Intercommunity and temporal variation of eleven essential and five toxic elements in human placentas from deliveries in thirteen arctic and sub-arctic areas of Russia and Norway†
J. O.
Odland
*a,
E.
Nieboer
ab,
N.
Romanova
c,
D.
Hofoss
a and
Y.
Thomassen
c
aInstitute of Community Medicine, University of Tromso, N-9037 Tromso, Norway. E-mail: jon.oyvind.odland@ism.uit.no; Fax: + 47 77 64 48 31; Tel: + 47 77 64 48 16
bDepartment of Biochemistry, McMaster University, Hamilton, ON, Canada
cNational Institute of Occupational Health, Oslo, Norway
First published on 17th December 2002
Abstract
Research is described that constitutes an extension of an earlier paper (J. Environ. Monit., 2001, 3, 177–184), in which concentrations were measured in 263 human placentas of 11 essential elements (P, Ca, Mg, Cu, S, Na, Fe, Zn, K, Se, Mn) and 5 toxic elements (Ba, Sr, Pb, Ni, Cd). The additional data considered derive from earlier visits to 4 of the original 6 communities and 3 others, all but one of which are located in northern Norway and neighbouring areas of Russia. This more than doubled the number of placental samples available (263 to 571). Unfortunately, the personal, life-style and morphometric information obtained for the first study group was not available for the additional mothers. Country differences were evident for all elements except Ba, Fe and Zn; Cd, Cu, Mn, Na, Se, Ni, Pb, Sr and S were higher and K, P, Ca and Mg were lower in Russia (p < 0.03). Not unexpectedly, the highest median lead concentration was observed for the largest city in the western arctic region of Russia, namely Murmansk. Similarly, the higher median nickel level observed for Russia reflects the established observation that urinary nickel concentrations are higher in the Russian than in the Norwegian communities. Even though sampling was performed at different times of the year and before and after a 3-year interval in four centres, inter-collection differences were of relatively small magnitude and appear not to be linked to seasonal or temporal changes. Principal component analysis (PCA) confirmed the prominence of Factor 1, which grouped those metals that are known to form insoluble phosphate complexes and whose concentrations showed a dependence on gestational age and maternal smoking in the earlier study. It is concluded that PCA is a powerful statistical tool for exploring and identifying fundamental pathways and processes involved in governing the inorganic elemental composition of placental tissue. It also has the potential of identifying study limitations and quality assurance shortfalls. Further our findings show promise that placental concentrations of toxic elements may serve as an index of exposure and of nutritional intake for selected essential micro-elements.
Introduction and objectives
In an earlier paper, the authors employed factor analysis to group 16 placental elements in a restricted study group from arctic and sub-arctic communities in Norway and Russia.1 It was part of a number of publications which focused on health-related issues such as examining associations between pregnancy outcome and maternal or neonatal body burdens of toxic and essential elements.2–4 Blood lead levels of children living in remote areas of the Kola Peninsula of Russia were also measured.5Placental accumulation of toxic elements as a potential indicator of exposure has been considered since the 1960s,6–8 and extensive information is available on its basic elemental composition.9 However, the scientific value of some studies has been limited by the diversity in tissue sampling and preparation protocols, as well as inherent analytical limitations.6,9,10 Even though improvements in methodology are evident in recent publications,1,6 the use of the element content of this tissue as an indicator of nutritional intake or environmental exposure remains somewhat controversial and perhaps underdeveloped.11
In our previous report,1 we limited our scope to placentas obtained from women for whom personal and life-style particulars were available through the administration of a questionnaire by local midwives and gynecologists. The objective of this paper is to extend our analysis and modelling of 11 essential and 5 toxic elements in placenta to a considerably larger study group (n = 263 to 571), which permits seasonal variation and differences between communities to be explored.
Community and subject recruitment
Personal contacts with colleagues in the different delivery departments were established, and all procedures and protocols were provided in Norwegian, English and Russian. The Russian geographic sites in our study were Nikel, Murmansk, Monchegorsk, Kirovsk, Apatity, and Arkhangelsk (see Fig. 1). Nikel is a community close to the Norwegian–Russian border with primary nickel-refining operations as the main employer. The population has decreased from 23 000 to 15 000 from the time of sampling until today because of reduced activity at the nickel refinery. Murmansk is the biggest city in the western Russian arctic (450 000 inhabitants) and is an important port. Although marine and military activities have been the primary focus, socio-economic and political changes have occurred during the last 10 years. Kirovsk and Apatity are neighbouring towns close to the Khibiny mountains (20 000 and 80 000 inhabitants, respectively), with apatite mining as the main activity. Monchegorsk (65 000 inhabitants) is the biggest nickel refining center in the Kola Peninsula. Arkhangelsk, located south of the Arctic Circle at 64° latitude, is the most populous city on the White Sea, with 419 000 inhabitants.12 The surrounding area features five pulp and paper plants of substantial size, but has no metal producing industry. The Norwegian cities were Kirkenes, Hammerfest and Bergen (Fig. 1). Kirkenes (4500 inhabitants) is located near the Norwegian–Russian border, 50 km from Nikel. The Kirkenes delivery department receives women from the eastern part of Finnmark (total population 28 000), the area geographically adjacent to the Russian border. Hammerfest (12 000 inhabitants) is a coastal city in Finnmark; its hospital delivery department accepts women from the western part of Finnmark (a total of 45 000 people residents), including the main native Saami centres in Finnmark County. Tromsø (60 000 inhabitants) is the biggest city of northern Norway and features the most northern university in the world and bustling marine activity. Bergen (60° latitude) is the second largest Norwegian city in the south-western part of Norway (total population 220 000), with no heavy industry. It was included because it represents a sub-arctic, urban community. | ||
| Fig. 1 Map of the study area indicating the location of the Russian cities (Nikel, Murmansk, Monchegorsk, Kirovsk, Apatity and Arkhangelsk) and those in Norway (Bergen, Hammerfest, Kirkenes and Tromsø). | ||
In most communities, placenta samples were collected from 50 consecutive patients presenting themselves to the hospital delivery departments in each location. The enrolment and sampling were completed in the following time periods (see Table 1): the first sampling from Nikel, Kirkenes, Hammerfest and Bergen in April–September 1991; Kirovsk/Apatity (common delivery department at that time) and Murmansk in September–December 1991; Arkhangelsk in April–May 1993; second sampling from Nikel, Kirkenes, Hammerfest, and Bergen, as well as the Monchegorsk and Tromsø sampling in the period November 1993 to June 1994. The restricted cohort previously studied by the authors1 consisted of the following five communities: Arkhangelsk, Nikel II, Monchegorsk, Bergen II, Hammerfest II, and Kirkenes II (see Table 1). For this subgroup, a questionnaire was completed to obtain morphometric and anamnestic information. The women were asked to join the study by means of completing a consent form. None of the delivering women refused to join the study. The 13 delivering women included for Tromsø were living in Målselv, a small rural community 120 km from the center of Tromsø. The reason for including this group was the measurement there of metal contaminants in water, air, birds, and animals under the auspices of the Arctic Monitoring and Assessment Programme (AMAP). Our study was approved by The Regional Ethical Committee, University of Tromsø, Norway, the Norwegian Data Inspectorate and the Regional Health Administrations of Murmansk and Arkhangelsk Counties.
| ID (N) | Town | Latitude | Population | Sampling time | Main occupation |
|---|---|---|---|---|---|
| A (1) | Arkhangelsk | 64° | 419 000 | April–May 1993 | Pulp and paper, marine |
| K/A (2) | Kirovsk/Apatity | 67° | 20 000/80 000 | September–December 1991 | Apatite mining/production |
| N1 (3) | Nikel I | 69° | 23 000 | April–September 1991 | Nickel refining |
| N2 (4) | Nikel II | 69° | 23 000 | March–June 1994 | Nickel refining |
| Mu (5) | Murmansk | 69° | 450 000 | September–December 1991 | Marine, military |
| Mo (6) | Monchegorsk | 68° | 60 000 | March–June 1994 | Nickel processing and refining |
| B1 (7) | Bergen I | 60° | 220 000 | April–September 1991 | Marine, university, trade |
| B2 (8) | Bergen II | 60° | 220 000 | June 1994 | Marine, university, trade |
| H1 (9) | Hammerfest I | 70° | 12 000 | April–September 1991 | Marine, fisheries |
| H2 (10) | Hammerfest II | 70° | 12 000 | December 1993–January 1994 | Marine, fisheries |
| K1 (11) | Kirkenes I | 69° | 4 500 | April–September 1991 | Mining, trade |
| K2 (12) | Kirkenes II | 69° | 4 500 | November 1993–January 1994 | Mining, trade |
| T (13) | Tromsø | 69° | 60 000 | November 1993 | Marine, fisheries, university, trade |
Materials and methods
Full details for the protocols and methodologies employed have been described previously by the authors, including references.1 An overview is given here.Questionnaire
The questionnaire was administered only to the restricted cohort described in the former paper by local midwives or gynecologists and sought personal and life-style information, which was supplemented by data from the delivery records such as date of birth, gestational age, weight and length of the baby, and weight of placenta. The informed consent form and collection of anamnestic information were completed before the delivery process started in order to minimize maternal stress.Specimen collection and storage
Cord blood and the first neonatal urine void were collected, while maternal blood, serum and urine were obtained 1–2 days after the births. After delivery, the midwife or gynecologist collected the whole placenta using latex or polyethylene gloves and placed it on a clean polyethylene sheet. The cord was detached at the point of entry to the placenta, and gently squeezed to remove excess blood. The placentas were cut with a custom-made titanium knife. Care was taken to collect only trophoblastic (embryonic) tissue, by limiting sampling to a single peri-insertional site. Three cubes of placental tissue, each of 20 cm3, were removed and transferred to containers that tested free of detectable amounts of Cd, Ni, Pb, and Zn. The placenta samples were immediately frozen at −20 °C, and after transport were stored in a −70 °C freezer until further preparation. After thawing, the three tissue portions were homogenized in a miniprocessor in which the original stainless steel rotation knife had been substituted with a titanium knife. The homogenized tissue was divided into two equal portions, which were stored in separate plastic containers at −70 °C until lyophilization prior to analysis.Sample preparation and instrument analysis
Accurately weighed amounts of around 0.5 g of the lyophilized placenta samples were digested with 2.5 ml 65% sub-boiled nitric acid in propylene digestion tubes which, after degasification at room temperature overnight, were heated at 95 °C for 1 h in a laboratory oven. After cooling and appropriate dilution, the Cd, Pb, Ni and Se were measured by electrothermal atomic absorption spectrometry (ETAAS) calibrated with matrix-matched standards under stabilized atomiser conditions using palladium chemical modification.Ba, Ca, Fe, K, Mg, Na, P, S, Cu, Mn, Sr, and Zn in the placenta solutions were measured after 1∶3 dilution with a multielement internal standard solution containing Eu (for Ba, Ca, Cu, Sr, and K), Ni (for Fe, Mg, and P), Cd (for Zn), Li (for Na and P), Pt (for Cu, Mn, and S) and Rh (for Ca, Cu, and Zn) by inductively coupled argon plasma atomic emission spectrometry (ICP-AES) employing calibration with nitric acid-matched standard solutions under standard plasma conditions.
Quality assurance protocols involved assessment of emission line reproducibility (1–3%), recovery of analyte spikes (average of 99.4%), the use of certified and non-certified quality control samples (±10% deviation from the expected/certified values), and day-to-day variations between 2.4 and 3.5% (ICP-EAS) and ±10% (ETAAS).
Statistics
The SPSS statistical package was employed for the Principal Component Analysis (PCA). The factor analysis involved the varimax rotation technique.1 Through a linear combination of the original variables (i.e., elemental contents in the present case), it creates new ones (referred to as factors or axes) that may help in the interpretation of the results. For the ANOVA, Epi Info 6, Version 6.04a, July 1996 (World Health Organization Geneva, Switzerland) was used. The non-parametric Wilcoxon rank sum test was employed for those elements with skewed frequency distributions. Concentrations below the detection limit (DL) were arbitrarly assigned the value of 1/2 the DL.Results
Frequency distribution
Mean concentrations (± standard deviation, s) for the elements exhibiting normal frequency distributions are presented in Tables 2 and 4. In both these tables, the levels are compared to those for the first Kirkenes collection to explore geographical and seasonal differences. It was selected because the study was initiated there. In addition in Table 4, the data for two separate collections are compared for the Norwegian communities of Bergen, Hammerfest and Kirkenes, and for Nikel in Russia. Comparable compilations are provided for those elements demonstrating skewed distribution patterns in Tables 3 and 5, for which medians and ranges are reported. The division of the elements by frequency distribution has not changed from that reported for the restricted group.1| Elements/units (dry weight) | Total (n = 571) | Russia (n = 249) | Norway (n = 322) | A (n = 50) | K/A (n = 41) | Mu (n =45) | Mo (n = 25) | K1a (n = 50) | T (n = 13) |
|---|---|---|---|---|---|---|---|---|---|
| Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | |
| a One “nonsense” value of 55.8 µg g−1 was excluded for copper. b Russia–Norway. c A–K1. d K/A–K1. e Mu–K1. f Mo–K1; two-tailed t-test; the Tromsø data are too few for statistical comparisons. | |||||||||
| Cd/µg g−1 | 0.04 (0.02) | 0.04 (0.02) | 0.03 (0.02) p < 0.001b | 0.03 (0.02) p = 0.28c | 0.05 (0.02) p < 0.001d | 0.05 (0.02) p = 0.002e | 0.04 (0.02) p = 0.29f | 0.04 (0.02) | 0.04 (0.01) |
| Cu/µg g−1 | 5.62 (0.95) | 5.92 (0.89) | 5.39 (0.92) p < 0.001b | 6.01 (0.89) p = 0.016c | 6.51 (0.87) p = 0.99d | 6.12 (0.71) p = 0.52e | 5.93 (0.64) p = 0.02f | 6.51 (1.14) | 5.11 (0.36) |
| Fe/mg g−1 | 0.65 (0.15) | 0.64 (0.15) | 0.65 (0.15) p = 0.56b | 0.55 (0.14) p = 0.67c | 0.67 (0.13) p < 0.001d | 0.70 (0.16) p < 0.001e | 0.57 (0.11) p = 0.85f | 0.56 (0.16) | 0.68 (0.07) |
| K/mg g−1 | 10.28 (1.01) | 10.12 (0.98) | 10.41 (1.02) p < 0.001b | 9.88 (1.09) p = 0.88c | 10.74 (0.79) p < 0.001d | 9.97 (0.71) p = 0.81e | 9.84 (1.09) p = 0.81f | 9.92 (1.29) | 10.63 (0.45) |
| Mn/µg g−1 | 0.22 (0.12) | 0.26 (0.13) | 0.19 (0.09) p < 0.001b | 0.28 (0.16) p < 0.001c | 0.28 (0.11) p < 0.001d | 0.29 (0.14) p < 0.001e | 0.28 (0.13) p < 0.001f | 0.18 (0.09) | 0.21 (0.06) |
| Na/mg g−1 | 10.41 (1.16) | 10.52 (1.19) | 10.31 (1.12) p = 0.03b | 10.80 (1.33) p = 0.06c | 10.60 (0.93) p = 0.25d | 10.30 (0.96) p = 0.50e | 10.28 (1.35) p = 0.75f | 10.37 (0.97) | 10.14 (0.81) |
| S/mg g−1 | 7.25 (0.46) | 7.33 (0.49) | 7.19 (0.44) p < 0.001b | 7.47 (0.50) p = 0.002c | 7.49 (0.53) p = 0.002d | 7.31 (0.37) p = 0.07e | 7.16 (0.58) p = 0.88f | 7.14 (0.53) | 7.31 (0.18) |
| Se/µg g−1 | 1.05 (0.16) | 1.07 (0.19) | 1.03 (0.14) p = 0.02b | 0.92 (0.09) p < 0.001c | 1.28 (0.17) p < 0.001d | 1.17 (0.13) p = 0.001e | 1.14 (0.11) p = 0.88f | 1.09 (0.12) | 1.28 (0.07) |
| Zn/µg g−1 | 57.17 (11.53) | 57.10 (14.07) | 57.24 (9.13) p = 0.89b | 71.84 (22.72) p < 0.001c | 53.07 (5.02) p = 0.43d | 55.12 (8.76) p = 0.63e | 54.08 (7.42) p = 0.76f | 54.27 (8.54) | 57.89 (5.25) |
| Elements/units (dry weight) | Total (n = 571) | Russia (n = 249) | Norway (n = 322) | A (n = 50) | K/A (n = 41) | Mu (n = 45) | Mo (n = 25) | K1 (n = 51) | T (n = 13) |
|---|---|---|---|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | |
| a Russia–Norway. b A–K1. c K/A–K1. d Mu–K1. e Mo–K1; Wilcoxon two-sample test; the Tromsø data are too few for statistical comparisons. | |||||||||
| Ba/µg g−1 | 0.04 (0.002–2.77) | 0.04 (0.002–2.77) | 0.04 (0.003–1.36) p = 0.50a | 0.06 (0.002–1.11) p = 0.19b | 0.02 (0.02–0.29) p = 0.98c | 0.08 (0.02–2.77) p = 0.02d | 0.03 (0.02–0.49) p = 0.50e | 0.02 (0.02–0.95) | 0.07 (0.02–0.45) |
| Ca/mg g−1 | 3.53 (0.67–54.50) | 2.82 (0.67–49.95) | 4.08 (0.67–54.50) p = 0.02a | 2.65 (0.67–49.95) p = 0.45b | 1.70 (0.78–19.71) p = 0.08c | 2.91 (0.74–39.82) p = 0.77d | 4.39 (0.69–25.63) p = 0.97e | 3.63 (0.68–54.50) | 4.66 (2.27–19.08) |
| Mg/mg g−1 | 0.48 (0.29–1.31) | 0.47 (0.29–1.20) | 0.49 (0.30–1.31) p = 0.007a | 0.46 (0.36–1.20) p = 0.41b | 0.48 (0.39–0.71) p = 0.89c | 0.48 (0.34–1.11) p = 0.35d | 0.48 (0.37–0.90) p = 0.48e | 0.47 (0.32–1.31) | 0.51 (0.43–0.74) |
| Ni/µg g−1 | 0.02 (0.004–0.38) | 0.02 (0.005–0.28) | 0.01 (0.004–0.38) p < 0.001a | 0.03 (0.005–0.12) p = 0.004b | 0.03 (0.005–0.16) p = 0.04c | 0.03 (0.01–0.06) p = 0.005d | 0.02 (0.01–0.07) p = 0.54e | 0.02 (0.004–0.30) | 0.02 (0.004–0.03) |
| Pb/µg g−1 | 0.09 (0.03–2.60) | 0.11 (0.03–2.60) | 0.07 (0.03–2.39) p < 0.001a | 0.11 (0.05–0.57) p < 0.001b | 0.11 (0.05–0.47) p < 0.001c | 0.17 (0.08–2.60) p < 0.001d | 0.12 (0.04–0.31) p < 0.001e | 0.07 (0.05–0.66) | 0.09 (0.06–0.13) |
| P/mg g−1 | 8.92 (4.54–31.71) | 8.59 (4.54–30.33) | 9.40 (5.50–31.71) p = 0.002a | 8.69 (5.88–30.33) p = 0.36b | 8.51 (6.54–16.24) p = 0.36c | 8.98 (5.94–25.45) p = 0.99d | 8.59 (6.64–19.23) p = 0.97e | 8.81 (5.50–31.71) | 9.55 (8.15–16.46) |
| Sr/µg g−1 | 0.95 (0.11–19.53) | 1.06 (0.22–17.39) | 0.84 (0.11–19.53) p = 0.002a | 1.450 (0.38–17.39) p < 0.001b | 0.93 (0.26–6.86) p = 0.43c | 1.03 (0.22–14.28) p = 0.10d | 1.32 (0.22–9.48) p = 0.14e | 0.70 (0.11–19.53) | 0.92 (0.56–4.00) |
| Elements/units (dry weight) | N1 (n = 50) | N2 (n = 38) | B1 (n = 50) | B2 (n = 50) | H1 (n = 58) | H2 (n = 50) | K1b (n = 51) | K2 (n = 50) |
|---|---|---|---|---|---|---|---|---|
| Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | Mean (s) | |
| a The first set of p-values refer to the comparison between the first and second collection in the same community; the second set of p-values for the comparison with the K1 mean; two-tailed t-test. b One “nonsense” value of 55.8 µg g−1 was excluded for copper. | ||||||||
| Cd/µg g−1 | 0.04 (0.02) p = 0.10 | 0.04 (0.02) p = 0.30; p = 0.62 | 0.04 (0.03) p = 0.87 | 0.03 (0.02) p = 0.17; p = 0.09 | 0.03 (0.01) p = 0.19 | 0.03 (0.01) p = 0.08; p = 0.006 | 0.04 (0.02) | 0.03 (0.01) p = 0.13 |
| Cu/µg g−1 | 5.82 (0.94) p = 0.001 | 5.04 (0.42) p < 0.001; p < 0.001 | 4.98 (0.74) p < 0.001 | 5.47 (0.77) p = 0.002; p < 0.001 | 5.45 (0.62) p < 0.001 | 4.90 (0.74) p < 0.001; p < 0.001 | 6.51 (1.14) | 5.06 (0.48) p < 0.001 |
| Fe/mg g−1 | 0.69 (0.15) p < 0.001 | 0.65 (0.13) p = 0.30; p = 0.003 | 0.75 (0.12) p < 0.001 | 0.67 (0.14) p = 0.004; p < 0.001 | 0.66 (0.16) p = 0.001 | 0.62 (0.15) p = 0.15; p = 0.08 | 0.56 (0.16) | 0.64 (0.13) p = 0.007 |
| K/mg g−1 | 9.96 (1.02) p = 0.84 | 10.31 (0.90) p = 0.10; p = 0.11 | 10.24 (0.91) p = 0.15 | 10.76 (0.71) p = 0.002; p < 0.001 | 10.23 (0.84) p = 0.12 | 10.60 (1.21) p = 0.07; p = 0.007 | 9.92 (1.29) | 10.67 (0.92) p = 0.001 |
| Mn/µg g−1 | 0.23 (0.11) p = 0.02 | 0.21 (0.13) p = 0.45; p = 0.19 | 0.24 (0.09) p = 0.002 | 0.20 (0.08) p = 0.04; p = 0.16 | 0.20 (0.10) p = 0.16 | 0.15 (0.10) p = 0.02; p = 0.22 | 0.18 (0.09) | 0.14 (0.07) p = 0.01 |
| Na/mg g−1 | 10.44 (1.24) p = 0.75 | 10.71 (1.29) p = 0.33; p = 0.16 | 9.92 (0.96) p = 0.02 | 10.32 (0.87) p = 0.03; p = 0.81 | 10.36 (1.18) p = 0.97 | 10.40 (1.50) p = 0.86; p = 0.88 | 10.37 (0.97) | 10.56 (1.16) p = 0.37 |
| S/mg g−1 | 7.18 (0.48) p = 0.72 | 7.34 (0.39) p = 0.10; p = 0.06 | 7.10 (0.50) p = 0.70 | 7.25 (0.30) p = 0.08; p = 0.21 | 7.20 (0.41) p = 0.51 | 7.15 (0.56) p = 0.56; p = 0.96 | 7.14 (0.53) | 7.27 (0.28) p = 0.13 |
| Se/µg g−1 | 1.01 (0.14) p = 0.002 | 0.93 (0.11) p = 0.004; p < 0.001 | 0.99 (0.16) p < 0.001 | 1.00 (0.10) p = 0.73; p < 0.001 | 1.08 (0.12) p = 0.86 | 1.02 (0.12) p = 0.007; p = 0.004 | 1.09 (0.12) | 0.96 (0.09) p < 0.001 |
| Zn/µg g−1 | 51.70 (6.11) p = 0.09 | 52.80 (7.56) p = 0.46; p = 0.40 | 54.24 (9.39) p = 0.99 | 55.20 (7.90) p = 0.58; p = 0.57 | 59.63 (8.96) p = 0.002 | 58.66 (9.21) p = 0.58) p = 0.01 | 54.27 (8.54) | 60.92 (9.52) p < 0.001 |
| Elements/units (dry weight) | N1 (n = 50) | N2 (n = 38) | B1 (n = 50) | B2 (n = 50) | H1 (n = 58) | H2 (n = 50) | K1 (n = 51) | K2 (n = 50) |
|---|---|---|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | |
| a The first set of p-values refer to the comparison between the first and second collections in the same community; the second set of p-values is for the comparison with the K1 mean; Wilcoxon two-sample test. | ||||||||
| Ba/µg g−1 | 0.02 (0.02–0.86) p = 0.51 | 0.05 (0.02–0.70) p = 0.04; p = 0.18 | 0.04 (0.02–0.94) p = 0.06 | 0.03 (0.02–1.36) p = 0.25; p = 0.33 | 0.04 (0.02–1.00) p = 0.42 | 0.06 (0.003–0.73) p = 0.19; p = 0.05 | 0.02 (0.02–0.95) | 0.04 (0.02–1.26) p = 0.13 |
| Ca/mg g−1 | 2.08 (0.69–36.02) p = 0.07 | 3.75 (0.97–27.76) p = 0.01; p = 0.78 | 4.41 (0.68–50.48) p = 0.80 | 2.89 (0.80–45.47) p = 0.15; p = 0.32 | 4.76 (0.67–36.38) p = 0.23 | 4.32 (0.89–29.67) p = 0.75; p = 0.24 | 3.63 (0.68–54.50) | 3.55 (0.90–46.14) p = 0.60 |
| Mg/mg g−1 | 0.45 (0.31–0.96) p = 0.15 | 0.47 (0.29–0.88) p = 0.35; p = 0.84 | 0.48 (0.34–1.30) p = 0.27 | 0.46 (0.37–1.13) p = 0.15; p = 0.91 | 0.52 (0.36–1.04) p = 0.04 | 0.50 (0.30–0.98) p = 0.37; p = 0.29 | 0.47 (0.32–1.31) | 0.49 (0.35–1.16) p = 0.24 |
| Ni/µg g−1 | 0.01 (0.01–0.28) p = 0.43 | 0.02 (0.009–0.10) p = 0.08; p = 0.69 | 0.01 (0.004–0.38) p = 0.49 | 0.01 (0.004–0.24) p = 0.98; p = 0.32 | 0.02 (0.004–0.12) p = 0.26 | 0.01 (0.004–0.10) p = 0.003; p = 0.18 | 0.02 (0.004–0.30) | 0.004 (0.004–0.09) p = 0.004 |
| Pb/µg g−1 | 0.12 (0.03–1.44) p < 0.001 | 0.09 (0.05–0.34) p = 0.048; p = 0.01 | 0.07 (0.04–0.53) p = 0.64 | 0.07 (0.04–2.39) p = 0.32; p = 0.46 | 0.09 (0.06–0.25) p = 0.002 | 0.06 (0.03–0.15) p < 0.001; p = 0.002 | 0.07 (0.05–0.66) | 0.06 (0.04–0.26) p = 0.02 |
| P/mg g−1 | 8.25 (5.42–21.85) p = 0.04 | 8.91 (4.54–19.87) p = 0.087; p = 0.74 | 9.03 (6.34–29.23) p = 0.29 | 9.16 (6.84–29.65) p = 0.32; p = 0.78 | 9.80 (6.18–23.66) p = 0.20 | 9.65 (6.01–21.92) p = 0.95; p = 0.26 | 8.81 (5.50–31.71) | 9.57 (6.35–27.15) p = 0.30 |
| Sr/µg g−1 | 0.66 (0.22–14.08) p = 0.74 | 1.23 (0.31–9.60) p = 0.008; p = 0.009 | 0.78 (0.14–7.44) p = 0.89 | 0.75 (0.14–9.87) p = 0.87; p = 0.94 | 0.99 (0.16–8.08) p = 0.23 | 0.99 (0.15–6.34) p = 0.98; p = 0.12 | 0.70 (0.11–19.53) | 0.81 (0.17–7.06) p = 0.48 |
Geographical variations by country and community
In addition to the results summarized in Tables 2 to 5, concentration means, medians and confidence intervals are graphically presented for all 13 communities in Fig. 2 for cadmium, iron and selenium. The corresponding plots for the remaining 13 elements are available as electronic supplemetary information (ESI)† to this article. On average, country differences are evident for all elements except Ba, Fe and Zn, with Cd, Cu, Mn, Na, Se, Ni, Pb, Sr and S being higher, or marginally so, in Russia and K, P, Ca and Mg lower or somewhat less there (p ≤ 0.03; see Tables 2 and 3). This trend is generally corroborated by inspection of the individual community results relative to concentrations for the first Kirkenes collection (see Tables 2 to 5) and of the plots in Fig. 2 (also see ESI†). Cd concentrations seem marginally higher for Kirovsk/Apatity and Murmansk samples (p < 0.002); Pb for Murmansk (p < 0.001); and Sr for Arkhangelsk (p < 0.001). Collectively, the findings may have been complicated by some obvious outliers: Zn and Sr (Arkhangelsk), Pb (Bergen II, Kirkenes I), Ni (Bergen I, Kirkenes I), and Cu (Kirkenes I).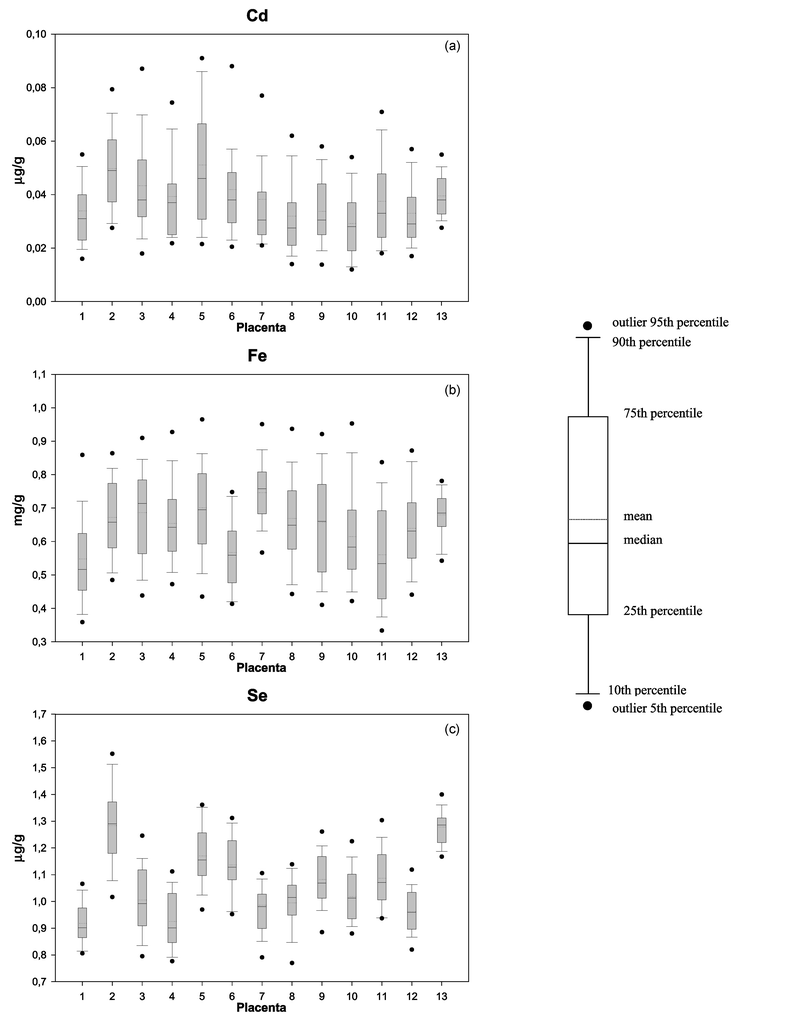 | ||
| Fig. 2 Graphical presentation of the observed concentrations of cadmium (a), iron (b) and selenium (c) in placentas from all 13 collections/communities. Town 1: Arkhangelsk (n = 50); Town 2: Kirovsk/Apatity (n = 41); Town 3: Nikel I (n = 50); Town 4: Nikel II (n = 38); Town 5: Murmansk (n = 45); Town 6: Monchegorsk (n = 25); Town 7: Bergen I (n = 50); Town 8: Bergen II (n = 50); Town 9: Hammerfest I (n = 58); Town 10: Hammerfest II (n = 50); Town 11: Kirkenes I (n = 51); Town 12: Kirkenes II (n = 50); Town 13: Tromsø (n = 13). Comparable plots are available for the other 13 elements as ESI†. All data points are plotted in the figures, including those that might be considered outliers. | ||
Seasonal and temporal variation
As shown in Table 1, sampling was performed at different times of the year, as well as before and after almost a 3-year interval for the communities of Nikel (Russia), Bergen, Hammerfest and Kirkenes (Norway). The results are summarized in Tables 4 and 5 (also see Fig. 2). The most consistent changes for the 1991–1994 interval were decreases in Cu (Nikel, Hammerfest, Kirkenes), Mn (Bergen, Hammerfest, Kirkenes) and perhaps Se, Ni and Pb (Hammerfest and Kirkenes), and an increase in K (Bergen, Hammerfest and Kirkenes). Because of the prolonged interval between the first and second collections, it is difficult to link these trends to changes in season. Even though there are some statistically significant (p ≤ 0.05) inter-collection differences, these are relatively small in magnitude.Interelemental correlation
The interelemental correlations (Table 6) for P, Ca, Mg, Sr, Ba, Ni and Zn are again the strongest (r = 0.40–0.98), with those for Mn a little weaker in this grouping (r = 0.2–0.3). The results are very similar to those for the restricted group of our study.1 The only major change is for Pb, for which the interelemental correlation coefficients with P, Mg, Ca, Sr, Ba and Ni remain significant (p < 0.001), even though they have been reduced in magnitude by about a factor of 3 (i.e.r around 0.2 rather than 0.6). As before,1 the interactions for Fe were negative except for its association with K.| P | Mg | Ca | Sr | Ba | Na | K | Mn | Fe | Ni | Cu | Zn | Cd | Pb | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a p ≤ 0.001. b p ≤ 0.005. c p ≤ 0.01. d p ≤ 0.025. | |||||||||||||||
| Mg | 0.98a | ||||||||||||||
| Ca | 0.97a | 0.94a | |||||||||||||
| Sr | 0.85a | 0.82a | 0.88a | ||||||||||||
| Ba | 0.76a | 0.73a | 0.77a | 0.74a | |||||||||||
| Na | −0.01 | 0.06 | −0.07 | −0.03 | −0.06 | ||||||||||
| K | −0.15 | −0.09 | −0.33a | −0.33a | −0.24a | 0.33a | |||||||||
| Mn | 0.26a | 0.29a | 0.21a | 0.32a | 0.20a | 0.02 | 0.04 | ||||||||
| Fe | −0.25a | −0.26a | −0.22a | −0.22a | −0.13b | −0.16a | 0.18a | −0.18a | |||||||
| Ni | 0.54a | 0.54a | 0.58a | 0.48a | 0.45a | −0.04 | −0.30a | 0.14a | −0.18a | ||||||
| Cu | −0.11d | −0.10 | −0.10d | −0.07 | −0.07 | 0.04 | 0.01 | 0.01 | −0.02 | 0.01 | |||||
| Zn | 0.57a | 0.59a | 0.52a | 0.50a | 0.40a | 0.18a | 0.04 | 0.28a | −0.31a | 0.29a | 0.05 | ||||
| Cd | −0.03 | −0.02 | −0.05 | −0.01 | −0.03 | −0.04 | 0.02 | 0.29a | −0.04 | 0.00 | 0.19a | −0.04 | |||
| Pb | 0.17a | 0.16a | 0.18a | 0.22a | 0.19a | −0.07 | −0.11c | 0.17a | −0.05 | 0.22a | 0.04 | 0.05 | 0.15 | ||
| S | 0.03 | 0.05 | −0.07 | 0.02 | −0.04 | 0.34a | 0.42a | 0.23a | −0.02 | −0.06 | 0.07 | 0.22a | 0.04 | −0.03 | |
| Se | −0.01 | 0.03 | −0.07 | −0.10d | −0.07 | 0.11d | 0.21a | 0.23a | −0.07 | 0.02 | 0.09 | −0.04 | 0.23a | −0.04 | 0.30a |
Factor analysis
The principal component (PCA) analysis of the full study group and for the Norwegian and Russian communities separately are presented in Table 7. Between 32.5 and 36.2% of the total variance is explained by Factor 1, with the elements P, Ca, Mg, Sr, Ba, Zn, Ni, Mn and Pb (in decreasing order) significantly related to the loading. Factor 2 explains 12.1 to 13.3% of the variance; Factor 3, 8.1 to 11.5%; and Factor 4, 7.5 to 9.0%. The cumulative variance explained by the four axes is 63.4 to 65.6% and is thus nearly independent of whether all the communities are considered, or only the Russian or Norwegian ones. Factor analysis of the elemental compositions for individual communities differ considerably independent of country (data not shown), with the loadings reflecting either the combined, Russian or Norwegian pattern reported in Table 7. In case of Factor 2, a difference occurs for the loading of Cu (which is considerable for Russia, but not Norway) and perhaps Mn (stronger presence in the Norway set). The other elements that load highly on Factor 2 are S, Na, Zn, K and Se. For Russia and the combined set, Factor 3 is dominated by Cd, Mn and Se, with Pb and Cu also related. By contrast, for Norway the strong negative weighting of Axis 3 for Fe, and the positive Na association and lack of loading by Cd and Mn are idiosyncratic. Cd and Mn dominate Factor 4 for the Norwegian group, with Cu (and perhaps Pb) having a smaller positive relation. By contrast, in case of the Russian set and the combined data, iron positively dominates Axis 4; a positive Pb association and negative loading from Zn (and perhaps Cu; combined set only) also occur. A unique feature at the community level was that only for Monchegorsk and the second collection in Nikel did nickel relate in a major way to both Factors 1 and 4.| Element | Factor 1 | Factor 1 | Factor 1 | Factor 2 | Factor 2 | Factor 2 | Factor 3 | Factor 3 | Factor 3 | Factor 4 | Factor 4 | Factor 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Rotation converged in 8 iterations (combined set, n = 571), 6 iterations (Russia, n = 249) and 7 iterations (Norway, n = 322). Bold type denotes major contribution, arbitrarily set at ≥ 0.29 or ≤ −0.29. | ||||||||||||
| Russia | Norway | Combined | Russia | Norway | Combined | Russia | Norway | Combined | Russia | Norway | Combined | |
| P | 0.97 | 0.98 | 0.97 | — | — | — | — | — | — | — | — | — |
| Ca | 0.97 | 0.97 | 0.96 | −0.12 | −0.16 | −0.14 | — | — | — | — | — | — |
| Mg | 0.95 | 0.97 | 0.95 | — | — | — | — | — | — | — | — | — |
| Ba | 0.81 | 0.86 | 0.83 | — | −0.13 | −0.10 | — | — | — | — | — | — |
| Sr | 0.94 | 0.92 | 0.90 | — | −0.15 | — | — | — | — | −0.12 | — | — |
| Pb | 0.32 | 0.22 | 0.25 | — | −0.24 | −0.14 | 0.31 | −0.11 | 0.39 | 0.46 | 0.26 | 0.43 |
| Ni | 0.56 | 0.64 | 0.62 | — | −0.28 | −0.22 | — | 0.20 | 0.12 | — | – | – |
| Cu | −0.20 | −0.17 | −0.15 | 0.45 | −0.21 | −0.12 | 0.45 | 0.32 | 0.46 | — | 0.46 | −0.29 |
| S | — | — | — | 0.76 | 0.77 | 0.77 | 0.16 | 0.17 | 0.16 | — | — | — |
| Na | — | — | — | 0.66 | 0.60 | 0.59 | −0.20 | 0.30 | — | −0.22 | −0.17 | −0.38 |
| Fe | −0.14 | −0.29 | −0.26 | — | — | — | −0.10 | −0.75 | −0.15 | 0.85 | — | 0.80 |
| Zn | 0.47 | 0.81 | 0.64 | 0.23 | 0.34 | 0.29 | — | — | — | −0.51 | — | −0.29 |
| K | −0.16 | −0.27 | −0.24 | 0.75 | 0.77 | 0.77 | 0.16 | −0.27 | — | 0.18 | — | 0.21 |
| Se | — | — | −0.02 | 0.29 | 0.34 | 0.37 | 0.66 | 0.62 | 0.53 | 0.18 | 0.12 | — |
| Cd | — | — | — | −0.12 | — | — | 0.79 | — | 0.75 | — | 0.79 | — |
| Mn | 0.39 | 0.29 | 0.33 | 0.11 | 0.31 | 0.24 | 0.60 | — | 0.60 | −0.28 | 0.57 | — |
| Variance (%) | 32.48 | 36.24 | 33.58 | 12.40 | 13.25 | 12.11 | 11.50 | 8.14 | 10.24 | 8.96 | 8.00 | 7.45 |
| Cumulative variance (%) | 32.48 | 36.24 | 33.58 | 44.88 | 49.49 | 45.69 | 56.38 | 57.63 | 55.93 | 65.34 | 65.64 | 63.38 |
Discussion
Geographical and seasonal variation
The observation that on average the concentrations of the macro-nutrients Mg, Ca and P were higher in Norway is consistent with their association in the restricted cohort1 with smoking. Based on a questionnaire survey, the frequency of cigarette smoking among the Norwegian mothers was 36.2%, compared to 23.1% in Russia.3 This suggests that the placental levels of Mg, Ca and P may be a more sensitive index of smoking than the actual tissue Cd concentration, which was similar for the two countries in the earlier study (p = 0.08) but somewhat higher in Russia for the expanded cohort (p < 0.001; Table 2). The relatively higher Cd levels observed for Kirovsk/Apatity and Murmansk may reflect special point sources such as large coal-burning heating plants; alternatively, smoking frequencies might have been different compared to other communities.An intriguing issue is the relatively elevated placental levels of nickel in the Russian group. At first glance, one might assign this to the presence of nickel refineries at Nikel and Monchegorsk. Indeed, Ni makes a unique contribution to Factor 4 only for these two communities. However, we2 and others13 have consistently shown that Russian subjects on average have higher levels of urinary nickel than individuals living in comparable communities in Norway. This finding is independent of nickel refineries as a point source, which do contribute. As yet, we have not found a good explanation for this phenomenon.
Higher tissue concentrations of Pb in the Russian group is consistent with the more extensive use of gasoline with lead additives there during the 1991–1994 period compared to Norway. Since Murmansk is the largest city in the western arctic region of Russia, and is larger than all communities surveyed, it is not surprising that the highest average lead concentration was observed there.
Selenium tissue levels were quite comparable in the Russian and Norwegian groups, which is consistent with the observation for the restricted cohort3 of similar serum selenium concentrations.
Except for a small number of isolated and random differences between the first and second collections for Kirkenes (Cu, Ni and Zn) and for Nikel and Bergen (Ca), temporal dependences of the elements examined were not strong. In the absence of more systematic trends and questionnaire information for the first collection, these are difficult to interpret. Nevertheless, the trend to lower lead levels in the placenta collected in 1994 compared to 1991 for the towns of Nikel (p ≤ 0.05), Hammerfest (p < 0.001), and Kirkenes (p < 0.02) is consistent with a more drastic apparent decrease in aerial deposition of lead in the northern communities than for the southern city of Bergen.14
Grouping of the elements
As previously explained,1 the grouping of factors by PCA is essentially a statistical device, facilitating interpretations by inference. It is a recognized statistical tool in other fields of research and is employed in the systematic grouping of large data sets with many variables.15The additional data clearly confirm the prominence of Factor 1 and the corresponding loading dominance by those elements that exhibit skewed concentration frequency distributions. As documented for the restricted cohort,1 the placental concentrations for members of the Factor 1 group exhibited dependences on gestational age and maternal smoking. These observations were interpreted to reflect interstitial mineralization (likely as insoluble phosphate complexes) due to the calcification of smoke-related necrotic placental tissue.1
Although the positive involvement of Fe and Pb in Factor 3 or 4 constitutes a departure from the earlier study,1 as before the loadings for Factors 2, 3 and 4 involve a limited number of macronutrients, the essential micronutrients, and the toxic metal Cd. Factor 2 is dominated by the macro-elements S, Na, and K. Formerly,1 they were assigned primarily to Axes 2 or 3, or to both. The micro-nutrients Cu and Se have input to Factor 3 that is independent of country; earlier1 this pairing occurred for Axis 4. Cd and Mn fell on Axis 4 previously,1 while here they contribute to either Axis 3 (Russia and combined data) or Axis 4 (Norway). Clearly relative to our earlier results,1 the more comprehensive PCA analysis summarized in Table 7 demonstrates that axes which explain relatively small percentages of the variance are subject to loading instability and cross-over. This seems to apply to Axes 2, 3 and 4. A macro-element involved in this vacillation appears to be iron. It makes a strong positive contribution to Factor 4 for Russia and the combined data, while negative loadings occur for Norway and the restricted cohort.1 Examining the PCA analysis results for individual communities indicates that Fe has major positive inputs to Axis 3 for Hammerfest I and Kirkenes II and to Axis 4 for Arkangelsk, Nikel I and Murmansk. As postulated in the preceeding paper,1 the most obvious interpretation is that iron serves as a signature element for the presence of residual blood in the intervillous space or fetal blood vessels. Relative to the observed overall placental concentration of 0.65 mg g−1 (Table 2), iron levels in whole blood are of comparable magnitude.16 This suggests the possibility of inadequate removal of blood from the placentas in the mentioned collections prior to sampling or that differential inclusion of intervillous space and fetal blood vessels occurred. The former is perfused with maternal blood and the latter with fetal blood.
The interelemental correlation coefficients for Fe summarized in Table 6 provide some support for the above interpretation. As before,1 Fe is negatively correlated with all elements except K. The latter element may be considered an intracellular marker. The pairing of Fe and Pb may also be indicative of the presence of excess blood in some of the placental tissues sampled, as Pb resides in the red blood cells with very little occurring in serum/plasma.17
The dominance of Factor 1 clearly reaffirms the power of PCA in the modelling and understanding of the fundamental biological/pathological processes that appear to regulate placental composition of essential and toxic elements. It permits a more sophisticated analysis of interelemental correlations by seeking out elements that fall into groups and thus have something in common. However as the above discussion illustrates, for minor axes that only explain around 10% of the variance interpretations are less straight forward. An examination of PCA output indicates that in this situation the assignment of elements to specific factors is strongly dependent on the number of axes considered in the modelling. Nevertheless, the iron issue suggests that more consistent sampling protocols may well reduce the uncertainty in axis assignment. Consequently, improved quality assurance and standardization of collection and sampling protocols may well afford additional insight through PCA.
Concluding remarks
The study revealed country differences for all elements except Ba, Fe and Zn. The enhanced placental levels of nickel in the Russian group are consistent with previous reports that, independent of nickel refineries as a point source, Russian subjects have higher concentrations of urinary nickel than individuals living in comparable communities in Norway. Higher tissue concentrations of Pb in the Russian group appear to reflect the use of lead additives in gasoline during the 1991–1994 period, while the marginally higher Cd levels observed in some of the Russian communities may reflect special point sources such as large coal-burning heating plants, although differential smoking frequencies cannot be ruled out.Even though sampling was performed at different times of the year and before and after a 3-year interval in four centres, inter-collection differences were of relatively small magnitude and appear not to be linked to seasonal or temporal changes.
The PCA modelling clearly permits a reduction in the number of variables. In the present application, four new variables represented the original 16 elements and explained 65.6% of the variability. We believe we have demonstrated that PCA is a powerful tool for exploring and identifying fundamental processes and pathways involved in governing the inorganic elemental composition of placental tissue. However, the introduction of inadvertent and systematic errors must be minimized for this to succeed, since the mathematical construction of the new variables appear to be sensitive to such input. To optimize the application of PCA, strict quality assurance measures must be implemented that specify “best” practices for the collection and handling of placenta; the isolation and sampling of trophoblastic tissue; its storage and transport; and perhaps tissue pre-treatment and quality control in the analytical steps. Our earlier study1 has demonstrated the relevance and necessity of collecting personal, morphometric and life-style particulars in order to conduct the appropriate statistical analyses.
Our findings also show promise that placental concentrations of toxic elements may serve as an index of exposure and of nutritional intake for selected essential micro-elements.
Acknowledgements
This work has been supported by the University of Tromsø, Steering Group of Medical Research in Finnmark and Nordland, the Royal Norwegian Department of Foreign Affairs, East-European Secretariate, the Barents Secretariate, and the Norwegian State Pollution Control. The authors wish to thank the staff at the delivery departments of Nikel Hospital, Murmansk Regional Hospital, Monchegorsk Hospital, Apatity Hospital, Arkhangelsk Regional Hospital, Kirkenes Hospital, Hammerfest Hospital, the Regional University Hospital of Tromsø, and Haukeland University Hospital, Bergen for excellent cooperation. Aknowledgement is also extended to Knut Dalaker, Kåre Augensen, Babill Stray-Pedersen, Alexander Duriagin, Elvira Khotova, Leonid Zhivakov, Irina Perminova, Gunhild Sand, Per Einar Fiskebeck, and Marie Hallonen for their generous support in different phases of the project.References
- J. O. Odland, E. Nieboer, N. Romanova, Y. Thomassen, D. Hofoss and E. Lund, J. Environ. Monit., 2001, 3, 177 RSC.
- J. O. Odland, E. Nieboer, N. Romanova, Y. Thomassen, T. Norseth and E. Lund, J. Environ. Monit., 1999, 1, 153 RSC.
- J. O. Odland, E. Nieboer, N. Romanova, Y. Thomassen, J. Brox and E. Lund, Acta Obstet. Gynecol. Scand., 1999, 78, 605 CrossRef CAS.
- J. O. Odland, E. Nieboer, N. Romanova, Y. Thomassen and E. Lund, Acta Obstet. Gynecol. Scand., 1999, 78, 852 CrossRef CAS.
- J. O. Odland, I. Perminova, N. Romanova, Y. Thomassen, L. J. S. Tsuji, J. Brox and E. Nieboer, Ecosyst. Health, 1999, 5, 75 CrossRef.
- G. V. Iyengar and A. Rapp, Sci. Total Environ., 2001, 280, 195 CrossRef CAS.
- G. V. Iyengar and A. Rapp, Sci. Total Environ., 2001, 280, 207 CrossRef CAS.
- G. V. Iyengar and A. Rapp, Sci. Total Environ., 2001, 280, 221 CrossRef CAS.
- K. S. Subramanian and I. Y. Iyengar, ACS Symp. Ser., 1997, 654, 115 Search PubMed.
- E. A. Manci and W. R. Blackburn, Placenta, 1987, 8, 497 CAS.
- B. J. Lagerkvist, S. Sandberg, W. Frech, T. Jin and G. F. Nordberg, Arch. Environ. Health, 1996, 51, 389 Search PubMed.
- J. C. Hansen, A. Gilman, V. Klopov and J. O. Odland, AMAP Assessment Report: Arctic Pollution Issues, Arctic Monitoring and Assessment Programme (AMAP), Oslo, 1998, ch.12, pp. 775–844 Search PubMed.
- T. Smith-Sivertsen, V. Tchachtchine, E. Lund, V. Bykov, Y. Thomassen and T. Norseth, Environ. Health Perspect., 1998, 106, 503 Search PubMed.
- AMAP Assessment Report: Arctic Pollution Issues, Arctic Monitoring and Assessment Programme (AMAP), Oslo, 1998 Search PubMed.
- R. A. Reyment, K. G. Jøreskog and L. F. Marcus, Applied Factor Analysis in the Natural Sciences, Cambridge University Press, Cambridge, UK, 1993, pp. 1–70 Search PubMed.
- C. A. Burtis and E. R. Ashwood, Tietz Textbook of Clinical Chemistry, W. B. Saunders, Philadelphia, 2nd edn., 1994, pp. 2059–2062 Search PubMed.
- C. Minoia, E. Sabbioni, P. Apostoli, R. Pietra, L. Puzzoli, M. Gallorini, G. Nicolaou, L. Alessio and E. Capodaglio, Sci. Total Environ., 1990, 95, 89 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Graphs of element concentrations from all 13 communities. See http://www.rsc.org/suppdata/em/b2/b206776p/ |
| This journal is © The Royal Society of Chemistry 2003 |
