Anti-microbial activities of ionic liquids
Juliusz Pernak*a, Kinga Sobaszkiewicza and Ilona Mirskab
aPoznan University of Technology, Sklodowskiej-Curie 2, 60-965, Poznań, Poland
bK. Marcinkowski University of Medicinal Sciences, Sieroca, 10, 61-771, Poznań, Poland. E-mail: juliusz.pernak@put.poznan.pl
First published on 2nd December 2002
Abstract
Ionic liquids (ILs) are shown to display anti-microbial activity with the activities being greatly affected by alkyl chain lengths. Shorter substituents on the cation result in a lack of activity against cocci, rods and fungi. ILs containing 10, 11, 12 and 14 carbon atoms in an alkoxy group show very high anti-microbial activities. The use of microorganisms in the IL require consideration of their minimum inhibitory concentration (MIC) values.
Green ContextAs often is the case with more interesting new areas of technology a high level of research activity leads to spin-off benefits outside of the main focus of effort. The dramatic growth in interest is the use of ionic liquids as alternative ‘greener’ solvents has led to such added benefits with somewhat unexpected applications being reported in synthesis and in biotechnology. Here we can read about their use as anti-microbial agents. The ILs studied are shown to be active against cocci, rods and fungi. The research reveals a relationship between the structure of the cation and the anti-microbial activities.JHC |
Introduction
Ionic liquids (ILs), a class of neoteric solvent, have attracted increasing interest over recent years as excellent alternatives to organic solvents in homogeneous and biphasic processes. These solvents can be used as media for a wide range of chemical reactions which have been the subject of several excellent reviews.1–7 ILs are non-volatile, non-flammable, have low melting points, high thermal stability in a wide temperature range and relatively low viscosity. They can be used as replacements for selected organic solvents and have shown promise toward important applications such as synthesis,1–10 separation and extraction processes.11–14 Investigation of this group of compounds as solvents is in its very early stages. Before the full potential of these ILs are realized, much more information about them as solvent systems must be obtained. The polarity,15–17 physical18–20 and thermodynamic21,22 properties of ILs have been investigated. ILs have been proposed as green solvents for organic synthesis due to the ease with which they can be recycled and reused whilst offering inert, dipolar media compatible with much of conventional chemistry.1One of the most exciting recent developments is the use of enzymes and other types of biotransformations in ILs. Reactions have been carried out in a biphasic H2O–IL system23,24 and show higher final yields; also an anhydrous system has been reported.25–32 The lipase reaction in ILs can be scaled up without major difficulty.27 The enzyme suspended in the IL could be reused three times with less than 10% loss of activity per cycle and the enantioselectivity was not influenced.26 The enzymes are stable in solvents such as 3-butyl-1-methylimidazolium hexafluorophosphate or tetrafluroborate. Based on these initial studies the use of enzymes in ionic liquids would appear to open up a new field of non-aqueous enzymonology. The most important finding of the preliminary reports is the fact that many enzymes retain their activity in ILs. In the literature there are examples of the use of a whole-cell biotransformation in an IL for the bacterium Rhodococcus R312 that contains the nitrile hydratase enzyme23 or Baker’s yeast.33 The studies of ABE fermentation with 3-octyl-1-methylimidazolium hexafluorophosphate present at saturation level suggest that the IL suppresses biological activity in the system.34
In this paper we report our results of the investigation of anti-microbial activities of ILs.
Results and discussion
A series of 3-alkoxymethyl-1-methylimidazolium salts of [Cl]− (1), [BF4]− (2) and [PF6]− (3) were prepared (Table 1). All chlorides synthesized were very hygroscopic. The [BF4]− and [PF6]− salts were obtained by simple metathesis reactions from the corresponding chlorides using aqueous KBF4 or KPF6. The [BF4]− salts have a greater miscibility with water than the corresponding [PF6]− salts. The water miscibility of 3-alkoxymethyl-1-methylimidazolium [BF4]− and [PF6]− depends on the length of the alkoxymethyl chain. The water soluble ones are those salts with short chains (propoxymethyl, butoxymethyl and pentyloxymethyl). Water was present in all salts of the type we studied even after a moderate drying procedure (drying at 70 °C for 4 h on a vacuum line). The water content in the dried ILs depends on the length of the alkyl chain and was found to be between 2200 and 400 ppm.19 All the salts prepared are air-stable under ambient conditions and may be handled under normal laboratory conditions. Molecular states of water in ILs have been investigated based on the 1-alkyl-3-methylimidazolium salts.35 It has been shown that in these ILs water moleculas absorbed from the air are present mostly in the ‘free’ state, bound via H-bonding with [PF6]− and [BF4]− with the concentration of dissolved water in the range 0.2–1.0 mol L−1. It has been concluded that most of the water molecules at these concentrations exist as 1∶2 H-bonded complexes: anion⋯HOH⋯anion.| Chlorides | Tetrafluoroborates | Hexafluorophosphates | ||||||
|---|---|---|---|---|---|---|---|---|
| R | Mp/°C | R | Mp/°C | R | mp/°C | |||
| Grease = heavy oil. | ||||||||
| 1a | C3H7 | Oil | 2a | C3H7 | Liquid | 3a | C3H7 | Liquid |
| 1b | C4H9 | Oil | 2b | C4H9 | Liquid | 3b | C4H9 | Liquid |
| 1c | C5H11 | Oil | 2c | C5H11 | Liquid | 3c | C5H11 | Liquid |
| 1d | C6H13 | Grease | 2d | C6H13 | Liquid | 3d | C6H13 | Liquid |
| 1e | C7H15 | Grease | 2e | C7H15 | Liquid | 3e | C7H15 | 37–38 |
| 1f | C8H17 | Grease | 2f | C8H17 | Liquid | 3f | C8H17 | 48–50 |
| 1g | C9H19 | Grease | 2g | C9H19 | Liquid | 3g | C9H19 | 47–49 |
| 1h | C10H21 | Grease | 2h | C10H21 | 56–57 | 3h | C10H21 | 46–47 |
| 1i | C11H23 | 66–68 | 2i | C11H23 | 61–62 | 3i | C11H23 | 52–53 |
| 1j | C12H25 | 67–70 | 2j | C12H25 | 62–64 | 3j | C12H25 | 61–63 |
| 1k | C14H29 | 70–73 | 2k | C14H29 | 65–67 | 3k | C14H29 | 67–69 |
| 1l | C16H33 | 73–75 | 2l | C16H33 | 68–69 | 3l | C16H33 | 71–73 |
All synthesized imidazolium salts were tested for anti-microbial activity against cocci, rods and fungi. Minimum inhibitory concentration (MIC) values and minimum bactericidal or fungicidal concentration (MBC) values are given in Tables 2–4. Also the MIC and MBC values of benzalkonium chloride (BAC) were determined and shown in Table 2. The calculated average MIC and MBC values for cocci, rods and fungi are presented in Figs. 1–3 as a relationship between the alkyl chain length and anti-microbial activity. As shown by the results, the salts studied are active against cocci, rods and fungi. Some of them exhibited strong activity and wide anti-bacterial action. Their activities are greatly affected by the alkyl chain length in the alkoxymethyl substituent but do not depend on the type of anion. The MIC values of imidazolium chlorides indicate the anti-microbial activities for the same imidazolium salts with [BF4]− or [PF6]−. For the studied salts the same correlation is observed for the MIC and MBC values. The most active salts against cocci and rods have an alkoxy group which contains 10, 11, 12 or 14 carbon atoms. The curves in Figs. 1 and 2 demonstrate the optimum anti-microbial efficiency, the most effective group being dodecyloxymethyl. Activity against fungi is significantly different with the curves in Fig. 3 indicating that no optimum activity was clear with the salts under study here. Though the observed increase of activity with number of carbon atoms in the alkoxy group will not be unlimited the hydrophobic chain has the important function of adsorbing onto the surface of the microbial cell.
| Chloride | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | 1a–1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | 1k | 1l | BACb | |
| a In μM, the number of microorganisms in 1 mL range from 104 to 105.b BAC, benzalkonium chloride.c No data available (opacity of solution). | ||||||||||||
| Cocci | ||||||||||||
| M. luteus | MIC | >8600 | 4300 | 2030 | 960 | 230 | 108 | 52 | 25 | —c | —c | 7 |
| MBC | >8600 | 4300 | 4060 | 3800 | 910 | 217 | 207 | 49 | 91 | 336 | 11 | |
| S. epidermidis | MIC | >8600 | 4300 | 1000 | 480 | 110 | 54 | 52 | 25 | —c | —c | 3 |
| MBC | >8600 | 4300 | 2030 | 1900 | 1820 | 867 | 103 | 49 | 91 | 1340 | 3 | |
| S. aureus | MIC | >8600 | 4300 | 500 | 480 | 228 | 54 | 52 | 25 | —c | —c | 7 |
| MBC | >8600 | 8600 | 1000 | 960 | 1820 | 867 | 826 | 99 | 91 | 168 | 7 | |
| S. aureus MRSA | MIC | >8600 | 8600 | 4060 | 3800 | 1820 | 433 | 207 | 99 | —c | —c | 7 |
| MBC | >8600 | 8600 | 8100 | 7680 | 3640 | 867 | 413 | 395 | 363 | 671 | 11 | |
| E. hirae | MIC | >8600 | 8600 | 8100 | 1900 | 228 | 108 | 103 | 99 | —c | —c | 11 |
| MBC | >8600 | 8600 | 8100 | 7680 | 3640 | 433 | 207 | 197 | 91 | 168 | 22 | |
| Rods | ||||||||||||
| E. coli | MIC | >8600 | 8600 | 8100 | 3800 | 1820 | 433 | 413 | 99 | 181 | 671 | 7 |
| MBC | >8600 | 8600 | 8100 | 7680 | 1820 | 867 | 413 | 395 | 363 | 2680 | 11 | |
| P. vulgaris | MIC | >8600 | 8600 | 4060 | 3800 | 1820 | 217 | 207 | 197 | 181 | 336 | 22 |
| MBC | >8600 | 8600 | 8100 | 3800 | 1820 | 867 | 413 | 197 | 363 | 336 | 22 | |
| K. pneumoniae | MIC | >8600 | 8600 | 8100 | 3800 | 1820 | 433 | 207 | 197 | 181 | 168 | 11 |
| MBC | >8600 | 8600 | 8100 | 7680 | 3640 | 1733 | 413 | 395 | 181 | 336 | 11 | |
| P. aeruginosa | MIC | >8600 | 8600 | 4060 | 3800 | 1820 | 867 | 826 | 395 | 726 | 2680 | 54 |
| MBC | >8600 | 8600 | 8100 | 7680 | 3640 | 1733 | 1653 | 790 | 5800 | >5370 | 205 | |
| Fungi | ||||||||||||
| C. albicans | MIC | >8600 | 8600 | 4060 | 1900 | 1820 | 433 | 413 | 197 | 91 | 84 | 7 |
| MBC | >8600 | 8600 | 8100 | 3800 | 3640 | 867 | 413 | 395 | 181 | 84 | 11 | |
| R. rubra | MIC | >8600 | 8600 | 2030 | 1900 | 910 | 217 | 103 | 99 | 45 | 42 | 11 |
| MBC | >8600 | 8600 | 4060 | 3800 | 3640 | 867 | 207 | 197 | 91 | 42 | 11 | |
| Tetraflouroborates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | 2a–c | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | |
| a In μM, the number of microorganisms in 1 mL range from 104 to 105.b No data available (opacity of solution) | ||||||||||
| Cocci | ||||||||||
| M. luteus | MIC | >7000 | 3500 | 3360 | 800 | 192 | 46 | 44 | 21 | —b |
| MBC | >7000 | 7000 | 3360 | 1600 | 1540 | 368 | 88 | 21 | 39 | |
| S. epidermidis | MIC | >7000 | 3500 | 1680 | 400 | 192 | 46 | 44 | 21 | —b |
| MBC | >7000 | 7000 | 3360 | 800 | 1540 | 368 | 177 | 85 | 158 | |
| S. aureus | MIC | >7000 | 7000 | 840 | 800 | 384 | 92 | 22 | 21 | —b |
| MBC | >7000 | 7000 | 1680 | 3200 | 1540 | 736 | 353 | 85 | 79 | |
| S. aureus MRSA | MIC | >7000 | 7000 | 3360 | 3200 | 1540 | 368 | 177 | 85 | —b |
| MBC | >7000 | 7000 | 6700 | 6400 | 3070 | 736 | 353 | 680 | 632 | |
| E. hirae | MIC | >7000 | 3500 | 3360 | 1600 | 384 | 92 | 88 | 85 | —b |
| MBC | >7000 | 7000 | 6700 | 6400 | 3070 | 1471 | 177 | 340 | 158 | |
| Rods | ||||||||||
| E. coli | MIC | >7000 | 7000 | 6700 | 3200 | 1540 | 368 | 177 | 170 | 316 |
| MBC | >7000 | 7000 | 6700 | 6400 | 3070 | 1471 | 177 | 170 | 158 | |
| P. vulgaris | MIC | >7000 | 7000 | 6700 | 3200 | 1540 | 184 | 177 | 170 | 158 |
| MBC | >7000 | 7000 | 6700 | 3200 | 3070 | 736 | 353 | 170 | 316 | |
| K. pneumoniae | MIC | >7000 | 7000 | 6700 | 1600 | 3070 | 368 | 177 | 170 | 158 |
| MBC | >7000 | 7000 | 6700 | 6400 | 3070 | 736 | 353 | 340 | 158 | |
| P. aeruginosa | MIC | >7000 | 7000 | 6700 | 3200 | 1540 | 1471 | 707 | 340 | 632 |
| MBC | >7000 | 7000 | 6700 | 6400 | 3070 | 1471 | 1413 | 680 | 1260 | |
| Fungi | ||||||||||
| C. albicans | MIC | >7000 | 7000 | 3360 | 1600 | 770 | 736 | 707 | 340 | 158 |
| MBC | >7000 | 7000 | 6700 | 3200 | 1540 | 1471 | 1413 | 340 | 158 | |
| R. rubra | MIC | >7000 | 7000 | 3360 | 1600 | 192 | 184 | 177 | 21 | 20 |
| MBC | >7000 | 7000 | 6700 | 3200 | 1540 | 736 | 177 | 85 | 39 | |
| Hexafluorophosphates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | 3a–c | 3d | 3e | 3f | 3g | 3h | 3i | 3j | 3k | |
| a In μM, the number of microorganisms in 1 mL range from 104 to 105.b No data available (opacity of solution) | ||||||||||
| Cocci | ||||||||||
| M. luteus | MIC | >5850 | 2900 | 2800 | 1350 | 330 | 160 | 152 | 37 | —b |
| MBC | >5850 | 5850 | 5600 | 2700 | 1300 | 630 | 152 | 73 | 138 | |
| S. epidermidis | MIC | >5850 | 2900 | 1400 | 680 | 330 | 80 | 76 | 18 | —b |
| MBC | >5850 | 5850 | 5600 | 2700 | 1300 | 1250 | 303 | 147 | 138 | |
| S. aureus | MIC | >5850 | 2900 | 2800 | 1350 | 650 | 160 | 19 | 18 | —b |
| MBC | >5850 | 5850 | 5600 | 2700 | 2600 | 1250 | 607 | 73 | 69 | |
| S. aureus MRSA | MIC | >5850 | 5850 | 2800 | 2700 | 1300 | 630 | 607 | 73 | —b |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 2500 | 1214 | 587 | 551 | |
| E. hirae | MIC | >5850 | 2900 | 2800 | 2700 | 330 | 630 | 152 | 37 | —b |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 2500 | 607 | 587 | 275 | |
| Rods | ||||||||||
| E. coli | MIC | >5850 | 5850 | 5600 | 2700 | 1300 | 1250 | 607 | 73 | 138 |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 1250 | 607 | 293 | 275 | |
| P. vulgaris | MIC | >5850 | 5850 | 5600 | 2700 | 1300 | 314 | 303 | 147 | 138 |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 2500 | 1214 | 293 | 551 | |
| K. pneumoniae | MIC | >5850 | 5850 | 5600 | 2700 | 2600 | 630 | 152 | 147 | 138 |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 2500 | 1214 | 147 | 138 | |
| P. aeruginosa | MIC | >5850 | 5850 | 5600 | 2700 | 2600 | 1250 | 1214 | 587 | 1100 |
| MBC | >5850 | 5850 | 5600 | 5400 | 2600 | 2500 | 1214 | 587 | 2200 | |
| Fungi | ||||||||||
| C. albicans | MIC | >5850 | 5850 | 2800 | 1350 | 1300 | 630 | 607 | 587 | 138 |
| MBC | >5850 | 5850 | 5600 | 2700 | 2600 | 1250 | 1214 | 587 | 275 | |
| R. rubra | MIC | >5850 | 5850 | 2800 | 1350 | 650 | 314 | 152 | 37 | 17 |
| MBC | >5850 | 5850 | 5600 | 2700 | 2600 | 630 | 303 | 587 | 138 | |
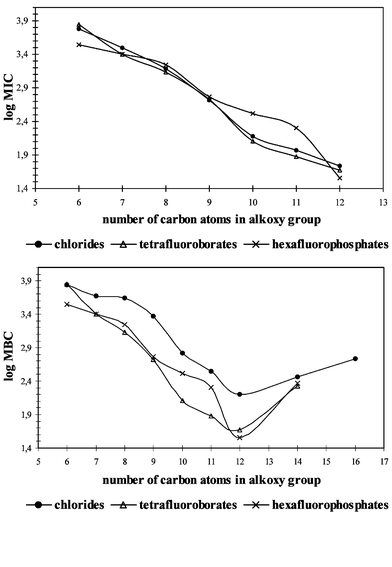 | ||
| Fig. 1 Mean MIC and MBC values for cocci. | ||
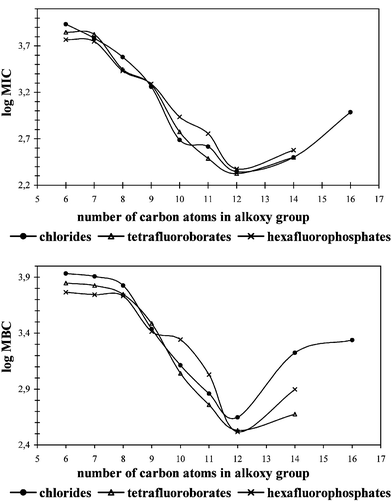 | ||
| Fig. 2 Mean MIC and MBC values for rods. | ||
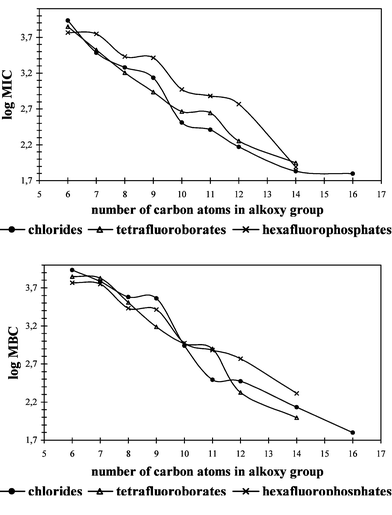 | ||
| Fig. 3 Mean MIC and MBC values for fungi. | ||
The activity of salts 1j, 2j, and 3j approach the activity of commercially available benzalkonium chloride (BAC in which R represents a mixture of alkyls from C8H17 to C18H37). The ILs with short substituents (propoxymethyl, butoxymethyl and pentyloxymethyl) are not active against bacteria and fungi.
In summary we found that ILs showed anti-microbial activities. There is a relationship between the structure of the cation and the anti-microbial activities. These salts with short substituents are not active against bacteria and fungi. The results indicate that salts containing 10, 11, 12 and 14 carbon atoms in the alkoxy group show very high anti-microbial activities. The use of microorganisms in ILs requires consideration of their MIC values.
Experimental
3-Alkoxymethyl-1-methylimidazolium chlorides (1), tetrafluoroborates (2) and heksafluorophosphates (3) were prepared according to the published method.36Anti-microbial actibity
Anti-microbial activity was determined by the tube dilution method. A series of imidazolium salt dilutions were prepared on Müller–Hinton broth medium (bacteria) or Sabouraud broth medium (fungi). A suspension of the microorganisms, prepared from 24 h cultures of bacteria in the Müller–Hinton broth medium and from 48 h cultures in the Sabouraud agar medium for fungi at a concentration of 106 cfu mL−1, were added to each dilution in a 1∶1 ratio. Growth (or lack thereof) of the microorganisms was determined visually after incubation for 24 h at 37 °C (bacteria) or 48 h at 28–30 °C (fungi). The lowest concentration at which there was no visible growth (turbidity) was taken as the MIC. Then from each tube, one loopful was cultured on an agar medium with inactivates (0.3% lecithin, 3% polysorbate 80 and 0.1% cysteine L) and incubated for 48 h at 37 °C (bacteria) or for 5 days at 28–30 °C (fungi). The lowest concentration of the salt supporting no colony formation was defined as the MBC.Microorganisms used: eleven standard strains representative of cocci; Micrococcus luteus ATCC 9341, Straphylococcus epidermidis ATCC 12228, Staphylococcus aureus ATCC 6538, Staphylococcus aureus MRSA, Enterococcus hirae, rods; Escherichia coli NCTC 8196, Proteus vulgaris NCTC 4635, Klebsiella pneumoniae ATCC 4352, Pseudomonas aeruginosa ATCC 15442, fungi; Candida albicans ATCC 10231, Rhodotorula rubra (Demml 1889, Lodder 1934). Standard strains were supplied by the National Collection of Type Cultures (NCTC) London and American Type Culture Collection (ATCC). The R. rubra was taken from the Department of Pharmaceutical Bacteriology, K. Marcinkowski University of Medical Sciences, Poznan.
Acknowledgments
This investigation received financial support from the Polish Committee of Scientific Research Grant KBN No 4 T09B 008 22.References
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef.
- J. Dupont, C. S. Consorti and J. Spencer, J. Braz. Chem. Soc., 2000, 11, 337 Search PubMed.
- C. M. Gordon, Appl. Catal. A: General, 2001, 222, 101 CrossRef CAS.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC.
- C. S. Consorti, R. F. de Souza, J. Dupont and P. A. Z. Suarcz, Quim. Nova, 2001, 24, 830 Search PubMed.
- J. L. Scott, D. R. MacFarlane, C. L. Raston and C. M. Teoh, Green Chem., 2000, 2, 123 RSC.
- S. Toma, B. Gotov, I. Kmentova and E. Solcaniova, Green Chem., 2000, 2, 149 RSC.
- Mizushima Eiichiro, Hayashi Teruyuki and Tanaka Masato, Green Chem., 2001, 3, 76 RSC.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis, Jr. and R. D. Rogers, Chem. Commun., 2001, 135 RSC.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, S. T. Griffin and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3596 CrossRef CAS.
- A. E. Visser, R. P. Swatloski and R. D. Rogers, Green Chem., 2000, 2, 1 RSC.
- A. E. Visser, R. P. Swatloski, S. T. Griffin, D. H. Hartman and R. D. Rogers, Sep. Sci. Technol., 2001, 36, 785 Search PubMed.
- A. J. Carmichael and K. R. Seddon, J. Phys. Org. Chem., 2000, 13, 591 CrossRef CAS.
- K. A. Fletcher, I. A. Storey, A. E. Hendricks, S. Pandey and S. Pandey, Green Chem., 2001, 3, 210 RSC.
- S. V. Dzyuba and R. A. Bartch, Tetrahedron Lett., 2002, 43, 4657 CrossRef CAS.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156 RSC.
- S. V. Dzyuba and R. A. Bartch, ChemPhysChem., 2002, 3, 161 CrossRef CAS.
- J. K. Shah, J. F. Brennecke and E. J. Maginn, Green Chem., 2002, 4, 112 RSC.
- A. Heintz, D. V. Kulikov and S. P. Verevkin, J. Chem. Eng. Data, 2002, 47, 849 CrossRef CAS.
- S. G. Cull, J. D. Holbrey, V. Vargas-Mora, K. R. Seddon and G. J. Jye, Biotechnol. Bioeng., 2000, 69, 227 CrossRef CAS.
- M. Erbeldinger, A. J. Mesiano and A. J. Russell, Biotechnol. Progr., 2000, 16, 1129 CrossRef CAS.
- R. M. Lau, F. van Rantwijk, K. R. Seddon and R. A. Sheldon, Org. Lett., 2000, 2, 4189 CrossRef CAS.
- S. H. Schöfer, N. Kaftzik, P. Wassrescheid and U. Kragl, Chem. Commun., 2001, 425 RSC.
- K. W. Kim, B. Song, M. Y. Choi and M. J. Kim, Org. Lett., 2001, 3, 1507 CrossRef CAS.
- T. Itoh, E. Akasaki, K. Kudo and S. Shirakami, Chem. Lett., 2001, 262 CrossRef CAS.
- S. Park and R. J. Kazlauskas, J. Org. Chem., 2001, 66, 8395 CrossRef CAS.
- M. T. Reetz, W. Wiesenhöfer, G. Francio and W. Leitner, Chem. Commun., 2002, 992 RSC.
- P. Kielbasinski, M. Albrycht, J. Luczak and M. Mikolajczyk, Tetrahedron: Asymmetry, 2002, 13, 735 CrossRef CAS.
- S. J. Nara, J. R. Harjani and M. M. Salunkhe, Tetrahedron Lett., 2002, 43, 2979 CrossRef CAS.
- J. Howarth, P. James and J. Dai, Tetrahedron Lett., 2001, 42, 7517 CrossRef CAS.
- A. G. Fadeev and M. M. Meagher, Chem. Commun., 2001, 295 RSC.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192 RSC.
- J. Pernak, A. Czepukowicz and R. Poźniak, Ind. Eng. Chem. Res., 2001, 40, 2379 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
