Change in levels of persistent organic pollutants in human plasma after consumption of a traditional northern Norwegian fish dish—Mølje (cod, cod liver, cod liver oil and hard roe)
Torkjel M.
Sandanger
*ab,
Magritt
Brustad
b,
Eiliv
Lund
b and
Ivan C.
Burkow
ac
aNorwegian Institute for Air Research, The Polar Environmental Centre, No-9296 Tromsø, Norway. E-mail: torkjel.sandanger@nilu.no; Fax: + 47 77 75 03 76; Tel: + 47 77 75 03 92
bInstitute of Community Medicine, University of Tromsø, No-9037 Tromsø, Norway
cNorwegian Institute of Fisheries and Aquaculture Research, No-9291 Tromsø, Norway
First published on 8th January 2003
Abstract
The traditional northern Norwegian fish dish “mølje”, consisting of boiled cod, cod liver, cod liver oil and hard roe, is still consumed frequently during the winter months January to March. The liver of the cod is rich in lipids and the levels of persistent organic pollutants (POPs) are relatively high. To better understand the short-term consequences of this traditional meal on the plasma levels of PCBs and p,p′-DDE, individual intake of liver and cod liver oil during one meal was measured. Blood samples were collected from 33 participants before the meal, and then 4 h, 12 h and 5 days after it. Lipid-weight and wet-weight levels of 10 PCB congeners and p,p′-DDE were determined in the plasma samples and the food. The plasma levels of p,p′-DDE was found to increase significantly from 0 to 4 h, both when expressed as wet-weight (35% change) and lipid-weight (20% change). The corresponding changes (0–4 h) in wet-weight levels of the most prevalent PCB congeners were non significant. By contrast, PCB congeners with low levels in the food showed a significant drop in lipid-weight levels during the first 4 h. The observed changes were independent of amount consumed. Significant differences in fasting and non-fasting samples were found for most PCBs and p,p′-DDE. For the lipid weight levels of sum PCBs there was a significant decrease of 16% from non-fasting to fasting samples. To obtain reliable data on human levels of POPs it is, on the basis of these findings, recommended that blood samples should be collected from fasting individuals and both wet-weight and lipid-weight levels should be reported.
Introduction
The Arctic has some of the cleanest environments in the world. Despite this fact high levels of persistent organic pollutants (POPs) have been found in environmental samples from this region. Long-range transportation of contamination from more contaminated areas is believed to be responsible, considering the few local sources.1 Due to their persistence, the POPs bioaccumulate, permitting transfer to humans harvesting from the top of the food chain. Use of local and indigenous food is a common characteristic of people in Arctic communities, and a wide range of animals and plants are consumed.2 Of these local food items, animals from the marine food chains have been found to be the most important sources of exposure to POPs.3 A considerable part of the traditional diet is rich in marine lipids and also POPs.“Mølje” consisting of boiled cod, cod liver, hard roe, and fresh cod liver oil from the boiling of the cod liver, was traditionally a considerable part of the diet during the winter season among people living in the coastal areas of northern Norway. Cod liver and cod liver oil are rich in vitamin D and a dietary survey from northern Norway in 1931 did show that cod liver and fresh cod liver oil was the most important vitamin D and fat source.4 Although the cod is a lean fish, the liver is lipid rich containing up to 60% in raw liver.5 Today, the contribution of “mølje” in the daily northern Norwegian diet is less pronounced, but recent data from the nationwide Norwegian Women and Cancer Study (NOWAC) have shown that there are still places in northern Norway where it is frequently consumed (Lund, E. personal communication).
Considering the high lipid content of this meal, a short-term increase in plasma lipids following the meal is expected. This increase is further expected to affect the equilibrium of POPs between adipose tissue and plasma.6 This is supported by Haddad et al.,7 who have shown that the adipose tissue:blood-partitioning coefficient is equal to the ratio of lipids in adipose tissue and blood. The levels of POPs found in plasma are considered to reflect the body burden,8 but there have been discussions whether to use lipid-weight data or wet-weight data.6,7,9,10 The levels of POPs have thus been reported in different ways in different studies and some samples have been from fasting and some from non-fasting individuals, making comparison between studies difficult. Different methods for determining lipids in plasma complicate the issue more. Lipids might be quantified by gravimetric determination of the extractable organic material (EOM) or by a summation of the different enzymatically determined lipid classes.6 These methods appear to give different results and several studies are recommending the enzymatic summation method.6,11,12
Due to the high lipid content as well as “high” levels of POPs in cod liver, the intake of a meal of “mølje” is a good opportunity to study the short-term consequences on the equilibrium of POPs in plasma, both lipid-weight and wet-weight.
The aim of this study was to determine how much an intake of large amounts of marine lipids by consuming “mølje”, affects the levels of POPs in plasma and if they are influenced differently depending on their relative amounts in food.
Materials and methods
Study group
The majority (60.6%) of the 33 people participating were women; the average age was 42 (range: 28–65) years with an average body mass index (BMI) of 24.2 (range: 19.5–28.5). One participant (of Eastern European origin) was excluded from the statistical analyses of the data, since the person had p,p′-DDE levels four times higher than the average values (significant outlier). The participants were all working and living in Tromsø.The meal and the sampling
The participants were served the traditional hot northern Norwegian fish dish consisting of cod with liver, hard roe, fresh cod liver oil, and potatoes. The meal was prepared by boiling the cod and the hard roe separately in water. Fresh cod liver oil was obtained by boiling liver (in small pieces) in small amounts of water. Participants could eat as much as they wanted. The amount of liver and cod liver oil consumed by each participant was weighed and recorded separately.The study was carried out in the beginning of April 2000. The meal was served between 6 and 7 pm on Day 1. Blood samples were collected just before the meal (0 h), after 4 h, 12 h, and 5 days. The 12 h and 5 d blood specimens were taken in the morning before breakfast thus designated as fasting specimens compared to the 0 and 4 h as non-fasting. Participants were asked to maintain their ordinary diet during the study period, except not to have any meals between lunch and the cod dish served on Day 1. Body weight was measured on Day 5.
Analytical procedures
Blood samples were drawn from a cubital vein into 7 ml Vacutainer with ethylenediaminetetracetic acid (EDTA) as anticoagulant (Hemoguard, Becton Dickinson, Sweden). Plasma was separated by centrifugation at 2000 rev min−1 for 10 min (Kubota 2010 centrifuge, Kubota Corporation, Tokyo, Japan), and transferred to pre-cleaned vials, which were coded and kept frozen (−20 °C) until analysis.p,p′-DDE and 10 PCBs were included in this study. Because of the expected abundance below the detection limits, no other compounds like p,p′-DDT with metabolites, other pesticides and toxaphenes were included in the study. Inclusion of these components would have reduced the statistical power (data not shown) or our ability to detect changes.
The method employed for extraction and clean up was a slight modification of that developed at Centre de Toxicologie du Quebec, Canada.13 Plasma samples were extracted using liquid–liquid extraction with internal standards added before the first extraction. Specifically, 3 ml of plasma, 3 ml of ethanol and 3 ml of deionised water saturated with ammonium sulfate were extracted twice with 10 ml of n-hexane in a small glass tube. POPs were separated from the lipids using a tandem florisil column manually packed with 1.5 g of 0.5% deactivated florisil with 2 g of granulated sodium sulfate on top. Hexane:dichloromethane (3:1) was used as eluent. The columns were pre-washed using 10 ml of eluent before the sample was applied to the column, and the POPs were eluted using 11 ml of eluent.13 The collected fraction was evaporated to 0.5 ml using a Zymark Turbovap 500 Closed Cell Concentrator (Hopkinton, USA), followed by a gentle flow of nitrogen for reduction to 100 µl. Gas chromatography (GC) was performed using a Fisons 8060 Mega gas chromatograph (Milan, Italy). A 30 m DB-5 MS column (0.25 mm id and 0.25 µm film thickness; J&W Scientific, CA, USA) and a deactivated guard column (0.53 mm id, 2.5 m, J&W Scientific, CA, USA) were used for all analyses. The GC was further connected to a low-resolution Fisons MD 800 mass spectrometer (Milan, Italy). The internal standards used for quantification were C-13 labelled p,p′-DDE, PCB 101, 118, 141 and 178. Octachloronaphthalene (OCN) was added to calculate the recovery. The quantification was done using both negative chemical ionization (NCI) and positive electron-impact ionization (EI+) sources in the same MS. In both cases selected ion monitoring (SIM) mode was used. The different compounds were identified from their SIM masses and retention times. Peaks with differences in isotopic ratio greater than 20% compared with the quantification standard were rejected and not quantified. For every 10 samples, a blank was analysed to assess laboratory-derived (i.e., inadvertent) sample contamination. The limits of detection (LOD) were calculated using three times the area of the noise in 8 s (estimated peak width) at the given retention time, or if peaks were found in the blanks, three times the area of the blank. To account for the additional uncertainty with matrix effects, the limits of quantification were set to 3 times the LOD values.
Our laboratory participates in the AMAP's Human Health Ring test for human plasma samples. We have been participating in this programme from its outset. We have performed well throughout the participation performing a score of 98, 85 and 95 of a 100 in the last three inter-laboratory comparisons. We are still participating in this program.
The cod liver and cod liver oil was analysed according to a method published by Herzke et al.14 For the plasma samples, the amount of lipids were determined using the following summation formula: TL = 2.27 × TC + TG + 62.6 (mg dl−1).15 Here TL is total lipids, TC is total cholesterol, and TG is triacylglycerols. The levels of lipids were determined gravimetrically for 22 samples making comparison of the two methods possible.
Statistical analyses
Statistical analyses were carried out employing the SAS software package, version 6.12 (SAS Institute, 1996).16 One-way ANOVA was used when trying to determine the predictors of change in plasma levels of the different compounds. Change in plasma PCB and DDE levels by time was assessed by ANOVA with repeated measurement design, as well as paired sample t-test for comparison of two means for different sampling times.The data was not log transformed since most of the statistics were based on differences that did show a normal distribution.
The criteria of significance has conservatively been set to 99%, due to multiple comparisons and the high number of components, as well as the uncertainties in these types of analyses.
Results
Analytical aspects
For the analyses of the plasma samples the recovery rates were good for all internal standards with a range of 65–95% (results not shown). The calculated LOD and LOQ values are shown in Table 1. Some values were below the LOQ but they were included in the statistical analyses as they were, considering the strict definition of LOQ and the few levels in this range. The few values below LOD were set to half the LOD value before included. As for the PCB congeners the PCB 138 is not fully resolved from PCB 163, and thus reported as PCB 138/163. PCB 163 however accounts for less than 20% of the total peak area (results not shown).| Component | LOD/µg l−1 plasma | LOQ/µg l−1 plasma |
|---|---|---|
| p,p′-DDE | 0.121 | 0.363 |
| PCB 99 | 0.021 | 0.063 |
| PCB 101 | 0.010 | 0.030 |
| PCB 118 | 0.040 | 0.120 |
| PCB 138/163 | 0.053 | 0.159 |
| PCB 153 | 0.063 | 0.189 |
| PCB 156 | 0.015 | 0.045 |
| PCB 170 | 0.018 | 0.054 |
| PCB 180 | 0.030 | 0.090 |
| PCB 183 | 0.016 | 0.048 |
| PCB 187 | 0.018 | 0.054 |
Consumption
The amount of cod liver and cod liver oil eaten by the participants is shown in Table 2, together with the calculated amounts of PCBs and p,p′-DDE consumed. The fresh fish liver oil contained 98% lipids, whereas the pieces of cooked liver itself contained 65% lipids (results not shown).Lipids in plasma
The average amount of lipids in the plasma samples and the differences in levels are listed in Table 3. From 0 to 4 h, the amount of lipids increased significantly by an average of 11% and from 4 to 12 h the amount of lipids dropped significantly by 18%. From 12 h to 5 d there was no significant change in the levels. The difference of 10% observed between 0 and 12 h was also significant. The changes observed in the amount of total lipids are mainly caused by the changes in levels of triacylglycerols. All PCBs and p,p′-DDE did however show a better correlation with the total lipids than the triacylglycerols (data not shown).| 0 h/mg dl−1 | 4 h/mg dl−1 | 12 h/mg dl−1 | 5 d/mg dl−1 | 0–4 h % difference (p-value) | 4–12 h % difference (p-value) | 12 h–5 d % difference (p-value) | 0–12 h % difference (p-value) | |
|---|---|---|---|---|---|---|---|---|
| Arithmetic mean | 680.8 | 753.2 | 611.4 | 653.1 | 10.9 (p < 0.01) | −18.3 (p < 0.01) | 7.2 (p = 084) | −9.8 (p < 0.01) |
| Geometric mean | 669.2 | 739.9 | 602.1 | 644.3 | ||||
| Range | 464.4–954.5 | 460.9–1040.2 | 434.7–820.1 | 474.0–854.1 | −5.6–35.6 | −33.5– −3.2 | −3.6–27.2 | −27.5–7.4 |
| Standard deviation | 128.8 | 143.1 | 108.5 | 108.7 | 8.9 | 7.5 | 7.3 | 6.3 |
POPs in the meal and plasma
In the fish liver and liver oil, p,p′-DDE was the most abundant compound with wet-weight levels of 37.5 ng g−1 and 88.0 ng g−1 respectively (Table 4). Of the PCBs, PCB 138/163 and PCB 153 dominated, followed by 118 > 101 > 99. In human plasma, p,p′-DDE and PCB 153 were the most abundant compounds followed by PCB 180 > 138/163 > 170 >118, 187. Wet-weight data are given in Table 5 and lipid-weight data in Table 6.| Boiled cod liver/ng g−1 | Fresh cod liver oil/ng g−1 | |
|---|---|---|
| p,p′-DDE | 37.5 | 88.0 |
| PCB 99 | 11.9 | 28.2 |
| PCB 101 | 14.7 | 35.2 |
| PCB 118 | 27.0 | 54.7 |
| PCB 138/163 | 35.2 | 71.6 |
| PCB 149 | 6.6 | 14.2 |
| PCB 153 | 33.1 | 70.7 |
| PCB 156 | 2.0 | 4.4 |
| PCB 170 | 3.4 | 7.3 |
| PCB 180 | 7.1 | 15.4 |
| PCB 183 | 1.8 | 3.5 |
| PCB 187 | 4.4 | 9.5 |
| Sum PCB | 147 | 315 |
| 0 h/µg l−1 plasma | 4 h/µg l−1 plasma | 12 h/µg l−1 plasma | 5 d/µg l−1 plasma | |||||
|---|---|---|---|---|---|---|---|---|
| AMa (SDb) | GMc | AMa (SDb) | GMc | AMa (SDb) | GMc | AMa (SDb) | GMc | |
| a AM = arithmetic mean. b SD = standard deviation. c GM = geometric mean. | ||||||||
| p,p′-DDE | 0.87 (0.47) | 0.75 | 1.17 (0.71) | 0.99 | 0.67 (0.44) | 0.56 | 0.59 (0.31) | 0.51 |
| PCB 99 | 0.09 (0.04) | 0.08 | 0.09 (0.04) | 0.08 | 0.07 (0.04) | 0.06 | 0.07 (0.03) | 0.06 |
| PCB 101 | 0.05 (0.02) | 0.04 | 0.03 (0.02) | 0.03 | 0.02 (0.01) | 0.02 | 0.01 (0.00) | 0.01 |
| PCB 118 | 0.20 (0.08) | 0.18 | 0.18 (0.08) | 0.16 | 0.15 (0.07) | 0.13 | 0.14 (0.07) | 0.13 |
| PCB 138/163 | 0.54 (0.27) | 0.47 | 0.58 (0.29) | 0.51 | 0.49 (0.28) | 0.42 | 0.45 (0.23) | 0.39 |
| PCB 153 | 0.78 (0.42) | 0.67 | 0.82 (0.44) | 0.72 | 0.68 (0.39) | 0.58 | 0.65 (0.33) | 0.57 |
| PCB 156 | 0.09 (0.05) | 0.08 | 0.08 (0.04) | 0.07 | 0.07 (0.04) | 0.05 | 0.07 (0.04) | 0.06 |
| PCB 170 | 0.30 (0.15) | 0.26 | 0.27 (0.14) | 0.24 | 0.24 (0.15) | 0.20 | 0.25 (0.14) | 0.21 |
| PCB 180 | 0.65 (0.36) | 0.56 | 0.58 (0.31) | 0.50 | 0.52 (0.32) | 0.41 | 0.49 (0.27) | 0.42 |
| PCB 183 | 0.05 (0.03) | 0.04 | 0.05 (0.02) | 0.04 | 0.04 (0.03) | 0.04 | 0.04 (0.02) | 0.04 |
| PCB 187 | 0.17 (0.09) | 0.15 | 0.17 (0.09) | 0.15 | 0.14 (0.11) | 0.12 | 0.13 (0.07) | 0.12 |
| Sum PCB | 2.86 (1.44) | 2.50 | 2.84 (1.40) | 2.52 | 2.44 (1.38) | 2.14 | 2.30 (1.14) | 2.01 |
| 0 h/µg kg−1 lipids | 4 h/µg kg−1 lipids | H/µg kg−1 lipids | 5 d/µg kg−1 lipids | |||||
|---|---|---|---|---|---|---|---|---|
| AMa (SDb) | GMc | AMa (SDb) | GMc | AMa (SDb) | GMc | AMa (SDb) | GMc | |
| a AM = arithmetic mean. b SD = standard deviation. c GM = geometric mean. | ||||||||
| p,p′-DDE | 124.38 (54.59) | 112.17 | 149.49 (71.95) | 132.32 | 103.54 (51.43) | 90.61 | 87.72 (40.90) | 78.22 |
| PCB 99 | 12.89 (4.49) | 12.09 | 11.08 (4.37) | 10.25 | 10.27 (4.46) | 9.06 | 9.97 (4.01) | 9.20 |
| PCB 101 | 7.31 (3.42) | 6.34 | 4.41 (2.46) | 3.81 | 3.09 (1.24) | 2.79 | 1.81 (0.56) | 1.73 |
| PCB 118 | 29.01 (10.35) | 27.38 | 23.52 (9.94) | 21.14 | 23.32 (9.12) | 21.51 | 21.71 (9.96) | 19.65 |
| PCB 138/163 | 77.77 (35.57) | 70.20 | 73.89 (30.60) | 67.91 | 74.82 (32.05) | 68.87 | 67.13 (31.64) | 60.08 |
| PCB 153 | 112.43 (54.12) | 100.38 | 105.10 (46.48) | 95.47 | 103.93 (45.73) | 94.83 | 98.17 (46.44) | 87.49 |
| PCB 156 | 12.50 (6.12) | 11.24 | 10.64 (4.84) | 9.54 | 10.44 (5.71) | 8.70 | 9.88 (4.88) | 8.73 |
| PCB 170 | 43.34 (20.26) | 39.08 | 35.05 (16.07) | 31.65 | 36.70 (17.62) | 27.87 | 36.83 (19.89) | 32.11 |
| PCB 180 | 91.59 (47.10) | 81.46 | 74.97 (35.80) | 67.21 | 79.46 (39.16) | 67.30 | 74.04 (38.48) | 65.04 |
| PCB 183 | 7.46 (3.34) | 6.79 | 6.40 (2.64) | 5.87 | 6.67 (2.96) | 5.83 | 6.34 (2.90) | 5.66 |
| PCB 187 | 24.05 (21.85) | 21.85 | 21.63 (9.57) | 19.71 | 21.82 (10.42) | 19.44 | 20.17 (9.38) | 18.05 |
| sum PCB | 412.10 (185.05) | 374.63 | 366.17 (150.55) | 336.77 | 375.81 (151.94) | 347.15 | 345.20 (159.65) | 310.13 |
The most pronounced difference in congener pattern between human plasma and fish liver and oil samples was observed for PCB 180. It was the second most abundant PCB congener in human plasma, constituting 23% of the sum PCB value. In liver and fish liver oil, PCB 180 was the 6th most abundant congener constituting only 5% of the sum value. The food contained a higher proportion of lower chlorinated PCBs compared to the plasma samples.
Comparing the lipid-weight levels of the major compounds in plasma with the levels in liver and oil indicates that they are slightly lower in the food. For PCB 138/163 the levels were comparable.
Observed changes in plasma levels of p,p′-DDE and the PCBs
Using paired sample t-tests, several of the changes in the levels of PCB and p,p′-DDE were found to be significant. The average differences together with p-values are listed in Table 7. The p,p′-DDE showed the greatest fluctuations in levels (Fig 1). On a wet-weight basis all changes observed were significant except from 12 h to 5 d. From 0 to 4 h there was an increase of 35% followed by a decrease of 40% from 4 h to 12 h. Comparing the 0 h samples to the 12 h and 5 d samples showed a drop of 20 and 32% respectively. On a lipid-weight basis the changes were less pronounced even though they were all of significance.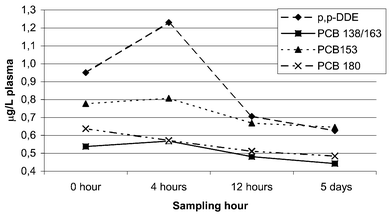 | ||
| Fig. 1 Wet-weight levels of the major compounds in plasma at the different sampling hours. | ||
| 0–4 h % change | 4–12 h % change | 12 h–5 d % change | 0–12 h % change | 0 h–5 d % change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wet weight | Lipid weight | Wet weight | Lipid weight | Wet weight | Lipid weight | Wet weight | Lipid weight | Wet weight | Lipid weight | |
| a p ≤ 0.01. | ||||||||||
| p,p′-DDE | 35a | 20a | −40a | −27a | −12 | −16a | −20a | −13a | −32a | −30a |
| PCB 99 | −2 | −13a | −21a | −4 | 2 | −3 | −25a | −18a | −25a | −22a |
| PCB 101 | −20a | −28a | −27a | −10a | −32a | −36a | −46a | −41a | −68a | −67a |
| PCB 118 | −8 | −18a | −18a | 1 | 0 | −5 | −28a | −22a | −29a | −26a |
| PCB 138/163 | 10 | −2 | −17a | 1 | −4 | −10a | −10 | −2 | −16a | −13a |
| PCB 153 | 8 | −4 | −18a | 0 | 0 | −6 | −12a | −5 | −15a | −12a |
| PCB 156 | −1 | −11 | −16a | 2 | 12 | 5 | −16a | −9 | −17a | −14 |
| PCB 170 | −3 | −13a | −10 | 9 | 5 | −2 | −13a | −6 | −13 | −10 |
| PCB 180 | −6 | −16a | −10 | 9 | −3 | −9 | −15a | −9a | −21a | −18a |
| PCB 183 | 2 | −12a | −10 | 9 | 1 | −5 | −7 | −5 | −12 | −12 |
| PCB 187 | 1 | −8a | −11 | 6 | 4 | −3 | −10 | −3 | −17a | −14a |
| Sum PCB | 2 | −9a | −16a | 2 | −1 | −7 | −15a | −8a | −18a | −16a |
The PCB congeners appear to behave differently and the fluctuations are smaller than for the p,p′-DDE (Table 7, Fig. 1). Looking at the wet-weight levels first, we found no significant changes for any of the major congeners or the PCBs sum from 0 to 4 h. From 4 to 12 h all concentrations drop between 10 and 27%, with the higher chlorinated congeners showing non-significant changes. Differences observed between 12 h and 5 d are in general non significant. From 0 h to 12 h and 5 d most congeners drop significantly. On a lipid-weight basis most congeners and sum PCBs drop significantly from 0 to 4 h with the PCB 138/163 and 153 as the only exception. No significant differences were observed for any of the major congeners from 4 to 12 h or 0 to 12 h. The only exception was for PCB 180 and sum PCBs from 0 to 12 h.
No significant predictors of the changes in plasma levels of POPs and lipids were found using ANOVA with a repeated measurement design. Individual intake, age, gender and BMI were included in the statistical model. As expected, the correlations between lipids in plasma and levels of POPs were significant (results not shown).
Discussion
Changes in plasma levels of PCBs and p,p′-DDE
Significant changes in levels of most of the studied compounds were found in connection to a meal of “mølje” even when the plasma levels were lipid adjusted. The study further found differences in levels of most compounds from fasting and non-fasting individuals. As for the set-up of the study the 0 h plasma sample should ideally have been fasting. This was however not possible when attempts were made to serve this in a traditional way and a blood sample 12 hours after the meal was required for D-vitamin analyses. It would also be hard to find volunteers to eat this heavy meal for breakfast. Further the full summation formula for the lipid determination in plasma should have been used, but due to missing free cholesterol values this was not possible (discussed further below).Based on ANOVA with a repeated measurement design significant (p < 0.01) changes with time were found for all compounds studied in this paper. There was however no relationship between individual consumption and concentration changes. Inclusion of BMI, age, and gender in the statistical model did not strengthen it. BMI, age and gender are factors known to affect the levels of POPs.17,18
The fact that changes in levels of either POPs or lipids were not at all related to individual intake, is surprising. There might be several reasons for this. Small sample size giving low statistical power might be one. There also seem to be great individual variation in rate of uptake. Another factor is the time before the second sample was taken. After a meal, the maximum amount of lipids in plasma is normally reached between five and six hours. Based only on one plasma sample 4 h after the meal it is impossible to conclude whether this was the peak concentration of the studied compounds. Further it is not possible to conclude if the peak concentration of POPs occurs at the same time as the lipid peak or if the concentration curves were different.
Previous studies have shown that absorption from the diet is >90% for persistent lipophilic compounds. For coefficients exceeding logKow = 7.5, absorption diminishes with increasing hydrophobicity.19 It has also been found that high intake of lipids and POPs increases uptake from the gastrointestinal tract.19,20 The relative uptake also seems to depend on the type of lipids consumed.21 In the present study the average amounts of p,p′-DDE and sum PCBs consumed was 6 µg and 23.8 µg respectively. In comparison the average daily intake of sum PCBs in Scandinavia is estimated to be 15–20 µg.22 Further, the concentration of p,p′-DDE in plasma was approximately 1 µg l−1 and there is about 3 l of plasma in an average body.23 This should give a total amount of approximately 3 µg of p,p′-DDE in the plasma before consumption of the meal (6 µg p,p′-DDE). This intake is thus expected to significantly alter the adipose tissue–plasma equilibrium. The fact that the level of p,p′-DDE actually increased with 35% on a wet-weight basis confirms this assumption. The level even increased more than the lipid levels, confirmed by the 20% lipid-weight increase. There was no significant increase for any of the PCB congeners with similar levels in plasma and food. For the higher chlorinated PCBs there was even a significant decrease on a lipid weight basis. These compounds also had lower concentrations in the food. No significant changes (0–4 h) on wet weight basis were observed. Explanations for these differences in concentration curves might be different uptake, distribution and different excretion rates (into bile) for the PCBs and p,p′-DDE. Several factors have been found to affect uptake, adsorption and distribution.21 Our findings are further supported by Schlummer et al.20 who found that absorption cannot be explained only on the basis of the gradient between the lipid-based food and blood concentrations. Kuwabara et al.24 found that digestion of PCB contaminated fish gave a significant increase after 5 h in the levels of PCBs that was not related to a similar increase in the amount of lipids.
For the PCB congeners the relative amounts in the food was of importance to the observed changes. From 0 to 4 h the levels of the three major PCB congeners in plasma, PCB 153, 180 and 138 developed differently. The lipid concentrations of PCB 153 and 138 were comparable in plasma, cod liver and cod liver oil, but no significant change in plasma levels was found. The lipid level of PCB 180 in the liver and oil was only 17% of the level in the plasma and a significant drop (−16%) from 0 to 4 h was observed. The other higher chlorinated PCBs with low levels in the food also exhibit significant decreases in plasma levels. Further evidence that the congener pattern in the food is reflected in the plasma, is given by a Finnish study where it was found that the PCDD/F pattern in plasma was dependent on the patterns in the fish consumed.25
The percent differences observed for the PCB congeners are small. The immediate changes caused by diet should, however, be considered when trends in human levels are monitored making sure that plasma samples are collected in the same manner under standardised dietary conditions.
Independent of the lipid rich meal, the 0 h and the 5 d samples were considered as non-fasting and fasting samples respectively. From Table 7 it is evident that the concentrations are different (in both units) in the two sample sets. These findings are in contrast to the findings by Phillips et al.6 and Longnecker et al.9 who found no significant differences in lipid-weight levels of POPs, between fasting and non-fasting samples. The study by Phillips et al.6 did however not take into consideration the levels of POPs in the meal itself and the effect that has on the plasma levels. In that study the meal was not expected to have “high” levels of POPs. Our results (0 h–4 h) clearly indicate that there will be fluctuations in levels of POPs depending on the food, the amount of POPs in the food and the physicochemical properties of the compounds consumed. The use of lipid-weight concentrations does however reduce the fluctuations for p,p′-DDE. Bergman et al.10 on the other hand claim that the lipid content in human plasma varies depending on the diet and how soon after the meal the blood sample is taken, and it is therefore more correct to express the concentration on a fresh-weight basis.
POPs in plasma in relation to other studies
Comparing the plasma levels in this study with levels in other studies yielded different results, depending on whether the samples were from the fasting or non-fasting individuals. In a study on 47 women in Vestvågøy, a small fishing village in northern Norway, the average levels of sum PCBs (7 congeners) and p,p′-DDE was 2.34 and 1.20 µg l−1 plasma respectively.18 The samples from the Vestvågøy study were from non-fasting individuals. The 0 h samples from the present study had sum PCB and p,p′-DDE levels of 2.88 and 0.87 µg l−1 respectively. In the 4 h samples, the levels were 2.86 and 1.17 µg l−1. As we see the concentrations in the two studies with non-fasting samples are comparable. The average wet-weight levels in the 5 d samples (fasting) of sum PCBs and p,p′-DDE were 2.31 µg l−1and 0.59 µg l−1 respectively. For p,p′-DDE it appears that the level is now only half of that in the Vestvågøy study.18The average concentrations of p,p′-DDE, PCB 153 and PCB 138/163 found in human plasma (5 d) were 88, 98 and 67 µg kg−1 lipid-weight respectively. These levels are comparable to Swedish and Norwegian data in the AMAP report 1998,2 but lower than what was found in Swedish men (p,p′-DDE, PCB 153, PCB 138; 290, 220, 160 µg kg−1 lipid-weight respectively) who reported no or low intake of fish from the Baltic Sea.26 By contrast for Swedish men reporting high intake of fish from the Baltic Sea, the average levels of DDE and PCB 153 were 4500 and 1000 µg kg−1 lipid.27
The levels of POPs found in this study are low and a meal of “mølje” gives a significant increase (after 4 h) only in the levels of p,p′-DDE. No association between fish consumption and levels of POPs was found in the northern-Norwegian fishing village Vestvågøy.18Based on this study, the findings of Furberg et al.18 and the discussion of possible health effects in the AMAP report2 there is no reason to believe that the levels found in these people and the intake of “mølje” can cause negative health effects related to POPs.
Lipids in plasma
Plasma lipids determined gravimetrically were 15% lower than the levels assessed using the summation formula. This is comparable to the 20% difference reported by Sjödin et al.26 They used the summation formula that included phospholipids and free cholesterol. One of the main advantages of using the summation method is that a defined total lipid value of known composition is obtained, rather than a simple weight of substances extracted by organic solvents.11 The correct working definition of what is determined gravimetrically refers to extractable organic material (EOM) rather than lipids. The gravimetric determination also requires greater sample volumes for accurate determinations. Thus, several studies are recommending the enzymatic summation method,11,28 with the following formula being the preferred one; TL = 1.677(TC − FC) + FC + TG + PL.6,11,12,28 Here FC is free cholesterol.The differences observed in lipid normalised levels of PCBs and p,p′-DDE between fasting and non-fasting samples, might have been reduced if the full summation formula had been used.6 For the free cholesterol Akins et al.11 have shown that this fraction ranged from 21.1% to 30.1% in their study, showing the importance of including it in the summation formula. Nevertheless, we suspect that the use of the complete formula would not have removed the statistically significant differences observed. The reason why the short formula for calculating lipids was being adopted was that data on the free cholesterol was not available for any of the samples.
POPs in cod liver
The sum PCBs levels found in the boiled cod liver (147 ng g−1 wet-weight) and cod liver oil (315 ng g−1 wet-weight) are comparable to levels previously reported in raw cod liver (240 ng g−1 wet-weight) from northern Atlantic cod by Kallenborn et al.29 but slightly lower than 660 ng g−1 wet-weight in liver from northern Atlantic cod, by SNT.5Conclusions
The concentrations of PCBs and p,p′-DDE in plasma change significantly after a meal rich in marine lipids and relatively high levels of these compounds. This was in particular the case for p,p′-DDE.As observed for the PCB congeners, this study indicates that the relative congener pattern of POPs in the food is reflected in the plasma sample, when this sample is taken 4 h after the meal.
Consequently, it seems essential to collect plasma specimens under fasting conditions and to continue to endorse the practice of reporting both unadjusted and lipid adjusted concentrations. One can then test when examining associations which units explains most of the variability.
A common approach for determining lipids in plasma should be employed to facilitate better comparison of data. The enzymatic summation method is here recommended.
Further studies are needed for better understanding of the kinetics of POPs during digestion and remobilisation from adipose tissue.
Acknowledgements
There is no doubt that the success of this study depended on the great effort and enthusiasm of the participants, for which we are thankful. Scientific and editorial contributions from Evert Nieboer and Jon Øyvind Odland as well as partial funding from The Barents Secretariat were appreciated.References
- I. C. Burkow and R. Kallenborn, Toxicol. Lett., 2000, 112–113, 87–92 CrossRef CAS.
- AMAP Assessment Report: Arctic Pollution Issues, Oslo: Arctic Monitoring and Assessment Programme (AMAP), 1998.
- J. C. Hansen, Toxicol. Lett., 2000, 112–113, 119–125 CrossRef CAS.
- J. Kloster, Acta Paediatr., 1931, 12, 1–82.
- Norwegian Food Control Authority (SNT), Assessment of Environmental Contaminants in Fish and Shellfish in Northern Norway, SNT-rapport 4, Oslo, 1997.
- D. L. Phillips, J. L. Pirkle, V. W. Burse, J. T. Bernert, L. O. Henderson and L. L. Needham, Arch. Environ. Contam. Toxicol., 1989, 18, 495–500 CAS.
- S. Haddad, P. Poulin and K. Krishnan, Chemosphere, 2000, 40, 839–843 CrossRef CAS.
- D. G. Patterson, L. L. Needham, J. L. Pirkle, D. W. Roberts, J. Bagby, W. A. Garrett, J. S. Andrews, H. Falk, J. T. Bernert, E. J. Sampson and V. N. Houk, Arch. Environ. Contam. Toxicol., 1988, 17, 139–143 CAS.
- M. P. Longnecker, L. Bernstein, C. L. Bird, A. K. Yancey and J. C. Peterson, Cancer Epidemiol. Biomarkers Prev., 1996, 5, 753–755 Search PubMed.
- A. Bergman, E. Klasson-Wehler and H. Kuroki, Environ. Health Perspect., 1994, 102, 464–469 Search PubMed.
- J. R. Akins, K. Waldrep and J. T. Bernert, Jr, Clin. Chim. Acta, 1989, 184, 219–226 CrossRef CAS.
- C. S. Cheek and D. F. Wease, Clin. Chem., 1969, 15, 102–107 CAS.
- I. Romieu, M. Hernandez-Avila, E. Lazcano-Ponce, J. P. Weber and E. Dewailly, Am. J. Epidemiol., 2000, 152, 363–370 CrossRef CAS.
- D. Herzke, G. W. Gabrielsen, A. Evenset and I. C. Burkow, Environ. Pollut., 2002, 121, 293–300 CrossRef CAS.
- L. L. Needham, V. W. Burse, S. L. Head, M. P. Korver, P. C. McClure, J. S. Andrews, D. L. Rowley, J. Sung and S. E. Kahn, Chemosphere, 1990, 20, 975–980 CrossRef CAS.
- SAS Institute Inc., SAS/STAT User's Guide, Version 6, Fourth Edition, Cary, NC: SAS Institute Inc., 1989.
- B. Deutch and J. C. Hansen, Int. J. Circumpolar Health, 1999, 58, 214–219 Search PubMed.
- S. Furberg, T. Sandanger, I. Thune, I. C. Burkow and E. Lun, J. Environ. Monit., 2002, 4, 175–181 RSC.
- G. A. Moser and M. S. McLachlan, Chemosphere, 2001, 45, 201–211 CrossRef CAS.
- M. Schlummer, G. A. Moser and M. S. McLachlan, Toxicol. Appl. Pharmacol., 1998, 152, 128–137 CrossRef CAS.
- R. J. Jandacek and P. Tso, Lipids, 2001, 36(12), 1289–1305 Search PubMed.
- P. Weihe, P. Grandjean, F. Debes and R. White, Sci. Total Environ., 1996, 186, 141–148 CrossRef CAS.
- M. Rowland and T. M. Tozer, Clinical Pharmacokinetics Concepts and Applications, ed. D. Balado, Lippincott, Williams and Wilkins, Philadelphia, 1995, p. 149 Search PubMed.
- K. Kuwabara, T. Yakushiji, I. Watanabe, S. Yoshida, K. Yoyama and N. Kunita, Bull. Environ. Contam. Toxicol., 1979, 21, 273–278 CAS.
- H. Kiviranta, T. Vartiainen and J. Tuomisto, Environ. Health Perspect., 2002, 110, 355–361 Search PubMed.
- A. Sjödin, L. Hagmar, E. Klasson-Wehler, J. Bjork and A. Bergman, Environ. Health Perspect., 2000, 108, 1035–1041 Search PubMed.
- L. Asplund, B. G. Svensson, A. Nilsson, U. Eriksson, B. Jansson, S. Jensen, U. Wideqvist and S. Skerfving, Arch. Environ. Health, 1994, 49, 477–486 Search PubMed.
- E. Grimvall, L. Rylander, P. Nilsson-Ehle, U. Nilsson, U. Stromberg, L. Hagmar and C. Ostman, Arch. Environ. Contam. Toxicol., 1997, 32, 329–336 CrossRef CAS.
- R. Kallenborn, I. C. Burkow, M. Schlabach and E. H. Jørgensen, Organohalogen Compd., 1997, 32, 252–256 Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |
