Preparation of optically active cis-4-methylcyclohex-4-ene-1,2-dicarboximides by a combination of Diels–Alder reaction and complexation with optically active hosts and enantioselective Diels–Alder reaction in inclusion crystals in a water suspension medium
Hisakazu Miyamoto*, Taku Kimura, Naoki Daikawa and Koichi Tanaka
Department of Applied Chemistry, Faculty of Engineering, Ehime University, Matsuyama, Ehime, 790-8577, Japan. E-mail: miyamoto@eng.ehime-u.ac.jp
First published on 28th November 2002
Abstract
Optically active Diels–Alder adducts were prepared using a one-pot preparative method and enantioselective Diels–Alder reaction with optically active hosts in a water suspension medium.
Green ContextDiels–Alder reactions are perfectly atom economic. They also have the potential for being enantioselective and forming complex multifunctional products from relatively simple raw materials. This paper shows that such procedures can take place readily in aqueous environments with good enantiomeric purity. This is achieved through the formation of inclusion complexes in chiral hosts suspended in water. Heating the filtered solid allowed the Diels–Alder reaction to proceed to give the chiral product in good yield. No organic solvents are used in this sequence, although extraction was carried out with ethyl acetate.DJM |
Introduction
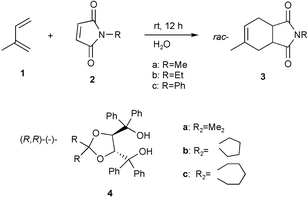
Optically active enantiomers of the cis-4-methylcyclohex-4-ene-1,2-dicarboximides, important as key starting materials for the synthesis of various bioactive compounds, have been prepared by biological synthesis. These, however, are neither always simple nor efficient. The Diels–Alder reaction is a simple and efficient tool to form the key step in the preparation of six-membered rings. Many procedures have been developed to increase the yields and selectivities of Diels–Alder reactions.1 Recently, organic reactions in aqueous media, particularly in water, have received much attention, because water is economical and harmless to the environment.2Optically active cis-4-methylcyclohex-4-ene-1,2-dicarboximides were prepared by Diels–Alder reaction using optically active hosts and enantioselective Diels–Alder reactions in inclusion crystals in a water suspension medium.
Results and discussion
We report a one-pot preparative method3 to prepare optically active compounds by a combination solid state Diels–Alder reaction in a water suspension medium and an enantioselective inclusion complexation of the product with an optically active host compound in the same aqueous medium. Heating in vacuo of the inclusion complex crystal isolated from the aqueous medium by filtration gave optically active product by distillation. Since no solvent is necessary throughout the reaction, inclusion complexation and isolation of the optically active product from the inclusion complex, this is genuinely sustainable and green chemistry. For example, when a mixture of N-ethylmaleimide 2b (0.2 g, 1.60 mmol), 2-methyl-1,3-butadiene 1 (0.400 g, 5.87 mmol), and water (2 mL) was stirred at room temperature for 12 h, rac-3b was produced. To a water suspension medium of rac-3b was added the optically active compound 4c4 (0.405 g, 080 mmol) and the mixture was stirred for 12 h to give a 1∶1 inclusion complex of 4c with (+)-3b. Heating the filtered inclusion complex in vacuo gave (+)-3b with 94% ee (0.043 g, 31% yield). From the filtrate left after separation of the inclusion crystals, (−)-3b with 37% ee (0.043 g, 28% yield) was obtained by extraction with ethyl acetate. By the same procedure, optically active 3a was prepared (Table 1). In the case of (+)-3b, the efficiency of enantiomeric resolution is very high. Optical resolution of 3a using 4b gave only the (−)-product. To clarify the results, we attempted to characterize the inclusion complex of 3a with 4b by X-ray analysis but unfortunately suitable single crystals could not be grown. Inclusion complexes of 3a with 4c, 3b with 4a, and 3c with 4a–c were not obtained (Table 1). Furthermore, optical resolution of rac-3c with 4c by recrystallization was successful giving (+)-3c with 85% ee in 47% yield. Since the optically active host remained after separation of the optically active guest from its inclusion complex by distillation it can be used repeatedly. This one-pot method in water is thus both ecological and economical.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion complexa | From complex | From filtrate | |||||||
| Dienophile | Host (% ee) | Host∶Guest | Mp/°C | Product | Yield (%) | Enantiomeric purityb (% ee) | Product | Yield (%) | Enantiomeric purityb (% ee) |
| a All crystals are colorless powders.b Enantiomeric purities were determined by HPLC. | |||||||||
| 2a | 4a (100) | 1∶1 | 118–120 | (+)-3a | 35 | 29 | (−)-3a | 40 | 59 |
| 2a | 4b (100) | 2∶1 | 132–135 | (−)-3a | 48 | 71 | (+)-3a | 40 | 63 |
| 2a | 4c (100) | rac-3a | 74 | 0 | |||||
| 2b | 4a | rac-3b | 69 | 0 | |||||
| 2b | 4b | 2∶1 | 123–125 | (+)-3b | 76 | 78 | (−)-3b | 48 | 50 |
| 2b | 4c | 1∶1 | 127–130 | (+)-3b | 31 | 94 | (−)-3b | 28 | 37 |
| 2c | 4a | rac-3c | 78 | 0 | |||||
| 2c | 4b | rac-3c | 80 | 0 | |||||
| 2c | 4c | rac-3c | 80 | 0 | |||||
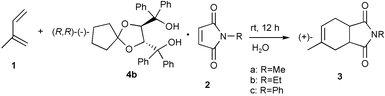
We also report enantioselective Diels–Alder reaction in inclusion crystals in a water suspension medium. A suspension of the powdered 2∶1 inclusion compound of 4b with 2c (2.00 g, 3.1 mmol) and 1 (1.00 g, 14.70 mmol) in water (15 mL) containing hexadecyltrimethylammonium bromide (0.04 g) as a surfactant to aid dispersion was stirred for 12 h. The solid reaction product was filtered off and the product dried. The filtered crystals included the product as shown by IR spectroscopy. The product in comparison with the starting material 2c has a greater affinity towards the optically active host 4b. Heating of the filtered crystals in vacuo gave (+)-3c with 11% ee in 81% yield. Since the optically active product was obtained the reaction occurred in the inclusion crystals. Optically active 3a and 3b were prepared by the same procedure (Table 2). 4b did not form inclusion complexes with 3b and 3c after recrystallization.
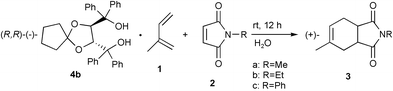
A suspension of the powdered 3∶1 inclusion compound of 4b with 1 (1.5 g, 5.5 mmol) and 2c (0.168 g, 0.99 mmol) in water (15 mL) containing hexadecyltrimethylammonium bromide (0.04 g) as a surfactant to aid dispersion was stirred for 12 h. The solid reaction product was filtered off and dried. The filtered crystals included the product as shown by IR spectroscopy. The product in comparison with the starting material 1 has a greater affinity towards the optically active host 4b. Heating of the filtered crystals in vacuo gave (+)-3c with 77% ee in 13% yield. Since the optically active product was obtained the reaction occurred in the inclusion crystals. By the same procedure, optically active 3a and 3b were prepared (Table 3). In the case of (+)-3c, using the inclusion complex of 4b with 1, the efficiency of the enantiomeric resolution is high. We found enantioselective Diels–Alder reaction in inclusion crystals in a water suspension medium gave optically active cis-4-methylcyclohex-4-ene-1,2-dicarboximides.
Conclusion
In conclusion, optically active Dies-Alder adducts were prepared by a one-pot preparative method and enantioselctive Diels–Alder reaction using optically active hosts in a water suspension medium.Experimental
General procedure for one-pot preparation of optically active Diels–Alder adducts by a combination of synthesis and enantiomeric resolution with optically active hosts in a water suspension medium
To a suspension of 2-methyl-1,3-butadiene 1 (0.40 g, 5.87 mmol) in 2 mL water was added N-ethylmaleimide 2b (0.20 g, 1.60 mmol), and the mixture was stirred at room temperature for 12 h to give rac-3b in 90% yield. To the water suspension medium of rac-3b was added powdered optically active host 4c (0.405 g, 0.80 mmol), and the suspension stirred at room temperature for 12 h, the product filtered off, and dried to give the 1∶1 inclusion complex of 4c with (+)-3b as a colorless powder (0.51 g, 75% yield, mp 127–130 °C). IR (Nujol): νmax 3409, 3305, 1684 cm−1. Anal. Calc. for C45H49NO6: C, 77.23; H, 7.06; N, 2.00. Found: C, 76.93; H, 7.12; N, 1.82%. Heating of the filtered inclusion crystals in vacuo (190 °C/22 mmHg) gave (+)-3b with 94% ee (0.047 g, 31% yield, [α]D +48 (c 0.13, MeOH). The filtrate remaining after separation of the inclusion crystals was extracted twice with 10 mL ethyl acetate. The ethyl acetate solution was dried over MgSO4, and evaporated to give (–)-3b with 37% ee (0.043 g, 28% yield, [α]D −19 (c 0.11, MeOH)).General procedure for enantioselective Diels–Alder reaction using the 2∶1 inclusion compound 2(4b)·2 and 1 in a water suspension medium
When a solution of 4b (2.10 g, 4.26 mmol) and 2c (0.37 g, 2.14 mmol) in diethyl ether (10 mL) was allowed to stand at room temperature for 12 h, a 2∶1 inclusion compound of 4b and 2c was obtained as a colorless powder (2.0 g, 81% yield, mp 120–123 °C), IR (Nujol): νmax 3421, 3233 cm−1. Anal. Calc. for C76H71NO10: C, 78.80; H, 6.18; N, 1.21. Found: C, 78.86; H, 6.37; N, 1.25%. A suspension of powdered 2(4b)·2c (2.00 g, 3.1 mmol) and 1 (1.00 g, 14.70 mmol) in water (15 mL) containing hexadecyltrimethylammonium bromide (0.04 g) as a surfactant was stirred for 12 h. The solid reaction product was filtered off and dried. Heating of the filtered crystals in vacuo gave (+)-3c with 11% ee (0.34 g, 81% yield, [α]D +7 (c 0.30, MeOH)).General procedure for enantioselective Diels–Alder reaction using the 3∶1 inclusion compound 3(4b)·1 and 2 in a water suspension medium
When a solution of 4b (2.60 g, 5.28 mmol) and 1 (0.68 g, 10 mmol) in diethyl ether (10 mL) was allowed to stand at room temperature for 20 h, a 3∶1 inclusion compound of 4b and 1 was obtained as a colorless powder (1.5 g, 55% yield, mp was not clear). IR (Nujol) νmax 3435, 3228 cm−1. A suspension of powdered 3(4b)·1 (1.5 g, 5.5 mmol) and 2c (0.168 g, 0.99 mmol) in water (15 mL) containing hexadecyltrimethylammonium bromide (0.04 g) as a surfactant was stirred for 12 h. The solid reaction product was filtered off and dried. Heating of the filtered crystals in vacuo gave (+)-3a with 77% ee (0.30 mg, 13% yield, [α]D +48 (c 0.13, MeOH)).References
- U. Pindur, G. Lutz and C. Otto, Chem. Rev., 1993, 93, 741 CrossRef CAS.
- S. Otto, J. B. F. N. Engberts and J. C. T. Kwak, J. Am. Chem. Soc., 1998, 120, 9517 CrossRef CAS.
- H. Miyamoto, S. Yasaka, R. Takaoka, K. Tanaka and F. Toda, Enantiomer, 2001, 6, 51 Search PubMed.
- D. Seebach, A. K. Beck, R. Imwinkelried, R. Rogo and A. Wannacott, Helv. Chim. Acta, 1987, 70, 954 CrossRef CAS; F. Toda and K. Tanaka, Tetrahedron Lett., 1988, 29, 551 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |