DOI:
10.1039/B207750G
(Paper)
Green Chem., 2003,
5, 71-75
Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides and methanol using heterogeneous Mg containing smectite catalysts: effect of reaction variables on activity and selectivity performance
Received 7th August 2002
First published on 18th November 2002
Abstract
This paper reports the effect of various reaction variables on the activity and selectivity performance on a two-step synthesis of dimethyl carbonate (DMC) and glycol from propylene oxide, carbon dioxide and methanol using a heterogeneous Mg containing smectite catalyst. The first step, the reaction of propylene oxide with CO2 to form propylene carbonate, and the second step, the transesterification reaction of the cyclic carbonate such as ethylene carbonate with methanol to DMC and ethylene glycol, have been studied. The catalyst was found to be effective for one-pot synthesis of DMC, i.e. the sequential reaction of the epoxide, CO2 and methanol.
Green ContextThe utilisation of dimethyl carbonate (DMC) in a variety of applications is growing, and clean routes to its synthesis are of great value. The work presented here is aimed at developing a viable route from carbon dioxide and methanol, and is based on the addition reaction of an epoxide with CO2 catalysed by various metal based catalysts. The cyclic carbonate (useful in its own right) can then be transformed into DMC either in a separate step, or in a one-step process. This concept eliminates the requirement for toxic and wasteful intermediates such as phosgene.DJM |
1 Introduction
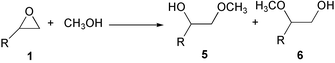
Dimethyl carbonate (DMC) synthesis using carbon dioxide is considered as one of the promising reactions in the development of environmentally benign processes based on the utilization of naturally abundant carbon resources such as carbon dioxide.1,2 DMC finds extensive applications as a solvent, an octane booster in gasoline to meet oxygenate specifications, and a starting material for organic synthesis via carbonylation and methylation, replacing poisonous phosgene and dimethyl sulfate.3,4 It is also used as a precursor for polycarbonate resins. DMC is synthesized by oxidative carbonylation of methanol (non-phosgene route) or by phosgenation of methanol. Both routes involve the use of poisonous and/or corrosive gases of chlorine, phosgene and carbon monoxide and there is a possibility of explosion hazards in the case of methanol carbonylation.5–7 There are several reports on direct synthesis of DMC from carbon dioxide and methanol in the presence of organometallic complexes,8-11 inorganic bases12–14 or zirconium oxide.15 However, most of these systems suffer from several drawbacks such as low yields and/or high cost of the starting materials and problems associated with catalyst–product separation due to the homogeneous nature of the catalysts. Recently, we have reported that DMC can be synthesized from epoxide compounds of ethylene oxide or propylene oxide by a two-step reaction in which the formation of cyclic carbonates is involved (Scheme 1) using basic metal oxide catalysts.16 In the first step, the epoxide reacts with CO2 producing a corresponding cyclic carbonate. In the second step, the carbonate is transesterified with methanol to DMC and a corresponding glycol. |
| | Scheme 1 | |
Smectite is a layered clay minerals, in which one layer consists of one octahedral sheet sandwiched by two tetrahedral sheets. The octahedral sheet contains divalent or trivalent cations such as Mg2+ and Al3+ surrounded by six oxygen atoms and the tetrahedral sheet contains Si4+ cations surrounded by four oxygen atoms. The trilayers are negatively charged and are held together by electrostatic interaction with exchangeable cations in the interlayer region. It is possible to introduce various transition metal cations in the octahedral sheet and alkali metal cations in the interlayer. We have earlier reported the synthesis of various smectites containing Ni and Mg that have both acidic and basic sites.17 The acidic and basic properties of the smectites are tunable,17 this being a great advantage for them to be used as catalysts. Recently, we have reported that Mg and/or Ni containing smectites catalysts are effective catalysts for both steps in Scheme 1 and the catalytic activity strongly depends on the amounts of alkali-metal atoms incorporated in the smectite catalysts18
In this work using a selected smectite catalyst, effects of reaction parameters have been studied in detail for the reaction of propylene oxide with CO2 to the corresponding cyclic carbonates and for the transesterification reaction of the ethylene carbonate with methanol to DMC and ethylene glycol. For the latter reaction, propylene oxide and other alcohols have been used as well. A combination of the two reactions, one-pot synthesis of DMC from propylene oxide, methanol and CO2, has also been examined.
Results and discussion
In our previous works,18 it has been reported that the catalytic activities for both the first and second steps of DMC synthesis are higher for a smectite catalyst containing a larger amount of alkali-metal atoms. Hence, most of the reaction experiments were carried out with the S-Mg-1 sample (see Experimental section).CO2 addition to propylene oxide
When the reaction of propylene oxide (PO) and CO2 was carried out with S-Mg-1, propylene carbonate (PC) was produced as a main product. Byproducts consisted of propylene glycol (PG) and oligomers of PO and/or PC. Fig. 1 illustrates the changes of the PO conversion and the selectivity for PC (mol of PC formed/mol of PO reacted) with time at 150 °C. The reaction progresses gradually and the PO conversion reaches 84% after 15 h, at which the PC yield is 57%. The selectivity for PC obtained at 3 h is lower than those obtained at the longer reaction times. It was found that the amount of PC increased with reaction time while that of PG little changed. Thus, the selectivity for PC obtained at 3 h is low but it increases to more than 60% at longer reaction times. |
| | Fig. 1 Variations of (A) PO conversion and (B) selectivity for PC with reaction time. PO, 57 mmol; catalyst, 0.5 g; CO2, 8 MPa; temperature, 150 °C. | |
Fig. 2 demonstrates the influence of CO2 pressure on the PO conversion and the selectivity for PC. The CO2 pressure does not significantly affect the conversion and the selectivity in the region between 3 and 10 MPa; however, both the conversion and the selectivity decrease sharply at 15 MPa. It is highly probable that the reaction system consists of three phases: CO2–liquid–solid at the lower pressures, while it should be in a homogeneous phase at 15 MPa. Such a phase change would cause an increase in the volume at the location where the reaction proceeds.14 Hence, the concentration of the catalyst and/or PO would be low at 15 MPa, resulting in low PO conversion at this pressure. As Fig. 2 shows, the lowest selectivity is obtained at 15 MPa. Such a high pressure might promote oligomer formation.
 |
| | Fig. 2 Effect of CO2 pressure on (A) PO conversion and (B) selectivity for PC. PO, 57 mmol; catalyst, 0.5 g; temperature, 150°C; time, 15 h.. | |
The effect of the reaction temperature on the reaction of PO and CO2 was investigated (Fig. 3) and the PO conversion was found to increase with temperature. However, the selectivity decreases at 170 °C and thus the optimum reaction temperature is 150 °C.
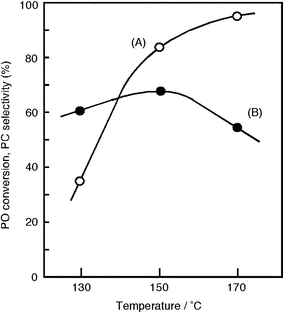 |
| | Fig. 3 Effect of temperature on (A) PO conversion and (B) selectivity for PC. PO, 57 mmol; catalyst, 0.5 g; CO2, 8 MPa; time, 15 h. | |
Transesterification of ethylene carbonate with methanol
Transesterification of ethylene carbonate (EC), i.e. the second step reaction in Scheme 1, was carried out under various conditions. Fig. 4 shows the yields of DMC and ethylene glycol (EG) vs. time. The yields of DMC and EG are almost the same as expected from Scheme 1 and reach constant values after about 2 h. The selectivities for DMC and EG (mol of DMC or EG formed/mol of EC reacted) were 100% for the initial 2 h. However, these selectivity values were found to decrease to 90% after 4 h, probably due to the decomposition of EC to EO.16,19Table 1 shows the effect of reaction temperature on the EC conversion and the DMC and EG yields. As the reaction temperature is increased, the conversion increases and the selectivities do not change much below 150 °C; however, the selectivity decreases from 90% at 150 °C to 80% at 175 °C. Thus, again, the optimum reaction temperature is 150 °C with respect to the DMC yield. |
| | Fig. 4 Variations of (○) DMC and (●) EG yields with reaction time. EC, 25 mmol; methanol, 200 mmol; catalyst, 0.25 g; temperature, 150 °C. | |
Table 1 Influence of temperature on the reaction of EC and methanol
| | | Yield (%) |
|---|
| Temperature/°C | EC conversion (%) | DMC | EG |
|---|
| EC, 25 mmol; methanol, 200 mmol; S-Mg-1, 0.25 g; 4 h. |
|---|
| 100 | 15 | 15 | 14 |
| 125 | 44 | 44 | 44 |
| 150 | 73 | 66 | 67 |
| 175 | 77 | 60 | 64 |
When the reaction was conducted with various amounts of EC at 150 °C for 4 h using a constant amount of methanol, the EC conversion decreased with increasing amount of EC. In a reverse manner, when the reaction was conducted with various amounts of methanol while keeping the amount of EC unchanged, the EC conversion increased with increasing the amount of methanol. Fig. 5 shows the relationship between the molar ratio of methanol to EC and the DMC yield. The selectivities to DMC and EG did not change with the molar ratio. Thus, both DMC and EG yields increase with the molar ratio. To obtain higher EC conversion, the reaction should be conducted with a high methanol to EC ratio.
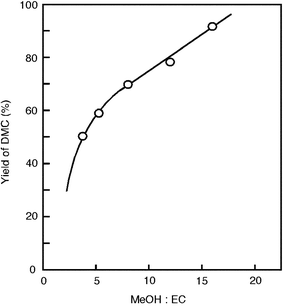 |
| | Fig. 5 Change of DMC yield with the molar ratio of methanol to EC. Catalyst, 0.25 g; temperature, 150 °C; time 4 h. | |
After a reaction run, the catalyst was separated by filtration, rinsed with acetone a few times and dried. Then the catalyst was reused for a subsequent run. Table 2 shows the reaction results with the same sample for the repeated runs. The activity of the smectite catalyst is seen to be stable upon recycling with no loss of activity being observed.
Table 2 Recycle use of the smectite catalyst for the reactionof EC and methanol
| | Yield (%) |
|---|
| Run | DMC | EG |
|---|
| EC, 25 mmol; methanol, 200 mmol; S-Mg-1, 0.25 g; 150 °C; 2 h. |
|---|
| 1 | 66 | 70 |
| 2 | 67 | 70 |
| 3 | 65 | 70 |
The effect of various alcohols was also investigated for the transesterification of ethylene carbonate (EC) and of propylene carbonate (PC). Fig. 6 gives conversion data obtained for methanol, ethanol and propanol. DMC, diethyl carbonate and dipropyl carbonate were obtained along with ethylene glycol and propylene glycol for the reaction of EC and PC, respectively. It is shown that the yield of dialkyl carbonate from EC as the starting material is always higher than that from PC, and that the reactivity decreases with increase in the number of carbon atoms of the alcohol for both EC and PC. The difference between EC and PC could be attributed to the steric hindrance effect caused by replacement of –H by the bulkier –CH3 group.
 |
| | Fig. 6 Transesterification of EC (white bars) and PC (black bars) with various alcohols. EC, 25 mmol; PC, 25 mmol; catalyst, 0.25 g; temperature, 150°C; time, 4 h. | |
One-pot synthesis of DMC
It is interesting to integrate these two reactions into a one-pot reaction (Scheme 2). Table 3 shows the results of this integration for propylene oxide. For comparison, our previous result obtained with MgO catalyst16 is also listed in Table 3. DMC, propylene glycol and propylene carbonate are formed with the side-products 1-methoxy-2-propanol and 2-methoxy-1-propanol. These by-products are formed from propylene oxide by methanolysis (Scheme 3). The selectivity for DMC depends on the catalyst used. The highest selectivity of 33.6% is obtained with S-Mg-1. This selectivity value is 2.5 times that obtained with MgO, which was considered to be the best catalyst among the metal oxide catalysts tested in the previous work.16 The difference between the catalysts would result from the difference in the amount and strength of basic sites existing on the catalysts. Although the best catalyst S-Mg-1 still gives the side products 1-methoxy-2-propanol and 2-methoxy-1-propanol, the results in Table 3 suggest that further controlling the basic properties of the catalyst could give more active and selective catalysts, contributing towards the development of one-step method for DMC, EG, EC synthesis from epoxide and carbon dioxide. |
| | Scheme 2 | |
Table 3 One-pot synthesis of DMC from PO, CO2 and methanol
| | | Selectivitya (%) |
|---|
| Catalyst | PO conversion (%) | 2 | 3 | 4 | 5 | 6 |
|---|
| PO, 21 mmol; methanol, 200 mmol; catalyst, 0.5 g; CO2, 8 MPa; 150 °C; 15 h. Mol of the product formed/mol of PO reacted.. |
|---|
| S-Mg-1 | 95 | 24 | 34 | 36 | 15 | 6 |
| S-Mg-2 | 95 | 20 | 4 | 5 | 43 | 28 |
| MgO | 99 | 14 | 14 | 15 | 22 | 30 |
 |
| | Scheme 3 | |
Conclusion
In conclusion, the effect of various reaction variables on the activity and selectivity performance on a two-step synthesis of dimethyl carbonate (DMC) and glycol from epoxide, carbon dioxide and methanol using a heterogeneous Mg containing smectite catalyst has been optimized. For the first step, the reaction of PO with CO2 to form PC, the optimum reaction temperature is 150 °C. The CO2 pressure does not have a significant influence on the yield of PC formation. For the second step, the transesterification reaction of the cyclic carbonate such as ethylene carbonate with methanol to give DMC and ethylene glycol, the optimum temperature and time are 150 °C and 2 h, respectively. The smectite catalyst was also found to be effective for the one-pot synthesis of DMC, i.e. the sequential reaction of the epoxide, CO2 and methanol. As compared with MgO, the smectite catalyst shows improved selectivity for DMC by avoiding the undesirable methonolysis reaction between PO and methanol.Experimental
Two Mg containing smectite catalysts (designated S-Mg-1 and S-Mg-2) were synthesized according to a hydrothermal method developed by Torri and Iwasaki.20 In brief, an alkali-metal solution was added to an acidic aqueous solution of sodium silicate and magnesium or nickel chloride. The precipitated hydrous oxide obtained, after being filtered off, was autoclaved at temperatures between 110 and 300 °C. The material obtained was dried in an oven at 110 °C for 15 h. The composition of the smectite catalysts prepared was determined by X-ray fluorescence. Numbers of constituent cations in the unit cell (Si∶Mg∶Na∶K) were 8∶6.44∶2.81∶0.13 for S-Mg-1 and 8∶6.17∶0.63∶0.02 for S-Mg-2. BET surface areas of S-Mg-1 and S-Mg-2 were 110 m2 g−1 and 339 m2 g−1, respectively.All recation experiments were carried out in a 50 mL autoclave reactor. The details of experimental setup have been reported earlier.16 Typical conditions and procedures are as follows: propylene oxide (57 mmol) and the catalyst (0.5 g) were charged into the reactor. Then CO2 was injected up to around 1 MPa. The reactor was heated to 150 °C and then liquid CO2 was further injected up to 8 MPa under stirring. The mixture was stirred for 15 h. After the reaction, the reactor was cooled to 0°C with ice-water and depressurized by a back pressure regulator. The liquid reaction mixture was analyzed by a gas chromatograph with a flame ionization detector and a mass spectrometer. The transesterification reactions were also conducted in a similar fashion. Ethylene carbonate (25 mmol), methanol (200 mmol), and the catalyst (0.25 g) were charged into the reactor. Then CO2 was injected up to around 1 MPa and the reactor was heated to 150 °C. Although CO2 is not required for the transesterification, the presence of CO2 prevents the decomposition of EC, as reported in our previous work.16 The reaction mixture was analyzed in similar procedures as described above.
Acknowledgement
We wish to thank for financial support obtained from Japan Science and Technology (JST) under the CREST program.References
-
(a) P. G. Jessop, T. Ikariya and R. Noyori, Science, 1995, 269, 1065 CAS;
(b) P. G. Jessop and W. Leitner, in Chemical Synthesis Using Supercritical Fluids, ed. P.G. Jessop and W. Leitner, Wiley-VCH, Weinheim, 1999, p. 351 Search PubMed;
(c) P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1999, 99, 475 CrossRef CAS.
- M. Aresta and A. Dibenedetto, J. Mol. Chatal. A: Chem., 2002, 182, 399 Search PubMed.
- P. Tundo and M. Selva, CHEMTECH, 1995, 31 Search PubMed.
- Y. Ono, CATTECH, 1997, 1, 31 Search PubMed.
- M. A. Pacheco and C. L. Marshall, Energy Fuels, 1999, 11, 2 CrossRef.
- M. Aresta and E. Quaranta, CHEMTECH, 1997, 32 Search PubMed.
- A. A. Shaikh and S. Sivaram, Chem. Rev., 1996, 96, 951 CrossRef CAS.
-
(a) J. Kizlink, Collect. Czech. Chem. Commun., 1993, 58, 1399 CrossRef CAS;
(b) J. Kizlink and I. Pastucha, Collect. Czech. Chem. Commun., 1994, 59, 2116 CrossRef;
(c) J. Kizlink and I. Pastucha, Collect. Czech. Chem. Commun., 1995, 60, 687 CrossRef CAS.
- T. Zhao, Y. Han and Y. Sun, Fuel Process. Technol., 2000, 62, 187 CrossRef CAS.
-
(a) T. Sakakura, Y. Saito, M. Okano, J. C. Choi and T. Sako, J. Org. Chem., 1998, 63, 7095 CrossRef CAS;
(b) T. Sakakura, J. C. Choi, Y. Saito, T. Masuda, T. Sako and T. Oriyama, J. Org. Chem., 1999, 64, 4506 CrossRef CAS;
(c) T. Sakakura, J. C. Choi, Y. Saito and T. Sako, Polyhedron, 2000, 19, 573 CrossRef CAS.
- D. Ballivet-Tkatchenko, O. Douteau and S. Stutzmann, Organometallics, 2000, 19, 4563 CrossRef CAS.
- N. S. Isaacs, B. O. Sullivan and C. Verhaelen, Tetradedron, 1999, 55, 11949 Search PubMed.
- S. Fang and K. Fujimoto, Appl. Catal. A, 1996, 142, L1 CrossRef CAS.
- S. Fujita, B. M. Bhanage, Y. Ikushima and M. Arai, Green Chem., 2001, 3, 87 RSC.
-
(a) K. Tomishige, T. Sakaihori, Y. Ikeda and K. Fujimoto, Catal. Lett., 1999, 58, 225 Search PubMed;
(b) Y. Ikeda, T. Sakaihori, K. Tomishighe and K. Fujimoto, Catal. Lett., 2000, 66, 59 Search PubMed;
(c) K. Tomishige, Y. Ikeda, T. Sakaihori and K. Fujimoto, J. Catal., 2000, 192, 355 CrossRef CAS.
- B. M. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Appl. Catal. A, 2001, 219, 259 CrossRef CAS.
-
(a) Y. Nishiyama, M. Arai, S. L. Guo, N. Sonehara, T. Naito and K. Torri, Appl. Catal. A., 1993, 95, 171 CrossRef CAS;
(b) M. Shirai, K. Aoki, T. Miura, K. Torri and M. Arai, Chem. Lett., 2000, 36 CrossRef CAS.
-
(a) S. Fujita, B. M. Bhanage, Y. Ikushima, M. Shirai, K. Torri and M. Arai, Catal. Lett., 2002, 70, 95 Search PubMed;
(b) B. M. Bhanage, S. Fujita, Y.-F. He, Y. Ikushima, M. Shirai, K. Torri and M. Arai, Catal. Lett., 2002, 83, 137 Search PubMed.
- R. M. Weinstein, US Pat., 481555, 1989.
-
(a) K. Torri and T. Iwasaki, Chem. Lett., 1986, 2021;
(b) K. Torri and T. Iwasaki, Clay Sci., 1987, 7, 1 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2003 |
Click here to see how this site uses Cookies. View our privacy policy here. 








