 min. It has been shown that the radicals undergo a fast reaction with the lignin portion in the pulp leading to a delignification of up to 50% in a single step.9,10 The redox systems of Vio as well as dmVio show a single electron oxidation peak at 1.02 V vs. NHE. The redox system is completely reversible in water as derived from cyclovoltammetric experiments.9,10
However, like in the enzymatic systems a loss of mediator during the electrochemical delignification is observed with Vio and dmVio. This loss limits the efficiency of the total process. We have now analyzed the primary molecular mechanisms of the radical catalyzed depolymerization of lignin in more detail.
Reaction of violuric acid and N,N′-dimethylvioluric acid radicals with lignin model compounds
In the following the reactions of violuric acid (Vio˙) and N,N′-dimethylvioluric acid (dmVio˙) radicals with lignin containing pulp is analyzed by means of lignin model compounds. The goal of the investigation is to detect the reaction sites as well as to determine the reaction rates of the Vio˙ and dmVio˙ radicals with the various structural groups in lignin.11For the investigations the model compounds shown in Table 1 were used. The compounds tested comprise classical lignin model compounds as well as monofunctional aromatic molecules. A focus is on guaiacol (Gua) and veratryl alcohol (VA) as representatives for phenolic and for non-phenolic structural lignin subunits. The dimeric lignin models are representatives of the O-4 linkage type, which frequently occurs in lignin, and show similar reactions but due to the multi-functionality of these compounds several chemical reactions occur in parallel and lead to a more complex analysis.
|
|
|||
|---|---|---|---|
| R1 | R2 | R3 | Compound |
| OH | OCH3 | H | Guaiacol |
| OH | OCH3 | CH2OH | Vanillyl alcohol |
| OH | OCH3 | GE | Guaiacolglyceryl-β-guaiacyl ether |
| OCH3 | OCH3 | H | Veratrole |
| OCH3 | H | H | Anisole |
| OCH3 | H | CH2OH | Anisyl alcohol |
| OCH3 | OCH3 | CH2OH | Veratryl alcohol |
| OCH3 | OCH3 | GE | Veratrolglyceryl-β-guaiacyl ether |
| OCH3 | OCH(OH)CH2OH | H | Guaiacolglyceryl ether |
| CH2OH | H | H | Benzyl alcohol |
Violuric acid (Vio) as well as N,N′-dimethylvioluric acid (dmVio) form almost identical radicals upon electrochemical oxidation (Fig. 1). From the EPR spectra their radical structure is derived with the unpaired electron localized on the NO group.12 The lifetime of these radicals in water at room temperature is on the order of several tens of minutes.9
 | ||
| Fig. 1 Structure of violuric acid (Vio) and dimethyl violuric acid (dmVio) and EPR-spectra of the corresponding iminoxy radicals. The radicals are the reactive species in the so-called delignification process. | ||
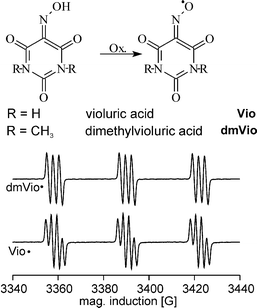
The reaction of Vio˙ with a number of test compounds is shown in Fig. 2. EPR was employed to monitor the time-dependence of the radical concentration in the reactions with different lignin model compounds. 50 mL of 100 mmol L−1 aqueous solutions (or suspensions where required) of the model compounds were added to 1 L of 0.5 mmol L−1 Vio solution (containing about 40% as Vio˙). The at least 10-fold excess of the model compound guarantees that the kinetics observed is corresponding to the different reactivities of the model compounds. No reaction is observed upon addition of cellobiose (1) but phenolic lignin model compounds (8–10) cause an immediate radical consumption. With non-phenolic aromatic model compounds (2–7) a slower reaction kinetics is observed.
 | ||
| Fig. 2 Time dependent decrease of the EPR radical signal of Vio˙ after the addition of different lignin model compounds. Before t = 0 the thermal decay of the Vio˙ radical in water is seen. At t = 0 the model compounds are added and a small change in the signal intensity due to dilution of the radical is observed for all compounds. | ||
The electrochemical formation of the mediator radicals Vio˙ and dmVio˙ is equivalent to the abstraction of a hydrogen atom from the oxime group. The fast reaction of Vio˙ with phenolic groups is due to hydrogen abstraction from the phenolic group which leads to the formation of a phenoxyl radical and recovery of Vio. The slower reaction of Vio˙ with the different aromatic model compounds is due to an oxidation of the aromatic ring. The rate constants correspond to the activation of the ring by its substituents and its thermodynamic oxidation potentials (Uox). This is seen from the initially same reaction rates of veratryl alcohol (Uox = 1.35 V vs. SCE) 7, a benzyl alcoholic compound, and veratrole (Uox = 1.27 V vs. SCE) 6, an activated aromatic compound. The comparison of the reaction rates of veratrole 6 and anise alcohol (Uox = 1.45 V vs. SCE) 5 show a clearly higher reaction rate with veratrol which proves that abstraction of benzylic hydrogen atoms is of minor importance. The structural unit where lignin is attacked with Vio˙/dmVio˙ is an aromatic system activated by two methoxy groups, a benzyl alcohol function or a phenolic group (Uox = 0.7 V vs. SCE). The spontaneous radical decrease observed with EPR after addition of pulp is related to the reaction with the phenolic subunits9 because they show the fastest reaction due to the acidic hydrogen atom.
In order to prove the selectivity of the radical the reaction with cellobiose 1 was tested which is a soluble model compound for the carbohydrate content in pulp. By adding cellobiose to a Vio˙ solution no reduction of the radical signal is observed. Thus no reaction takes place with the cellulose part of the pulp, which guarantees a high quality of the pulp produced. Viscosity investigations at electrochemically delignified pulp confirm the very good selectivity of the reaction.
Mediator recovery
As long as the reactions of the mediators would only comprise the above described mechanisms no mediator consumption should be observed. However, in the literature the irreversible loss of mediator is repeatedly reported [e.g. refs. 2 and 13]. The mediators analyzed in pure form by cyclovoltammetry reveal to be nicely reversible. The loss of mediator must be dependent on and due to the reaction partners during the delignification reaction.We have analyzed the recovery rate of Vio˙ for the reaction with equimolar solutions of the model compounds guaiacol and veratryl alcohol as well as for softwood Kraft pulp. RDE-voltammetry is a suitable tool to measure simultaneously the amount of Vio and Vio˙ with acceptable time resolution. The decrease of radical as well as the recovery of Vio from Vio˙ after the addition of lignin model compounds or pulp is quantitatively determined. The initial amount of mediator radical (Vio˙) should be theoretically 100% be converted back to mediator (Vio). The amount of mediator recovered in the experiment is given by the difference of mediator at the time where no more radical is detected (Vioend) minus the initial amount of mediator (Viostart). The recovery rate RR is then calculated from RR = ([Vio]end − [Vio]start)/[Vio˙]start. Recovery rates of Vio˙ to Vio with the lignin model compounds veratryl alcohol and guaiacol (Fig. 3) of 52% and 61%, respectively, are obtained. For softwood pulp a recovery rate of 66% is found. The reactions are completed within several minutes.
![Recovery of violuric acid from its radical upon reaction with the model compounds guaiacol and veratryl alcohol as well as softwood kraft pulp. The diagrams show the RDE-analyses of the reaction of Vio˙ with a = guaiacol, b = veratryl alcohol and c = pulp, respectively. The numbering corresponds to measurements at different times: (1) before (2) 30 s; (3) 3 min; (4) 7 min; (5) 11 min after the adding of the model compounds. The recovery rate RR is calculated from RR(t) = ([Vio]end
− [Vio]start)/[Vio˙]start. The end of the reaction is reached as soon as no remaining Vio˙ is detected.](/image/article/2003/GC/b208812f/b208812f-f3.gif) | ||
| Fig. 3 Recovery of violuric acid from its radical upon reaction with the model compounds guaiacol and veratryl alcohol as well as softwood kraft pulp. The diagrams show the RDE-analyses of the reaction of Vio˙ with a = guaiacol, b = veratryl alcohol and c = pulp, respectively. The numbering corresponds to measurements at different times: (1) before (2) 30 s; (3) 3 min; (4) 7 min; (5) 11 min after the adding of the model compounds. The recovery rate RR is calculated from RR(t) = ([Vio]end − [Vio]start)/[Vio˙]start. The end of the reaction is reached as soon as no remaining Vio˙ is detected. | ||
A more detailed analysis is required to investigate which processes occur in parallel. The turnover of each mediator molecule of Vio and dmVio may be calculated to range between two and three cycles in a real experiment. These values are in contradiction to the earlier finding that the mediators show an excellent electrochemical reversibility in aqueous solution. If the primary mediator reaction is not responsible for the apparently low mediator cyclicity then secondary reactions must occur which cause that mediator is removed from the solution and chemically attached to the model compounds or the pulp.
Formation of mediator-model compound adducts
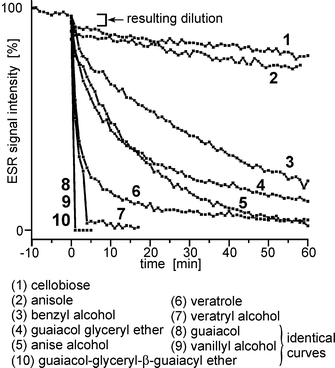
We have analyzed the soluble reaction products of Vio˙ and dmVio˙ radicals with lignin model compounds by time-dependent LC-MS analysis. It was found that in the case of Vio˙ an equimolar coupling product between Vio˙ and the substrate was formed (Fig. 4). The retention time indicates an increased polarity of the adduct in comparison to the lignin model substance. The molecular mass of the detected coupling product was 280 u which corresponds to the sum of Vio˙ (156 u) and guaiacol (124 u). In order to verify this result an analogous reaction with dmVio˙ was performed and a similar product with a molecular weight increased by 28 u (due to the two additional methyl groups) was obtained at 308 u. Both experiments indicate the formation of a 1∶1 adduct between mediator and guaiacol. | ||
| Fig. 4 Analysis of the products obtained from the reactions of mediator radicals Vio˙ and dmVio˙ with the model compounds guaiacol and veratryl alcohol. Shown is the total ion count (positive mode) chromatogram after 2 min and 24 h reaction time and the mass spectra of the formed adduct molecules after 2 min reaction time. In the last column the time dependent UV/Vis (solid line: 2 min reaction time, broken line 24 h reaction time) spectra are shown. | ||
However, also a striking difference is observed in that while the Vio adduct disappears after 24 h reaction time, the corresponding dmVio product is stable.
The formation of a covalent adduct is not only observed with the phenolic model compound guaiacol, but also with benzyl alcoholic model compounds like veratryl alcohol and also with activated aromatics like veratrole (not shown). In the case of veratryl alcohol the expected mass of the adduct molecule is 324 u with Vio and 352 u with dmVio. Both signals are observed, but the most intense ion signals correspond to an ion after an internal condensation reaction (308 u and 334 u).
After 24 h reaction time with both mediators (Vio and dmVio) no residual adduct molecules are detected which is in contrast to the phenolic lignin model compounds. In the Experimental section a more detailed summery of the reactant and product peaks and their identification is given (Table 2).
| Retention time/min | Mass/u | Substance | |
|---|---|---|---|
| a Most abundant ion. | |||
| Reactants | 9.2 | 156 | Vio (not shown) |
| 25.5 | 184 | dmVio | |
| 20.9 (UV) | No ion | Gua | |
| 29.8 | 167, 151a | VA | |
| Gua + Vio | 18.9 | 280,a 138 | Adduct |
| 33.3 (24 h) | 276,a 260 | Quinoid dimer | |
| Gua + dmVio | 25.7 | 184 | dmVio |
| 27.1 | 308,a 292, 138 | Adduct | |
| 32.6 924 h) | 276,a 260 | Quinoid dimer | |
| VA + Vio | 25.9 | 324, 306,a 182 | Adduct |
| 29.8 | 168, 151a | VA | |
| 32.5 | 167,a 151 | Veratric aldehyde | |
| VA + dmVio | 25.7 | 184 | dmVio |
| 29.4 | 352, 334a | Adduct | |
| 30.1 | 168, 151a | VA | |
| 32.8 | 167,a 151 | Veratric aldehyde | |
The formation of a semi-stable adduct also can be monitored by UV/Vis spectroscopy. From reaction of the radicals with the model compounds intensely redish colored solutions result, with an absorption maximum found at around 500 nm (Fig. 4). A continuous reduction of the absorption is observed which conforms to the semi-stability of the adducts found by LC-MS measurements.
From these experiments it is concluded that the reaction of mediators and model compounds lead to 1∶1 covalent adduct molecules. In most cases this adduct molecule is not stable and the adduct complex hydrolyses (type a), but in some cases, here e.g. dmVio and guaiacol, a stable adduct is formed (type b). The first mechanism (type a) could explain a transient loss of mediator, but the latter mechanism (type b) also may hold for an irreversible loss of mediator due to chemical attachment to the pulp.
Formation of model compound dimers
The next question to analyze is whether the observed reactions are due to the direct reaction of mediator radicals with model compounds or whether other mechanisms are involved. A good indicator for concomitant reactions is that the solutions (see Fig. 4) do not become completely colorless, because other colored compounds develop.The mass spectra indicate the formation of dimeric quinoid compounds from the lignin model compounds (Fig. 5). The same product is formed from reactions involving the different mediators Vio and dmVio. This corresponds with the reaction scheme that in a first step the model compound reacts with the Vio˙ under formation of an aromatic radical and in a second step a dimerisation with another model compound molecule occurs. Oxygen-rich quinoid systems are formed via reactions with the oxygen content of the aqueous solution.14–16 Performing MS/MS experiments with the product ion of mass 276 u leads to a major fragment with 244 u which correlates to the loss of molecular oxygen (Fig. 5) which proves the above mentioned mechanism.
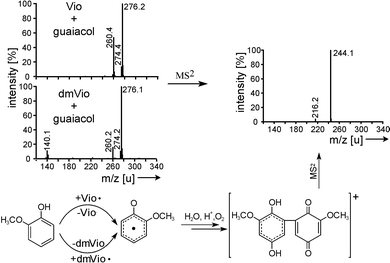 | ||
| Fig. 5 Formation of mediator type independent guaiacol dimers. The MS and MS/MS spectra of the molecule and a proposed reaction scheme is shown. | ||
The reaction mechanism found here is only triggered by the reaction of the mediator radical with the lignin model compound but the mediator does not take part in the second reaction step. Such type of reactions will cause undesired products but it would not cause any loss of mediator. Firthermore the dimerisation reaction is of little importance for the lignin in the pulp, as there is no free mobility of the aromatic subunits.
Reaction of model compound radicals with mediator molecules
From the experiments described the only possible conclusion is that the mediator radical is not covalently attached to the model compound but rather a second mediator molecule is reacting with the activated model compound.Hydrogen abstraction of the mediator-radical from lignin or the model compounds leads to the formation of secondary radicals which should be detectable by EPR spectroscopy as long as they have a sufficient lifetime. In non-protic organic solvents an extended lifetime of the secondary radicals is expected. For the detection of these secondary model compound radicals lyophilized Vio˙ and dmVio˙ was prepared and dissolved in cold acetonitrile (−40 °C). As a suitable lignin model compound the dimeric phenolic guaiacolglyceryl-β-guaiacyl ether was chosen. A small amount of the ether dissolved in cold acetonitrile was added to the mediator radical solution in acetonitrile. When Vio˙ was used no secondary radical signal could be detected after the addition of the model compound. Using dmVio˙ led to the formation a new radical signal, which clearly indicates a disappearance of the oxime structure (Fig. 6). The EPR signal of iminoxy radicals is split into three groups with a hyperfine constant ranging from 28 to 32 G due to an interaction with the oxime nitrogen atom (Fig. 6, upper curve).17 After the addition of guaiacolglyceryl-β-guaiacyl ether a radical signal is detected, which shows splitting into three groups of lines of same intensity. This indicates a coupling with a spin I = 1 atom like nitrogen. However, now the hyperfine constant is only approx. 10 G typical of N-hydroxy radicals.18 The further splitting of each group of lines into a doublet with a hyperfine constant of 2 G is caused by a proton at the α-carbon or a proton from a hydroxy group at the nitrogen atom. The detected radical signal belongs to the primary radical adduct between dmVio and the model substance guaiacolglyceryl-β-guaiacyl ether. With the formation of a covalent bond the oxime structure is lost.
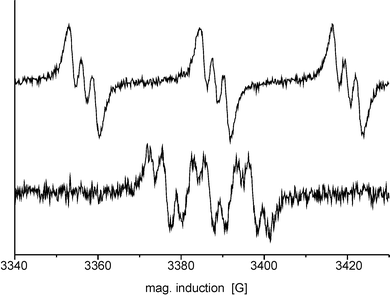 | ||
| Fig. 6 Radical reaction monitored by EPR in acetonitrile (−40 °C). (Upper curve) The EPR-signal of dmVio˙ with its large coupling constant is typical for an oxime radical. (Lower curve) After reaction of dmVio˙ with guaiacolglyceryl-β-guaiacyl ether the dmVio˙ signal disappears and a new radical signal with a much smaller coupling constant appears. | ||
In Fig. 7 a model for the structure of the adducts as well as their development is proposed on the basis of veratrol and guaiacol. Primarily a radical adduct is formed from the model compound radical obtained in the first step with a mediator molecule which leads to the formation of a N-hydroxy radical structure as seen in EPR. Due to the radical structure it is assumed that further internal reactions will occur, in particular ring-closure, before the radical character is lost via an inhibitor.
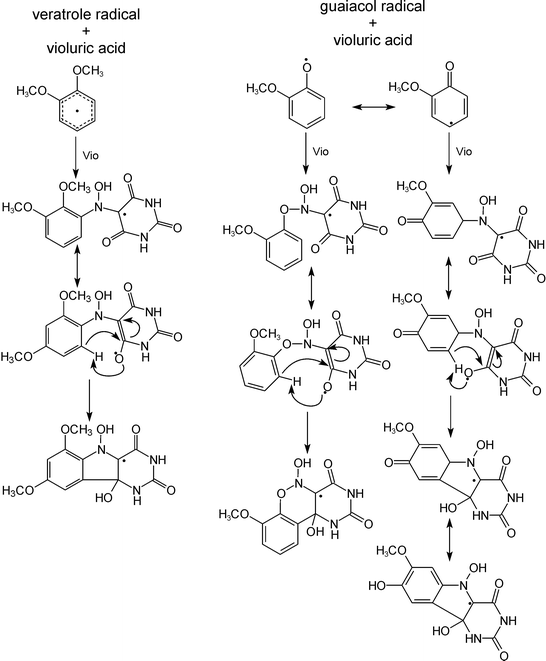 | ||
| Fig. 7 Proposed mechanism for the mediator–substrate adduct formation. From the LC-MS measurements a one-to-one stoichiometry between radical and lignin model compound is derived. | ||
The reactions cause the formation of semi-stable molecules. Using the proposed reaction model (Fig. 7) the fragmentation pattern of the APCI mass spectrum of the veratryl alcohol–Vio adduct may be interpreted (Fig. 8). The formed adduct releases H2O during ionization and a break down of the mediator part of the adduct is induced. Similar reactions occur in the reaction solution. They are responsible for the disappearance of the adduct signal within 24 h reaction time.
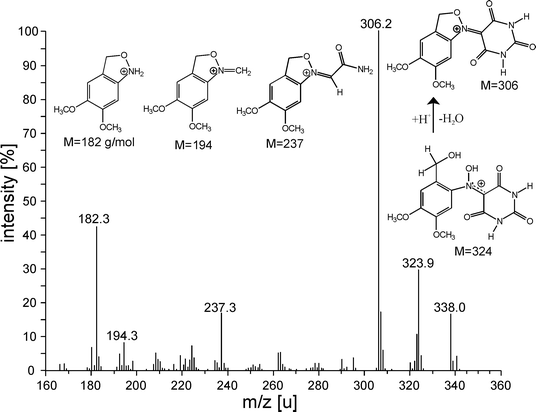 | ||
| Fig. 8 Shown is the APCI mass spectrum of the adduct of Vio˙ and veratryl alcohol. The fragmentation pattern observed fully corresponds with the ring formation theory. | ||
Summary
Electrochemical mediator-based (EMB) delignification is an attractive alternative to existing delignification processes based on chlorine. Theoretically this process should be possible without any consumption of chemicals. Thus it has the potential to be economically as well as environmentally attractive. Very little was known about the reaction of the activated mediator radicals with the lignin content in the pulp. We have investigated the key reactions of Vio˙ and dmVio˙ with lignin model compounds as well as native lignin by electrochemical, chromatographic and spectroscopic methods.It was found that the mediator radicals in aqueous solution react very rapidly with phenolic groups, and more slowly with activated aromatic rings under abstraction of hydrogen atoms. Hydrogen abstraction from the benzyl alcoholic carbon is of minor importance. No reaction with the cellulose content of pulp is expected as experiments with cellobiose have shown.
The reaction stoichiometry between the mediator radicals and the model compounds was found to be one to one.
The molecular cause for the loss of mediator is due to the formation of covalent adducts from mediator-activated model compound radicals (and their counterparts in the lignin network, respectively) with mediator molecules. The adduct molecules predicted by this model have been identified by LC-MS spectrometry. The adducts are colored semi-stable compounds. Primary radical N-hydroxy type adducts were identified by EPR measurements. As a consequence of the radical character further internal reactions are assumed which stabilize the adduct.
The findings reported in this paper point the way to an optimization of mediator-based enzymatic as well as electrochemical processes for pulp delignification. The interception of the induced secondary radicals by additives will increase the cyclicity of the mediator.
Experimental
Chemicals
Commercially available chemicals were purchased from Fluka–Sigma–Aldrich and used without further purification. Guaiacolglyceryl-β-guaiacyl ether was synthesized according to ref. 19. Veratrolglyceryl-β-guaiacyl ether was synthesized by reaction of guaiacolglyceryl-β-guaiacyl ether with dimethyl sulfate. The mediator dmVio was synthesized from dimethylbarbituric acid by oxidation with selenium dioxide followed by a reaction with hydroxylamine hydrochloride. All described reactions were performed in 0.1 M acetate buffer (pH 4.5).Methods
The X-band EPR-measurements were performed on a EPR spectrometer ESP 300 E from Bruker. A resonator from Varian equipped with a thin layer flow cell was used. The modulation amplitude was 2 G at a modulation frequency of 12.5 kHz. The mediator concentrations used were 2 mmol L−1. The solutions were circulated through the spectrometer by a peristaltic pump. The solution was pre-electrolyzed with Pt-electrodes at 1.5 V for 45 min at room temperature in order to achieve a radical content of about 40%. The radical concentration was determined by EPR which was calibrated by RDE-voltammetry. The half-life of the radicals under the conditions used here (room temperature, 0.1 M acetate-buffer pH 4.5) is more than one hour. After the electrolysation the very slow thermal decay of the radical population was measured (t = −10 to 0). Then the model compounds were added as a solution or suspension. In order to determine the reaction kinetics a reactivation of the mediator was not intended.For rotating disk electrode (RDE) measurements a BM-EDI101 electrode and CTV 101 speed control module from Radiometer Analytical were used. As a working electrode a glassy carbon disk with 3 mm diameter, as a counter electrode a glassy carbon rod with 5 mm diameter and a saturated calomel electrode (SCE) as a reference electrode were used. The electrode was rotated at 1000 rpm and the scan rate was 20 mV s−1 in the potential range from 0.1 to 1.1 V vs. SCE. All experiments were performed in 0.1 M acetate buffer (pH 4.5). For the determination of the mediator recovery rate for the reaction with guaiacol (UOx = 0.7 V vs. SCE) it is important to add only a small excess of guaiacol to minimize the error due to direct electrochemical oxidation of guaiacol. The RDE-measurements with pulp were done at a pulp consistency of 3% (1.3 g dried pulp in 40 ml 0.1 M acetate buffer pH 4.5). A direct oxidation of the pulp is not observable due to the fact that a direct contact of electrode and pulp scarcely occurs in the experiment.
LC-MS/MS measurements were done on a LCQduo from Thermoquest Finnigan equipped with an LC-system comprising of a P4000 gradient pump, a UV2000 UV/Vis detector and a RP18 Nucleosil column from Bischoff (Germany) (length 250 mm, 2 mm diameter, particle size 5μm). The mobile phase was a linear gradient from water containing 0.5% formic acid (A) and methanol, containing 10% water and 0.5% formic acid (B) (0–10 min: 100% A; 10–30 min: linear gradient 0% to 100% B; 30–60 min: 100% B). The concentration of the samples was 1–5 mmol L−1. APCI ionization (positive mode) with nitrogen as shooting gas was used. The radical solution in 0.1 M acetate buffer pH 4.5 was prepared electrochemically, and the radical concentration was determined by RDE-voltammetry. An excess of the model compounds in 0.1 M acetate buffer (pH 4.5) was added. Samples were taken from the reaction solution at different times and immediately frozen in liquid nitrogen. Then the samples were successively measured by LC-MS. In Table 2 the retention times and masses of the different peaks are given. The main peaks were identified by their mass spectra apart from the peak with the retention time 31.5 min when reacting Vio and dmVio with VA. The peak consists of a major ion with 182 u and a fragment with 151 u. The signal does not fit in its retention time and fragmentation pattern of veratric acid (m/z = 200, 183 (100%), 139, 117).
UV/Vis spectra were measured on a UV900 from Kontron. The probe was a diluted sample (1 to 1000) from the LC-MS investigations.
Acknowledgements
Stimulating discussions with D. Braga and N. Vlachopoulos as well as financial support from the Fond der Chemischen Industrie are gratefully acknowledged.References
- R. Bourbonnais and M. G. Paice, FEBS Lett., 1990, 267, 99–102 CrossRef CAS
.
- H. P. Call and I. Mücke, J. Biotechnol., 1997, 53, 163–202 CrossRef CAS
.
- M. Fabbrini, C. Galli and P. Gentili, J. Mol. Catal. B, 2002, 16, 231–240 CrossRef CAS
.
- R. Bourbonnais, D. Rochefort, M. G. Paice, S. Renaud and D. Leech, Tappi J., 2000, 83, 68–79 Search PubMed
.
- D. Rochefort, R. Bourbonnais, D. Leech, S. Renaud and M. Paice, J. Electrochem. Soc., 2002, 149, D15–D20 CrossRef CAS
.
- D. Rochefort, R. Bourbonnais, D. Leech and M. G. Paice, Chem. Commun., 2002, 1182–1183 RSC
.
- R. Bourbonnais and M. G. Paice, Biochim. Biophys.Acta, 1998, 1379, 381–390 CrossRef CAS
.
- K. Poppius–Levlin, W. Wang, T. Tamminen, B. Hortling, L. Viikari and M.-L. Niku–Paavola, J. Pulp Pap. Sci., 1999, 25, 90–94 Search PubMed
.
- H. C. Kim, M. Mickel, S. Bartling and N. Hampp, Electrochim. Acta, 2001, 47, 799–805 CrossRef CAS
.
- C. Padtberg, H. C. Kim, M. Mickel and S. Bartling, Tappi J., 2001, 84, 68 Search PubMed
.
- K. Li, F. Xu and K.-E. L. Eriksson, Environ. Microbiol., 1999, 65, 2654–2660 Search PubMed
.
- J. L. Brokenshire, J. R. Roberts and K. U. Ingold, J. Am. Chem. Soc., 1972, 94, 7040–7049 CrossRef CAS
.
- H. Jakob, M. D. Grosso, A. K. Küver, N. Nimmerfroh and H. U. Süss, Das Pap., 1999, 2, 85–95 Search PubMed
.
- J. Gierer, Wood Sci. Technol., 1986, 20, 1–33 Search PubMed
.
- J. Gierer, Holzforschung, 1990, 44, 387–394 Search PubMed
.
- J. Gierer, Holzforschung, 1982, 36, 43–51 Search PubMed
.
- C. Lagercrantz, Acta Chem. Scand., Ser. B, 1988, 42, 414–416 Search PubMed
.
- W. Lenk and M. Riedl, J. Phys. Org. Chem., 1990, 3, 687–693 CrossRef CAS
.
- F. Nakatsubo, K. Sato and T. Higuchi, Holzforschung, 1975, 29, 165–168 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2003 |