A convenient, rapid, highly selective and eco-friendly method for deprotection of aryl acetates using silica gel supported ammonium formate under microwave irradiation†
C. Ramesh, G. Mahender, N. Ravindranath and Biswanath Das†
Organic Chemistry Division-I, Indian Institute of Chemical Technology, Hyderabad-, 500007, India. E-mail: biswanathdas@yahoo.com; Fax: (91) 40-7173387; 7160512
First published on 18th November 2002
Abstract
Several aryl acetates were rapidly and selectively deprotected to the corresponding phenols in excellent yields using silica gel supported ammonium formate under microwave irradiation. The process is environmentally benign.
Green ContextRapid efficient deprotection methods are of use in synthetic chemistry. Phenols are sensitive substrates which often require protection by acetate functions to avoid oxidative decomposition. The combination of ammonium formate, silica and microwave irradiation is a very fast, energy efficient route to their liberation with little effect on other functional groups.DJM |
Introduction
Phenolic hydroxyl groups occur widely in various bioactive natural products. The protection of these groups is generally required for modifications and synthesis of these phenol-containing products.2 This protection is usually carried out by preparing the acetates of the compounds as the acetates can easily be formed and cleaved. Deprotection of aryl acetates can classically be carried out under acidic, basic or hydrogenatic conditions.2 However, these deprotection methods may affect various sensitive functional groups present in the compounds. The methods for selective deprotection of aromatic acetates are limited. Some methods utilizing specific micelles,3a Zn–MeOH,3b cyclodextrin,3c metallo-enzymes,3d metal complexes3e–g and antibodies3h have been reported for selective deprotection of aryl acetates in the presence of alkyl acetates. However, most of these methods are associated with different drawbacks including the uses of non-available and costly reagents, harsh reaction conditions, tedious experimental procedures, long reaction times and low yields. Recently a selective deprotection procedure using natural kaolinitic clay has been discribed4 but this clay is not commercially available and was collected from a specific geographical region. A microwave irradiation method using alumina has also been reported5 but the selectivity of deprotection of aryl acetates was found to be lost on increasing the irradiation time beyond a certain value. Both phenyl and octyl acetates were reported to undergo deprotection under microwave irradiation in the presence of alumina within 2 min. Thus the discovery of efficient, selective and rapid methods for deprotection of aromatic acetates is necessary.Results and discussion
During our recent work6 on the development of novel synthetic methodologies we observed that silica gel supported ammonium formate (HCOONH4) can catalyze efficiently and promptly the selective deprotection of aromatic acetates under microwave irradiation. HCOONH4 is generally used as an in situ hydrogen source for catalytic hydrogen transfer reduction. The combination of this reagent with palladium and charcoal is very selective for reduction of a variety of important functional groups.7 We discovered that aryl acetates, when irradiated with HCOONH4/SiO2 for 2 min under microwave irradiation, gave the corresponding phenols in excellent yields (Table 1).| Entry | Substrate | Product | Isolated yield of phenol (%) | Entry | Substrate | Product | Isolated yield of phenol (%) |
|---|---|---|---|---|---|---|---|
| 1 |  |  | 92 | 11 |  | 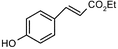 | 100 |
| 2 |  |  | 94 | 12 |  |  | 99 |
| 3 |  | 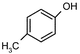 | 96 | 13 | 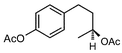 |  | 93 |
| 4 |  | 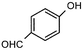 | 99 | 14 | 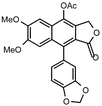 | 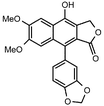 | 98 |
| 5 |  |  | 96 | 15 |  |  | 0 |
| 6 |  |  | 100 | 16 | 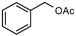 |  | 0 |
| 7 |  |  | 100 | 17 |  |  | 0 |
| 8 |  |  | 89 | 18 |  |  | 0 |
| 9 |  | 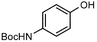 | 94 | 19 |  |  | 0 |
| 10 |  |  | 98 | 20 |  |  | 0 |
The method showed excellent selectivity for deprotection of aryl acetates as alkyl acetates remained totally unaffected. Even when the irradiation time was increased to 6 min no change of alkyl acetates was observed but irradiating for longer led to docomposition of the compounds. Double bonds, carbonyl groups, ether linkages, lactone rings and benzoate were also unchanged. The conversion can be conducted in the presence of other protecting groups such as N-acetyl, N-Boc and OTs groups. The presence of electron donating or withdrawing groups in the aryl acetates did not influence the yields of the phenols. The structures of the products were established from their spectral (1H NMR and MS) data and by comparison with authentic samples.
HCOONH4 is easily available, inexpensive and non-toxic. Although hygroscopic, in the presence of silica gel as solid support the reagent can conveniently be used for microwave irradiation. We have observed when silica gel alone was applied the yields of the generated phenols were low (42–47% after irradiation for 3 min) under our experimental conditions. HCOONH4 can also be used conventionally for deprotection of aryl acetates by treating the compounds with the reagent in MeOH at room temperature for 12 h to produce the corresponding phenols (yield 67–74%). The microwave irradiation method using silica gel supported HCOONH4 is better since it rapidly (2 min) generates the phenols with higher yields (89–100%) from the aromatic acetates. The experimental procedure is also simple.
In conclusion, a novel, simple and highly selective method has been developed for deprotection of aryl acetates using silica gel supported HCOONH4 under microwave irradiation. The utilization of a commonly used reagent, operational simplicity, rapid conversion and the excellent yields of the products, constitute the present method a useful alternative to the available deacetylation procedures of aromatic acetates. The advantages of both the solid supported reagent and microwave irradiation have been utilized. The developed process is environmentally benign.
Experimental
Typical procedure for deprotection of aryl acetates
HCOONH4 (63 mg, 5 mmol) was mixed thoroughly with silica gel (0.5 g, Merck, finer than 200 mesh) to form a powder. 4-Acetoxyphenylethyl acetate (222 mg, 1 mmol) was then added and mixed. The mixture was taken in a glass test tube (length: 15 cm, diameter: 1.5 cm) and placed at the centre of an alumina bath which was made by using a glass beaker (250 ml; length: 9.5 cm, diameter: 7.5 cm) three-quarters filled with alumina. The alumina bath was placed inside a microwave oven (BPL, BMO, 700T, 450 W) and the reaction mixture was irradiated for 2 min. The mixture was cooled, shaken with EtOAc (10 ml) and filtered. The filtrate was concentrated to afford the deprotected product, 4-hydroxyphenylethylacetate (176 mg, 98%).Spectral properties of some representative phenols are given below
Acknowledgements
The authors thank UGC and CSIR, New Delhi for financial assistance.References
- N. Ravindranath, C. Ramesh, M. R. Reddy and B. Das, Tetrahedron Lett., 2002, submitted ; IICT Communication No. 4589.
- (a) T. W. Greene and P. G. M. Wuts, Protecting Groups in Organic Synthesis, John-Wiley, New York, 2nd edn., 1995 Search PubMed; (b) P. G. Kocieneski, Protecting Groups, Thieme Verlag, Stuttgardt, 1994 Search PubMed.
- (a) T. Kunitake, Y. Okahata and T. Sakamoto, J. Am. Chem. Soc., 1976, 98, 7799 CrossRef CAS; (b) A. G. Gonzalez, Z. D. Jorge, H. L. Dorta and F. L. Rodriguez, Tetrahedron Lett., 1981, 22, 335 CrossRef CAS; (c) O. S. Tee, C. Mazza, R. Le Zano-Hemmer and I. B. Giorgi, J. Org. Chem., 1994, 59, 7602 CrossRef CAS; (d) M. R. Crampton, K. E . Holt and J. M. Perey, J. Chem. Soc., Perkin Trans. 2, 1990, 1701 RSC; (e) V. L. Beisselier, M. Postal and E. Dunach, Tetrahedron Lett., 1997, 38, 2981 CrossRef; (f) T. Koike and E. Kimura, J. Am. Chem. Soc., 1991, 113, 8935 CrossRef CAS; (g) J. Suh, Y. Cho and K. J. Lee, J. Am. Chem. Soc., 1991, 113, 4198 CrossRef CAS; (h) J. Guo, W. Huang and T. S. Scanlan, J. Am. Chem. Soc., 1994, 116, 6062 CrossRef CAS.
- B. P. Bandgar, L. S. Uppalla, A. D. Sagar and V. S. Sadavarte, Tetrahedron Lett., 2001, 42, 1163 CrossRef CAS.
- (a) S. V. Ley and D. M. Mynett, Synlett, 1993, 793 CrossRef CAS; (b) R. S. Varma, M. Varma and A. K. Chatterjee, J. Chem. Soc., Perkin Trans. 1, 1993, 999 RSC.
- (a) B. Das and B. Venkataiah, Synthesis, 2000, 1671 CrossRef CAS; (b) N. Ravindranath, C. Ramesh and B. Das, Synlett, 2001, 1777 CrossRef CAS; (c) K. V. N. S. Srinivas, E. B. Reddy and B. Das, Synlett, 2002, 625 CrossRef CAS.
- (a) S. Ram and R. E. Ehrenkaufer, Synthesis, 1988, 91 CrossRef CAS; (b) B. C. Ranu, A. Sarkar, S. Guchhait and K. Ghosh, Indian J. Chem. Soc., 1998, 75, 690 Search PubMed.
Footnote |
| † Part 20 in the series ‘Studies on Novel Synthetic Methodologies’, for part 19, see ref. 1 |
| This journal is © The Royal Society of Chemistry 2003 |

