Gold catalysis in the reactions of 1,3-dicarbonyls with nucleophiles
A. Arcadi, G. Bianchi, S. Di Giuseppe and F. Marinelli
Dipartimento di Chimica Ingegneria Chimica e Materiali della Facoltà di Scienze, Univeristà di L’Aquila, via Vetoio, Coppito Due, 67100, L’Aquila, Italy. E-mail: arcadi@univaq.it; Fax: +39-0862-433753; Tel: +39-0862-43373774
First published on 28th November 2002
Abstract
A new efficient gold(III) catalysed synthesis of β-enaminones from 1,3-dicarbonyl compounds and ammonia/amines providing an attractive and environmental friendly alternative to the more vigorous reagents and drastic conditions of the existing methodologies is described; the catalysis of gold(III) is also extended to reaction of cyclic 1,3-dicarbonyls with O-, P- and S-nucleophiles.
Green ContextSimplicity is an important feature of any truly green chemical process; reducing the auxiliaries present, the number of synthetic steps and the complexity of the process. β-Enaminones are normally synthesised in a process that involves a Dean–Stark reactor and hazardous solvents such as benzene. Here we have described a new catalytic route to these versatile synthetic intermediates. The method is based on a gold catalyst in ethanol as solvent and at room temperature.JHC |
Introduction
Currently, growing efforts of the scientific community of chemistry rely upon the development of economical processes based on the principles of green chemistry.1 Our research interest towards the realisation of new green catalytic systems led us to provide resource-saving synthetic methodologies through transition-metal catalysed domino processes.2 In particular, gold catalysts have been reported to allow sequential amination/annulation reactions of 2-propynyl-1,3-dicarbonyl compounds with primary amines, aminoalcohols and α-aminoesters.3 We described that NaAuCl4 is an efficient catalyst not only in the activation of the acetylenic bond towards the nucleophilic attack of O- or N-groups, but also, in the cases we examined, this catalyst showed the specific quality of accelerating the condensation of 1,3-dicarbonyls with N-nucleophiles. Moreover, Kobayashi et al.4 reported that AuCl3·3H2O exhibits higher catalytic activity compared to conventional Lewis acids in aza-Michael reactions of enones. Then, a further investigation of the gold-catalysed condensation of 1,3-dicarbonyl derivatives with amines to give β-enaminones promises to overcome some of the drawbacks caused by more vigorous reagents and drastic reaction conditions. The β-enaminones5 are an important class of organic synthetic intermediates, particularly in heterocyclic chemistry. Enaminones were usually prepared by a condensation reaction of amines and 1,3-dicarbonils. For ammonia and a large variety of primary and secondary amines this reaction occurred in benzene, under reflux using a Dean Stark trap.6 This procedure generally requires a large excess of amine: for instance the reaction with 1,3-dicarbonyls and ammonia requires a stream of gaseous ammonia. To avoid this problem the use of ammonium acetate7 as a source of ammonia, in benzene at reflux and in the presence of acetic acid, was described. For a more efficient condensation of cyclohexane-1,3-dione derivatives with low boiling amines the use of stoichiometric boron trifluoride etherate8 as an activator was reported. In recent years, environmentally benign synthetic methods have received considerable attention and new procedures for the synthesis of enaminones have been reported. On this subject, the use of montmorillonite K10 or SiO2 as catalysts combined with microwave irradiation9 and the condensation reaction of 1,3-dicarbonyls and amines in water10 as solvent has been investigated. This last procedure is restricted to a limited range of hydrosoluble and more reactive amines. Consequently, the development of clean, mild and efficient protocols for condensation reactions of 1,3-dicarbonyl derivatives with ammonia/amines is still a synthetic challenge.Herein we wish to report the results of the gold(III) catalysed synthesis of β-enaminones from 1,3-dicarbonyl compounds. The catalysis of gold(III) is also extended to reaction of cyclic 1,3-dicarbonyls with O-, P- and S-nucleophiles.
Results and discussion
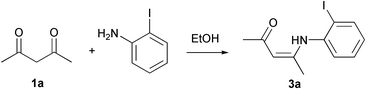
The condensation reaction of o-iodoaniline and acetylacetone 1a (Table 1) was selected as the model system and the preparation of the corresponding β-(2-halophenyl)amino-substituted α,β-unsaturated ketones 3a was attempted under different reaction conditions. The β-(2-halophenyl)amino substituted α,β-unsaturated ketones have been proven to be very useful for construction of aza heterocycles via radical11 or catalytic12 cyclization.The results reported in Table 1 show that without adding any catalyst the starting o-iodoaniline was recovered (90%) after reacting at 40 °C for 7 h in ethanol with 1a. By contrast, in the presence of a catalytic amount of NaAuCl4·2H2O the β-enaminone 3a has been isolated under the same reaction conditions with a 62% yield. Moreover, the gold(III)-catalysed amination reaction of 1,3-dicarbonyls can be carried out at room temperature. In these latter conditions 3a has been isolated in 60% yield by using a molar ratio 1,3-dicarbonyl∶amine of 1∶1 and in quantitative yield with a molar ratio 1,3-dicarbonyl∶amine = 1∶2. In addition, a screening on the efficiency of other transition-metal salts rivealed that gold(III) is more efficient than AgNO3, Na2PdCl4, CuI and ZnCl2 in the catalysis of condensation reactions of 1,3-dicarbonyls with amines. Although ZnCl2, AlCl3, TiCl4 and other Lewis acid have been reported to be efficient catalysts and water scavengers in the condensation of ketones with amines,13 to the best of our knowledge the gold-catalysed amination of ketones has not been investigated. On the other hand, the catalysis of organic reactions by gold-catalysts has received only little attention, this is especially true for homogeneous catalysis of organic reactions by gold catalysts.14
When the gold-catalysed amination was extended to other 1,3-dicarbonyl compounds the synthesis of β-enamino esters and enaminoketones 3 was accomplished with ammonia, primary and secondary amines according to Scheme 1.
 | ||
| Scheme 1 | ||
This procedure is quite general for a wide range of amines such as aliphatic, cyclic and aromatic amines. Our results are shown in Table 2 in comparison with the results reported in the literature by existing methodologies. It is clear from our results that the gold-catalysed condensation reaction of 1,3-dicarbonyls and amines to give 3 appears to provide a remarkably viable alternative route for the synthesis of enaminones and can be applied to a large variety of functionalized diketones or β-ketoesters. The catalysts NaAuCl4·2H2O is commercial available and has been used as purchased, without further purification. All the reactions were carried out at room temperature. It should be pointed out that in the reaction of arylalkyldicarbonyls with amines we observed the regioselective amination of the aliphatic carbonyl group (Table 2, entries 2, 4). In addition when the 1,1,1-trifluoroacetylacetone 1d and 1-(2-thenoyl)-3,3,3,-trifluoroacetone 1f were allowed to react with amines (Table 2 entries 9, 10) only the enaminones derived from the regioselective amination of the trifluoroacetyl group were observed. By contrast, 1d led to 3f through the exclusive amination of the acetyl group in the reaction with 4-aminophenazone.
| Entry | 1,3-Dicarbonyl | Amine | Product | Previously reported results by existing methodologies |
|---|---|---|---|---|
| a Yields refer to single runs, are given for pure isolated products, and are based on 1.b Unless otherwise stated, reactions were carried out at r.t. in ethanol under a nitrogen atmosphere using the following molar ratios: 1∶amine∶NaAuCl4 = 1∶1∶0.025.c Molar ratios: 1∶NH3∶NaAuCl4 = 1∶3∶0.025.d Molar ratios: 1∶NH3∶NaAuCl4 = 1∶2∶0.025. | ||||
| 1 |  |  |  | Benzene12 under reflux, 4-methylbenzensulfonic acid (cat.) (72%) |
| 2 |  | NH3 |  | CH3COO−NH4+, benzene7 under reflux, AcOH (70%) |
| 3 |  | NH3 |  | CH3COO−NH4+, benzene7 under reflux, AcOH (85%) |
| 4 | 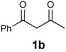 |  |  | |
| 5 |  | NH3 |  | CH3COO−NH4+, benzene7 under reflux, AcOH (90%) |
| 6 |  |  | 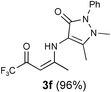 | |
| 7 |  |  |  | Benzene at reflux,15p-toluenesulfonic acid (cat.) (61%) |
| 8 |  | 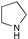 |  | Montmorillonite/microwave irradiation9 (95%) |
| 9 |  |  |  | |
| 10 |  | 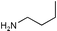 | 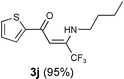 | |
Moreover we have developed an extremely simple approach to β-substituted cyclohexenones and cyclopentenones 4a–d (Table 3) from the corresponding 1,3-diketones.
| ||||
|---|---|---|---|---|
| Entry | Cyclic 1,3-dicarbonyls | Nucleophile | Solvent, temperature | Productab |
| a Yields refer to single runs, are given for pure isolated products, and are based on 1.b Unless otherwise stated, reactions were carried using the following molar ratios: 1∶NuH∶NaAuCl4 = 1∶1∶0.025.c The reactions were carried out at 40 °C using the following conditions: 1∶NaAuCl4 = 1∶0.025 in ethanol (2 mL). | ||||
| 1 |  |  | EtOH,c 40 °C |  |
| 2 |  | 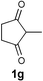 | EtOH,c 40 °C |  |
| 3 |  | 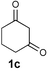 | CH3CN, 60 °C | 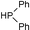 |
| 4 | 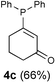 |  | CH3CN, r.t. |  |
Indeed, the gold(III) catalysis have been extended to the reaction of cyclic 1,3-dicarbonyls with ethanol, diphenylphosphine and 4-methoxybenzyl mercaptan. The reported procedure for β-substituted cyclohexenones involved the formation of enone mesilates16 which subsequently were transformed to the products 4 by reaction with nucleophiles.
In conclusion, we have developed by means of gold(III) catalysis a simple and green procedure for the synthesis of β-enaminones from 1,3-dicarbonyls and ammonia/amines that requires neither corrosive acid catalyst nor azeotropic conditions using a large excess of aromatic solvents such as benzene. Traditional stepwise formation of β-substituted cyclohexenones and cyclopentenones can be replaced by one-pot procedure.
Experimental
Typical procedure for the preparation of enaminones 3a–j
A 1∶1 mol ratio mixture of 1,3-dicarbonyl 1 and amine was allowed to react in ethanol at room temperature under nitrogen. The reaction was monitored by TLC or GC–MS. After completion, the solvent was removed trough evaporation and the reaction mixture was purified by flash chromatography (silica gel, n-hexane–ethyl acetate) to give enaminones 3.References
- P. Tundo, P. Anastas, D. StC. Black, J. Breen, T. Collins, S. Memoli, J. Miyamoto, M. Polyalkoff and W. Tumas, Pure Appl. Chem., 2000, 72, 1207 Search PubMed; Green Chemistry: Challenging Perspectives, ed. P. Tundo and P.T. Anastas, Oxford University Press, Oxford, 2000 Search PubMed.
- E. Rossi, A. Arcadi, G. Abbiati, O. A. Attanasi and L. De Crescentini, Angew. Chem., Int. Ed., 2002, 41, 1400 CrossRef CAS; A. Arcadi, S. Cacchi, S. Di Giuseppe, G. Fabrizi and F. Marinelli, Org. Lett., 2002, 4, 2409 CrossRef CAS; A. Arcadi, G. Cerichelli, M. Chiarini, S. Di Giuseppe and F. Marinelli, Tetrahedron Lett., 2000, 41, 9195 CrossRef CAS.
- A. Arcadi, S. Di Giuseppe, F. Marinelli and E. Rossi, Adv. Synth. Catal., 2001, 343, 443 CrossRef CAS; A. Arcadi, S. Di Giuseppe, F. Marinelli and E. Rossi, Tetrahedron: Asymmetry, 2001, 12, 2715 CrossRef CAS.
- S. Kobayashi, K. Kakumoto and M. Sugiura, Org. Lett., 2002, 4, 1319 CrossRef CAS.
- U. Kuckländer, in Enaminones as Synthones, ed. Z. Rapport, The Chemistry of Functional Groups, John Wiley and Sons, New York, 1994, Part 1, ch. 10 Search PubMed; P. Lue and J. V Greenhill., Adv. Heterocycl. Chem., 1997, 67, 207 Search PubMed; J. V Greenhill., Chem. Soc. Rev., 1977, 6, 277.
- J. V Greenhill, J. Chem. Soc. C, 1971, 2699 RSC; E. Roberts and E. E. Tutner, J. Chem. Soc., 1927, 1832 RSC; D. F Martin, G. A. Janusonis and B. B. Martin, J. Am. Chem. Soc., 1961, 83, 73 CrossRef.
- P. G. Baraldi, D. Simoni and S. Manfredini, Synthesis, 1983, 902 CrossRef CAS.
- C. P. Cartaya-Marin, D. G. Henderson and R. W. Soeder, Synth. Commun., 1997, 27, 4275 CAS.
- B. Rechsteimer, F. Texier-Boullet and J. Hamelin, Tetrahedron Lett., 1993, 34, 5071 CrossRef.
- H. A. Stefani, I. M. Costa and D. de O. Silva, Synthesis, 2000, 1526 CrossRef CAS.
- A. Navrro-Vàzquez, A. García and D. Domínguez, J. Org. Chem., 2002, 67, 3213 CrossRef CAS.
- T. Sakamoto, T. Nagano, Y. Kondo and H. Yamanaka, Synthesis, 1990, 215 CrossRef CAS.
- J. A. Mashall and C. A. Sehon, J. Org. Chem., 1995, 60, 5966 CrossRef CAS; J. H. Billman and K. M. Tai., J. Org. Chem., 1958, 23, 535 CrossRef CAS; K. Taguchi and F. H. Westheimer, J. Org. Chem., 1971, 36, 1570 CrossRef; I. Moretti and G. Torre, Synthesis, 1970, 141 CrossRef CAS; G. Borg, D. A. Cogan and J. A. Ellman, Tetrahedron Lett., 1999, 40, 6709 CrossRef CAS.
- A. S. K. Hashmi, T. M. Frost and J. A. Bats, Catal. Today, 2002, 72, 19 CrossRef CAS and references therein.
- P. W. Hickmott and G. Sheppard, J. Chem. Soc., Perkin Trans. 1, 1972, 1038 RSC.
- C. J. Kowalski and K. W. Fields, J. Org. Chem., 1981, 46, 197 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |