Solid oxide fuel cells
R. Mark Ormerod
Birchall Centre for Inorganic Chemistry and Materials Science, School of Chemistry and Physics, Keele University, Staffordshire, UK ST5 5BG. E-mail: r.m.ormerod@keele.ac.uk
First published on 14th November 2002
Abstract
Despite being first demonstrated over 160 years ago, and offering significant environmental benefits and high electrical efficiency, it is only in the last two decades that fuel cells have offered a realistic prospect of being commercially viable. The solid oxide fuel cell (SOFC) offers great promise and is presently the subject of intense research activity. Unlike other fuel cells the SOFC is a solid-state device which operates at elevated temperatures. This review discusses the particular issues facing the development of a high temperature solid-state fuel cell and the inorganic materials currently used and under investigation for such cells, together with the problems associated with operating SOFCs on practical hydrocarbon fuels.
 R. Mark Ormerod R. Mark Ormerod | Professor Mark Ormerod was born in Leeds, England in 1965. He graduated in Natural Sciences from the University of Cambridge in 1986, and then undertook his PhD in the Chemistry Department at Cambridge, under the supervision of Professor Richard Lambert, in the area of surface chemistry. He was subsequently awarded the Cambridge University Oppenheiner Research Fellowship, which he held until 1992, when he was appointed to a Lectureship in Physical and Inorganic Chemistry at Keele University. In 1997 he was awarded an EPSRC Advanced Research Fellowship and was promoted to Professor of Inorganic Materials Chemistry. His current research interests are centred on heterogeneous catalysis and inorganic materials chemistry, with a particular focus on solid oxide fuel cells and solid-state electrocatalysis. |
1 Introduction to fuel cells
Fuel cells are currently attracting tremendous interest because of their huge potential for power generation in stationary, portable and transport applications and our increasing need for sustainable energy resources.1,2 The combination of the high efficiency with which chemical energy is converted directly into electrical energy, and the very much lower emissions of sulfur and nitrogen oxides and hydrocarbon pollutants, and significantly reduced CO2 emissions, confers very significant environmental advantages on fuel cells over conventional power generation.Despite the fact that the fuel cell was discovered over 160 years ago, and the high efficiencies and environmental advantages offered by fuel cells, only now are fuel cells approaching commercial reality. The major factor underlying this is the cost of fuel cell technology. However, significant advances in the development of both materials with improved properties and in manufacturing processes in the last two decades have made fuel cells a realistic proposition to compete on a commercial footing with conventional power generation.
As noted above, fuel cells are far from being a new technology; the concept of the fuel cell was first demonstrated in 1839 by William Grove.3 While investigating the electrolysis of water, Grove observed that when the current was switched off, a small current flowed through the circuit in the opposite direction, as a result of a reaction between the electrolysis products, hydrogen and oxygen, catalysed by the platinum electrodes. Grove recognised the possibility of combining several of these in series to form a gaseous voltaic battery,4 and also made the crucially important observation that there must be a ‘notable surface of action’ between the gas, the electrode and the electrolyte phases in a cell.4 Maximising the area of contact between the gaseous reagent, the electrolyte and the electrode (the electrocatalytic conductor), the ‘three-phase boundary’, remains at the forefront of fuel cell research and development.
The term ‘fuel cell’ was first used some 50 years after Grove’s ‘gas battery’ by Mond and Langer in 1889,5 to describe their device which had a porous platinum black electrode structure, and used a diaphragm made of a porous non-conducting substance to hold the electrolyte.
1.1 Types of fuel cells
There is now a whole range of different types of fuel cells, which have been developed, which differ in the nature of the electrolyte. However, the basic operating principle of all types of fuel cell is the same, and is shown in Fig. 1. At the anode, a fuel such as hydrogen, is oxidised into protons and electrons, whilst at the cathode, oxygen is reduced to oxide species, and these then react to form water. Depending upon the electrolyte, either protons or oxide ions are transported through an ion conducting, but electronically insulating, electrolyte, while electrons travel round an external circuit delivering electric power.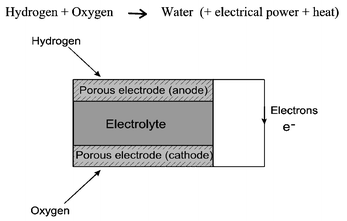 | ||
| Fig. 1 Schematic diagram showing the general operating principles of a fuel cell. | ||
There are five main types of fuel cell, summarised in Chart 1, which all have the same basic operating principle, namely two electrodes separated by an electrolyte. Ions move in one direction, which depends on the electrolyte, across the electrolyte to the opposite electrode, where reaction occurs, while the electrons flow round an external circuit, producing electric power. Each type of fuel cell is characterised by the electrolyte. It is generally considered that the two types of fuel cells most likely to succeed in achieving widespread commercial application are the polymer electrolyte membrane and the solid oxide fuel cell.
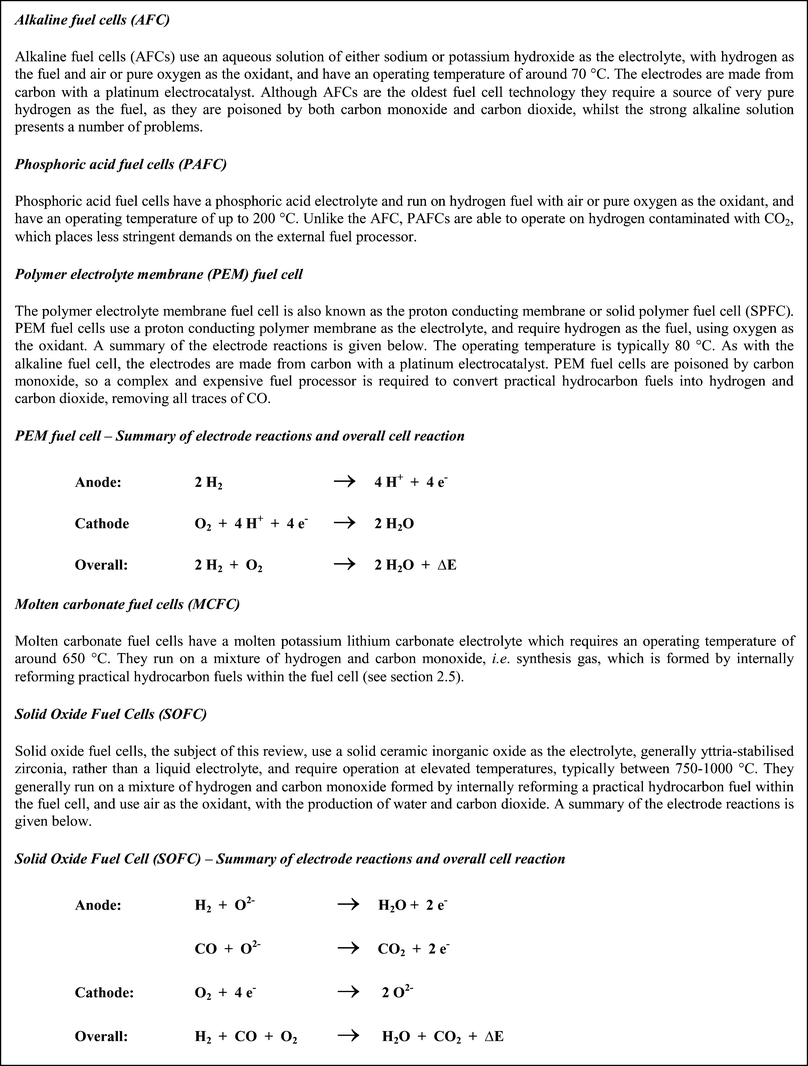 | ||
| Chart 1 Summary of the five main types of fuel cell and their principal characteristics. | ||
The most obvious difference in characteristics between the different types of fuel cell is the operating temperature, with molten carbonate and solid oxide fuel cells having elevated operating temperatures of ∼650 °C and 750–1000 °C, respectively, compared to the much lower operating temperatures of around 100 °C for alkaline and PEM fuel cells, and around 200 °C for phosphoric acid fuel cells. This difference in operating temperature has a number of implications for the applications for which particular fuel cell types are most suited.
1.2 Applications of fuel cells
The potential applications of fuel cells in society are ever increasing, driven by the different benefits which fuel cells bring to bear, such as environmental considerations (no NOx, SOx or hydrocarbon emissions and no or much reduced CO2 emissions), especially in urban areas where localised pollution is a major issue, and efficiency considerations (better utilisation of fossil fuels and renewable fuels, such as biogas and landfill gas). Their potentially high reliability and low maintenance coupled to their quiet operation and modular nature makes fuel cells particularly suited to localised power generation free from distributed networks, in ‘high quality’, uninterrupted power supplies, and in small-scale and remote applications. Applications range all the way from very small-scale ones requiring only a few Watts to large-scale distributed power generation of hundreds of MW.Fuel cells offer significantly higher power densities than batteries, as well as being smaller and lighter and having much longer lifetimes, so there is an increasing number of applications emerging where only a few Watts are required, such as palm-top and lap-top computers, mobile phones and other portable electronic devices, and computer systems in vehicles.
The combination of their high efficiency and significantly reduced emissions of pollutants mean that fuel cell powered vehicles are a very attractive proposition, especially in heavily populated urban areas. Low temperature fuel cells, in particular PEM fuel cells, are the most suited to transport applications, because of the need for short warm-up and cool-down times, and because there are no problems with temperature cycling. The concept of a fuel cell powered vehicle running on hydrogen, the so-called ′zero emission′ vehicle, is a very attractive one and is currently an area of intense activity for almost all the major motor manufacturers. Developing the infrastructure to supply hydrogen gas to vehicles would be a huge and very costly undertaking, and is therefore very unlikely in the shorter term. More likely liquid fuels such as petrol or methanol will be used with on-board fuel processors catalytically converting the fuel to produce hydrogen, and carbon dioxide.
In the case of public transport in cities, hydrogen is a realistic fuel since buses can be refuelled with hydrogen at a central depot. Fuel cell powered buses running on compressed hydrogen, such as the one shown in Fig. 2, are successfully operating in several cities around the world. The compressed hydrogen is stored in tanks in the roof, which can give a range of up to 300 km and a top speed of 80 km h−1.
 | ||
| Fig. 2 Fuel cell powered bus. | ||
Solid oxide fuel cells are particularly suited to combined heat and power (CHP) applications, ranging from less than 1 kW to several MW, which covers individual households, larger residential units and business and industrial premises, providing all the power and hot water from a single system. Such fuel cell CHP units offer significantly greater efficiency than the current situation where electricity is distributed from a small number of centralised power stations, whilst heating is supplied by decentralised boiler units in each house. Another advantage such CHP units offer for both domestic and commercial use is the reliability in the supply, which is becoming increasingly important, especially in certain commercial applications. Finally it should also be noted that in the power range 5–100 kW the existing technology is inefficient and displays extremely poor performance when operating at part-load.
Fuel cells, in particular SOFCs, offer potential for large scale distributed power generation (hundreds of MW), where the heat from the SOFC is used to drive a gas turbine to produce more electricity and increase the system efficiency to levels as high as 80%, significantly higher than any conventional electricity generation.
The modular nature of fuel cells makes them ideally suited for small-scale, stand-alone and remote applications, for example on gas pipelines, farms, caravans. The flexibility in the choice of fuel, and in particular the ability to operate SOFCs directly on practical hydrocarbon fuels, makes the SOFC particularly suited to such applications.
A rapidly developing market for fuel cells is in those applications where there is a real need for high quality, uninterrupted power supply. Such applications include information technology companies, airports and hospitals where there is a willingness to pay much higher prices for the guarantee of high quality, uninterrupted power to protect very valuable IT equipment or life-supporting equipment. Currently expensive surge protectors and stand-by emergency generators are required. Fuel cells are also well suited to high-current, low-voltage applications such as power tools, wheelchairs, electric bicycles, scooters and boats. Other applications include auxiliary power units in vehicles.
This review focuses on solid oxide fuel cells (SOFCs), and in particular the materials used in SOFCs, the function and properties of these materials, and practical problems associated with SOFCs, especially those of a chemical nature. Some of the challenges to be met and opportunities for SOFCs, together with some of the recent advances and developments in this rapidly developing area are reviewed.
2 Solid oxide fuel cells
2.1 Historical background
The solid oxide fuel cell was first conceived following the discovery of solid oxide electrolytes in 1899 by Nernst.7 Nernst reported that the conductivity of pure metal oxides rose only very slowly with temperature and remained only relatively low, whereas mixtures of metal oxides can possess dramatically higher conductivities. He noted that this result was in complete agreement with the known behaviour of liquid electrolytes, comparing the behaviour to that of aqueous salt solutions, which have very high conductivity, whereas the conductivities of pure water and pure common salt are both very low. Many mixed oxides which exhibit high conductivity at elevated temperatures were quickly identified, including the particularly favourable composition 85% zirconium oxide and 15% yttrium oxide, patented by Nernst in 1899. In this patent, Nernst suggested that zirconium oxide, stabilised with 15% yttrium oxide could be used as a glowing filament in lamps, the Nernst ′glower′. The Nernst lamp suffered from a number of practical disadvantages, and interest disappeared with the introduction of the first tungsten filament lamps in 1905. Nernst was convinced that his glowers were ionic conductors, and he proposed that in zirconia the oxidic additions were dissociated to some extent and were able to provide the necessary charge carriers.In 1905 Haber filed the first patent on fuel cells with a solid electrolyte, using glass and porcelain as the electrolyte materials, depending on the temperature of operation, and platinum and gold as the electrode materials.8 In 1916 Baur and Treadwell filed a patent on fuel cells with metal oxides as the electrodes and ceramic solids with salt melts in the pores as the electrolyte.
It was not until 1935 that Schottky suggested that yttria-stabilised zirconia could be used as a solid fuel cell electrolyte. In 1943 Wagner recognised the existence of vacancies in the anion sublattice of mixed oxide solid solutions and hence explained the conduction mechanism of the Nernst glowers, namely that they are oxide ion conductors.8 In 1937 Baur came to the conclusion, after many unsuccessful experiments with various types of liquid electrolytes that the solid oxide fuel cell had to be completely dry. Baur and Preis subsequently went on to demonstrate the solid oxide (or ceramic) fuel cell with an yttria-stabilised zirconia electrolyte, successfully running their cell at 1000 °C.8 Unfortunately the high operating temperature and the reducing nature of the fuel gas led to serious materials problems and despite a very significant search by Baur and other researchers for suitable materials, this was unsuccessful. This effectively hindered the development of the solid oxide fuel cell until the 1960s.
After 1960 various factors resulted in renewed interest in fuel cell technology, whilst advances in the preparation and production of ceramic materials led to a resurgence of interest in solid oxide fuel cells. In the early 1960s a rapidly increasing number of patents were filed relating to the development of SOFC technology. One of the problems with SOFCs at this time was their poor efficiency, as they had thick electrolyte layers and suffered from high losses because of their internal resistance. Continued advances in preparation and production methods through the 1970s led to the development of considerably thinner electrolytes, which gave a significant improvement in performance. In the last two decades numerous designs of SOFCs have been investigated, including various tubular and planar designs. Some of the subsequent developments and advances in SOFCs, and in particular the electrode and electrolyte materials employed, are discussed in section 2.4.
2.2 General introduction
The solid oxide fuel cell (SOFC) is characterised by having a solid ceramic electrolyte (hence the alternative name, ceramic fuel cell), which is a metallic oxide. The basic components of the SOFC are the cathode, at which oxygen is reduced to oxygen ions, which then pass through the solid electrolyte under electrical load, to the anode, where they react with the fuel, generally hydrogen and carbon monoxide, producing water and CO2, as well as electricity and heat. This is shown schematically in Fig. 3. The theoretical maximum efficiency is very high, in excess of 80%. As with Grove′s gas battery, multiple cells are connected electrically to produce larger quantities of power. This is achieved using an ′interconnect′, which is generally a conducting ceramic material (see section 2.4.4), though metallic interconnects have also been studied.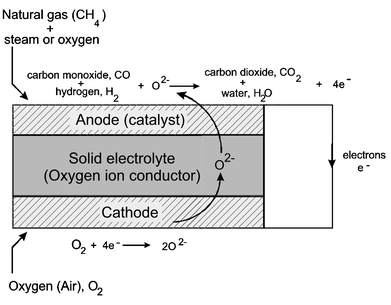 | ||
| Fig. 3 Schematic diagram showing the operating principles of a solid oxide fuel cell running on natural gas. | ||
The SOFC operates at elevated temperatures, conventionally between 800-1000 °C, though there is considerable interest in lowering the operating temperature of smaller SOFCs in particular to reduce costs, particularly of interconnect, manifolding and sealing materials (see sections 2.4.4 and 2.5). The elevated operating temperature of the SOFC has a number of consequences, the most important of which is that it presents the possibility of running the SOFC directly on practical hydrocarbon fuels without the need for a complex and expensive external fuel reformer, such as that which is necessary for PEM fuel cells. The hydrocarbon fuel is catalytically converted (internally reformed), generally to carbon monoxide and hydrogen (synthesis gas), within the actual SOFC, and the CO and H2 are then electrochemically oxidised to CO2 and water at the anode, with production of electrical power and high grade heat.
2.3 Applications and technological aspects of SOFCs
Internal reforming of the fuel can either be achieved indirectly using a separate fuel reforming catalyst within the SOFC, or directly on the anode. It is generally accepted that for SOFCs to ever be cost-effective, internal reforming of the fuel within the fuel cell is essential, since this both increases the efficiency and reduces the complexity of the system, and hence reduces costs.6The elevated operating temperature of the SOFC also leads to production of high temperature heat as a by-product in addition to the electrical power. This high quality heat is not wasted, but can be used in various ways, for example in combined heat and power systems, or to drive a gas turbine to generate more electricity. This significantly increases the overall efficiency of the SOFC compared to lower temperature variants.
The flexibility in the choice of fuel, the ability to operate SOFCs directly on practical hydrocarbon fuels, and the higher overall efficiency are three of the potential advantages of SOFCs over other types of fuel cells. Another particular advantage is their tolerance to carbon monoxide, which is electrochemically oxidised to CO2 at the anode, which contrasts markedly with PEM fuel cells, which are highly susceptible to poisoning by CO, and thus require complex and expensive external processing of hydrocarbon feeds to convert all the CO to CO2, which is then removed to leave very pure hydrogen as the fuel. SOFCs also show greater tolerance to impurities in the fuel, which poison other fuel cells, and to variations in the fuel composition, such that fuel processing requirements are less demanding. Other advantages include the fact that they do not contain any precious metals, which add significantly to the cost of the fuel cell, and the fact that the absence of any liquids in the cell eliminates potential problems due to corrosion and loss of electrolyte. In addition SOFC systems can be put together in various ways, some of which are considerably simpler than PEM fuel cell stacks. A prototype planar SOFC stack is shown in Fig. 4.
 | ||
| Fig. 4 A prototype planar SOFC stack. | ||
Thus, in principle, solid oxide fuel cell technology is both simpler and more efficient than that for other types of fuel cells. Unfortunately, however, the demands placed on the materials used as the electrolyte, the electrodes and the interconnect are severe. These will be discussed in section 2.4.
As noted in section 1.2 the elevated operating temperature of SOFCs makes them particularly suited to combined heat and power (CHP) applications, ranging from less than 1 kW to several MW, and for large scale distributed power generation (hundreds of MW), where the heat from the exhaust gas of the SOFC is used to drive a gas turbine and increase the system efficiency. For such applications long term durability is a key requirement, in addition to the cost compared to conventional stationary power generation systems. When the SOFC is integrated with a gas turbine, the temperature of the exhaust gas from the fuel cell stack should exceed about 850 °C. In the case of smaller CHP units the operating temperature of the SOFC does not need to be so high. As noted CHP units offer significantly greater efficiency than that achieved by having separate electricity and heating supplies, whilst the reliability of the supply is also an emerging advantage. Furthermore, in the power range 5–100 kW that many SOFC CHP units are being developed to meet, the existing technology is inefficient and displays extremely poor performance when operating at part-load.
The flexibility in the choice of fuel, coupled to the ability to operate SOFCs directly on practical hydrocarbon fuels, makes the SOFC particularly suited to small-scale, stand-alone and remote applications. For small-scale remote applications with no natural gas supply, bottled gas, namely propane and butane, are the most likely fuels.
One of the disadvantages of SOFCs for certain applications is the length of time that is generally required to heat up and cool down the system. This is a consequence of the need to use a relatively weak, brittle component as the substrate material and because of problems associated with thermal expansion mismatches. This restricts the use of SOFCs in applications that require rapid temperature fluctuations, which is particularly true for transport applications, where a rapid start-up and cool down is essential.
2.4 SOFC materials
As noted in sections 2.1 and 2.3, the SOFC places severe demands on the materials used as the electrolyte, the anode, the cathode and the interconnect. Each component must meet certain requirements and has more than one function. All components must possess chemical and physical stability in the appropriate chemical environment (oxidising and/or reducing), be chemically compatible with the other components, have proper conductivity, and have similar thermal expansion coefficients to the other components to avoid cracking or delamination during fabrication and operation. On top of this it is also important that the SOFC components are of low cost and are strong, yet easy to fabricate. Almost all SOFCs currently being developed employ an yttria-stabilised zirconia electrolyte, with a strontium-doped lanthanum manganite (La1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xSrxMnO3) cathode and a mixed nickel/yttria-stabilised zirconia (YSZ) cermet anode, and use doped lanthanum chromite (LaCrO3) as the interconnect. An electron micrograph of the cross-section of an SOFC developed by Siemens Westinghouse is shown in Fig. 5.
xSrxMnO3) cathode and a mixed nickel/yttria-stabilised zirconia (YSZ) cermet anode, and use doped lanthanum chromite (LaCrO3) as the interconnect. An electron micrograph of the cross-section of an SOFC developed by Siemens Westinghouse is shown in Fig. 5.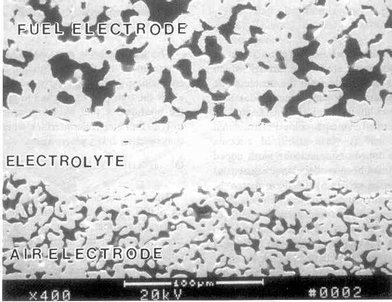 | ||
| Fig. 5 An electron micrograph of the cross-section of an SOFC developed by Siemens Westinghouse. | ||
This section focuses on the different inorganic materials used as anodes, cathodes, electrolytes and interconnects in SOFCs, and the strategy behind their selection and choice in terms of their chemical properties and the function they fulfil, with an emphasis on their chemistry. The development of new and novel materials with improved properties will be discussed. These include improvements in their electrochemical and catalytic properties, in their chemical and physical stability, and in their ability to withstand more rapid temperature fluctuations.
Almost all SOFC systems currently being developed employ an yttria-stabilised zirconia electrolyte. This is because in addition to having good oxygen ion conductivity, it shows good stability in both oxidising and reducing atmospheres and is unreactive towards other components used in the SOFC. It is also abundant, relatively low in cost and is strong whilst being easy to fabricate.
Pure ZrO2 is monoclinic at room temperature and undergoes phase transitions to a tetragonal structure above 1170 °C, and to the cubic structure above 2370 °C. Yttria, along with some other aliovalent oxides (Sc2O3, CaO, MgO and various rare-earth oxides), show a high solubility in ZrO2 and stabilise the zirconia in the cubic fluorite structure from room temperature to its melting point (2680 °C), and at the same time increase the concentration of oxygen ion vacancies, which significantly enhances the ionic conductivity. Stabilised zirconia has negligible electronic conductivity. It is generally found that the ionic conductivity is a maximum near the minimum level of dopant oxide required to fully stabilise the cubic phase. At higher dopant levels the ionic conductivity decreases, which is attributed to defect ordering, vacancy clustering or electrostatic interaction.9 Although some other oxide dopants produce cubic-stabilised zirconia with higher ionic conductivities than yttria-stabilised zirconia, yttria is the most widely used dopant for reasons of cost, availability and stability in oxidising and reducing atmospheres, and its chemical inertness towards other components in the SOFC. Typically the level of Y2O3 present in YSZ is around 8 mol%.
In addition to having a high ionic conductivity, negligible electronic conductivity, being stable in oxidising and reducing atmospheres, and being chemically unreactive towards the other cell components, the solid electrolyte must be completely non-porous to prevent mixing of the fuel and oxidant gas feeds.
Advances in the synthesis of zirconia powders and in the processing of ceramic powders, have enabled production of yttria-stabilised zirconia powders consisting of small, sub-micron, spherical particles with a narrow particle size distribution. Such particles have higher reactivity and a high packing density, enabling the complete densification of the powders to form a non-porous structure with a uniform microstructure at low sintering temperatures.10 The use of lower temperature sintering procedures has enabled electrolyte layers to be prepared which can withstand more rapid temperature fluctuations.
Conventional zirconia based SOFCs generally require an operating temperature above 850 °C. This high operating temperature places severe demands on the materials used as interconnects and for manifolding and sealing, and necessitates the use of expensive ceramic materials and specialist metal alloys (see section 2.4.4). There is therefore considerable interest in lowering the operating temperature of SOFCs to below 700 °C to enable the use of cheaper materials, such as stainless steel, and reduce fabrication costs, whilst maintaining high power outputs.
The operating temperature is principally governed by the nature of the electrolyte, i.e. its ionic conductivity, and the thickness of the electrolyte layer. There are therefore two possible approaches to lowering the operating temperature. The first is to reduce the thickness of the YSZ electrolyte layer, whilst the second is to search for alternative electrolyte materials with higher oxygen ion conductivities.
SOFCs currently being developed which do not rely on the solid electrolyte for structural support typically have an electrolyte layer around 30 μm thick. In such SOFCs the electrolyte must be supported on another substrate, which is in some cases the anode or the cathode. Whatever the support it must possess both mechanical strength and gas permeability. A great deal of effort has gone into the development of thinner solid electrolyte layers, which would enable the SOFC operating temperature to be lowered. Currently the limit on the thickness of dense impermeable electrolyte films that can be reliably produced using conventional, cheap ceramic fabrication routes is around 10–15 μm.
The search for, and study of, alternative solid electrolyte materials has been an active research area for many years. At present the two most promising alternative electrolytes to yttria-stabilised zirconia, which have been intensively studied in recent years, are gadolinia-doped ceria in particular11,12 and lanthanum gallate based structures.13 Both these electrolytes offer the possibility of lower temperature operation of SOFCs between 500 °C and 700 °C. Scandia-doped zirconia is also being investigated as an alternative to yttria-stabilised zirconia, since it has similar properties but exhibits higher ionic conductivities, though it is also more expensive than YSZ. Fig. 6 shows a plot of the ionic conductivities of different solid electrolyte materials.
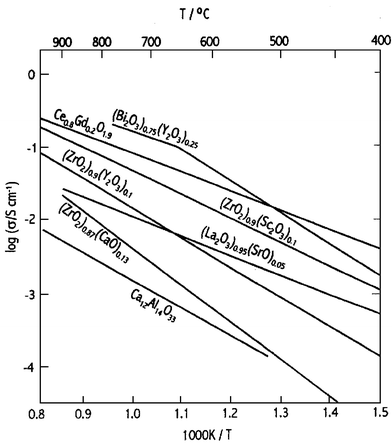 | ||
| Fig. 6 Specific ion conductivities of selected solid oxide electrolytes. | ||
Gadolinia-doped ceria (CGO) offers excellent promise as a potential electrolyte for lower temperature SOFCs. Unfortunately however, at elevated temperatures in a reducing atmosphere, such as that present at the anode, ceria undergoes partial reduction to Ce3+, which leads to electronic conductivity, which significantly lowers the efficiency of the SOFC, and also an undesirable structural change. Considerable effort has been devoted to minimising the electronic conductivity of doped ceria under reducing conditions. One solution is to use an additional ultra-thin interfacial electrolyte layer which prevents electronic transport,14 and can suppress the reduction of ceria under reducing conditions.11,12,15,16 If the operating temperature of the SOFC is lowered to around 500 °C then any electronic conductivity is sufficiently small that it can be neglected under these operating conditions. The main problem at such low operating temperatures lies with the development of sufficiently active cathode materials (see section 2.4.3).
Lanthanum gallate, LaGaO3, has attracted considerable attention in recent years as a promising alternative electrolyte for lowering the operating temperature of SOFCs.13 Increased conductivity is obtained by substituting both the trivalent lanthanum and gallium with divalent cations, generally strontium and magnesium, respectively:
La1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xSrxGa1 xSrxGa1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) yMgyO3 yMgyO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) x/2 x/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) y/2 (LSGM) y/2 (LSGM) |
A favoured composition in terms of ionic conductivity is La0.9Sr0.1Ga0.8Mg0.2O2.85. The ionic conductivity of LSGM, although significantly higher than YSZ, is slightly less than that of CGO at 500 °C.13 However, the potential range of operating temperatures of LSGM is greater than CGO because it does not suffer from the problems exhibited by CGO at higher temperatures associated with electronic conduction. Thus there is interest in the possibility of using LSGM at temperatures around 600–700 °C, which are currently too low to obtain adequate power densities with zirconia based SOFCs.
However, there are problems associated with the stability of certain compositions of LSGM. It has also proved difficult to prepare pure single phase electrolytes of LSGM, and additional phases including La4Ga2O9 and SrLaGa3O7, have been detected at the grain boundaries.13 Such phases raise doubts over the long term durability of SOFCs with LSGM electrolytes, and much further research into this material is still required.
Unlike the electrolyte, the anode must have a porous structure, which furthermore must be maintained at the high operating temperatures. This is achieved by dispersing the nickel with the solid electrolyte material to form a cermet, which maintains the porosity by preventing sintering of the nickel particles during operation, and also gives the anode a thermal expansion coefficient comparable to that of the solid electrolyte. The thermal expansion coefficient of nickel is appreciably different to that of yttria-stabilised zirconia, and thus adhesion of a pure nickel anode to the solid electrolyte would be a major problem during fabrication and firing, and temperature cycling during operation.10 The solid electrolyte can be considered as essentially analogous to the support in a supported metal catalyst, and hence may influence the catalytic behaviour of the anode. There is currently considerable interest in studying the catalytic properties of nickel based anodes, and modifying and optimising their composition,17 particularly for directly internally reforming SOFCs (see section 2.5.1).
The anode must be electrically conducting to function as an electrode. The conductivity of the nickel/solid electrolyte cermet depends on the nickel content. The threshold for electrical conductivity is about 30 vol% nickel, below this the conductivity of the cermet is effectively that of the solid electrolyte. Above this threshold the conductivity increases by about three orders of magnitude, as shown in Fig. 7, corresponding to electronic conduction through the nickel. The conductivity of the anode depends on its microstructure, in particular the size and particle size distribution of the solid electrolyte and nickel particles, and the connectivity of the nickel particles in the cermet.
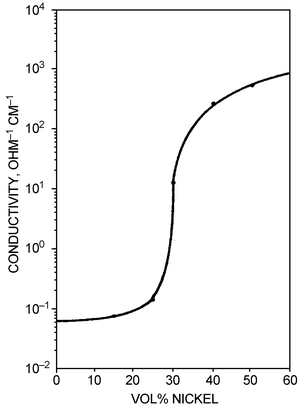 | ||
| Fig. 7 The electrical conductivity of a nickel/yttria-stabilised zirconia cermet anode as a function of nickel content.10 | ||
The proportion of nickel used in the anode cermet is also influenced by the thermal expansion mismatch with the solid electrolyte. The thermal expansion coefficient of a nickel/yttria-stabilised zirconia cermet increases linearly with the nickel content, and thus the higher the nickel content of the anode the greater the thermal mismatch with the electrolyte, and the greater the mismatch the more chance there is of cracking of the electrolyte or delamination of the anode during the fabrication process, and in operation during temperature cycling.10,18
Much attention has been given to overcoming problems associated with the thermal expansion mismatch, for example by minimising any processing flaws in the electrolyte, improving its fracture toughness, using graded anodes of different compositions and altering the thickness of the electrolyte and anode layers.18 Another approach is to incorporate small quantities of a third material into the anode in an attempt to match the thermal expansion coefficient with that of the electrolyte.
The nickel/YSZ anode cermet is generally made by physically mixing NiO and YSZ powders in a slurry. In an electrolyte or cathode supported SOFC, the anode is then applied to the solid electrolyte and fired in air, typically to around 1300 °C. The active anode is then formed by reduction of the NiO to nickel metal in situ when it is exposed to the fuel feed.
Another reason for wishing to lower the operating temperature of SOFCs is that at the higher operating temperatures sintering of the nickel particles over time is potentially a major problem. It has been found that the narrower the particle size distribution of the nickel particles the lower the rate of sintering, whilst the sintering rate increases with increasing nickel content and with increasing steam content in the fuel feed.
There is currently much interest in developing alternative anode materials to the nickel/YSZ cermet. A number of researchers have studied nickel/ceria cermet anodes for zirconia based SOFCs, in addition to being used for ceria–gadolinia based SOFCs.11,19,20 Nickel/ceria cermet anodes have been shown to still give sufficiently good performance in ceria based SOFCs operating at temperatures as low as 500 °C. Ceria has also been added to nickel/YSZ anodes to improve both the electrical performance and the resistance to carbon deposition.11,20 Although cobalt and ruthenium offer potential advantages over nickel, including high sulfur tolerance, and in the case of ruthenium, higher reforming activity and greater resistance to sintering, and cobalt/YSZ and ruthenium/YSZ anodes have been developed, the cost of these materials effectively precludes their use.
Various dopants have been incorporated into nickel/zirconia and nickel/ceria anodes, in an attempt to modify their behaviour, particularly in terms of their reforming activity and their resistance to carbon deposition and tolerance to sulfur, which lead to deactivation and loss of cell performance. These properties are particularly important for directly internally reforming SOFCs. Dopants that have been studied include molybdenum, gold, ruthenium and lithium.17,21
Electrically conducting oxides, which are stable under both oxidising and reducing conditions have been actively studied in recent years as potential alternative anode materials to nickel.11,22,23 In principle the use of such oxides as anodes would overcome many of the problems associated with nickel cermet anodes for use in direct reforming SOFCs, in particular those of carbon deposition, sulfur poisoning, and sintering and the possible undesirable formation of nickel oxide under oxidising conditions. Such oxides also offer potential for direct hydrocarbon oxidation (see section 2.5.2), whereby the hydrocarbon fuel is directly electrocatalytically oxidised by the oxygen ions which have passed through the solid electrolyte. Oxides that have been investigated as possible anode materials include materials based on lanthanum chromite, LaCrO3, and strontium titanate, SrTiO3. Doped lanthanum chromite and doped strontium titanate have also been studied. In the case of lanthanum chromite, calcium, strontium and titanium substituents have been used, e.g. La0.7Sr0.3Cr0.8Ti0.2O3, whilst for strontium titanate, niobium and lanthanum substitution has been investigated, e.g. Sr0.6Ti0.2Nb0.8O3 and La0.4Sr0.6TiO3. Mixed conducting oxides such as terbia- and titania-doped YSZ and yttria-doped ceria have also attracted some interest as potential anode materials.24 Such materials can significantly lower overpotential losses at the anode.
Some recent studies have identified alternative anodes which show considerable promise for the direct electrocatalytic oxidation of the hydrocarbon fuel by the electrochemically pumped oxygen ions, without the need for any co-fed oxidant.25,26 One of these anodes was copper-based and incorporated significant quantities of ceria in addition to YSZ, and the other involved adding yttria-doped ceria to nickel and YSZ, However, the conditions under which such anodes could be used for direct hydrocarbon oxidation may be a problem for their widespread application, whilst their long term performance in terms of deactivation resulting from carbon deposition is another concern. The development of such anodes is a very active area of current research.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xSrxMnO3, is the most commonly used cathode material for zirconia based SOFCs.
xSrxMnO3, is the most commonly used cathode material for zirconia based SOFCs.La1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xSrxMnO3 (LSM) xSrxMnO3 (LSM) |
LaMnO3 is a perovskite material with intrinsic p-type conductivity, the oxygen stoichiometry of which at high temperature is a function of the oxygen partial pressure, having an oxygen excess in an oxidising environment, whilst becoming oxygen deficient in a reducing environment. To be used as a cathode for SOFCs it is imperative that significant changes in the oxygen stoichiometry are avoided. LaMnO3 is generally produced with a lanthanum deficiency to prevent formation of La2O3, which can cause the cathode layer to disintegrate through hydration to La(OH)3.
The p-type conductivity of LaMnO3 is a consequence of the formation of cation vacancies, and hence the conductivity can be enhanced by the use of a lower valence ion as a dopant for either the A or B sites. The alkaline earth metals, magnesium, calcium, strontium and barium, have all been used as dopants, together with nickel.10,28 The divalent cation dopant substitutes for La3+ and increases the electronic conductivity by increasing the Mn4+ content, with conduction proceeding by small polaron hopping:
La3+1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xM2+xMn3+1 xM2+xMn3+1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xMn4+xO3 xMn4+xO3 |
Strontium-doped LaMnO3 (LSM) is the most commonly used cathode material in current zirconia based SOFCs. The conductivity of La1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xSrxMnO3 varies with strontium content, with an apparent optimum strontium level. However the optimal level of strontium is a matter of some conjecture, as a consequence of factors such as preparation method, firing conditions and hence the microstructure influencing conductivity. Strontium doping of LaMnO3 progressively increases the thermal expansion coefficient compared to undoped LaMnO3, and therefore increases the mismatch with the zirconia electrolyte.
xSrxMnO3 varies with strontium content, with an apparent optimum strontium level. However the optimal level of strontium is a matter of some conjecture, as a consequence of factors such as preparation method, firing conditions and hence the microstructure influencing conductivity. Strontium doping of LaMnO3 progressively increases the thermal expansion coefficient compared to undoped LaMnO3, and therefore increases the mismatch with the zirconia electrolyte.
In practice, two cathode layers are often employed, with the first layer being a mixture of LSM and yttria-stabilised zirconia, analogous to the NiO/YSZ cermet used as the anode. This improves the thermal match of the cathode with the zirconia electrolyte, and results in improved porosity and resistance to sintering, whilst still exhibiting the required electronic conductivity.10 The second cathode layer, often called the current collect layer, is then pure LSM. The addition of platinum to LSM cathodes has been shown to improve the cell performance, both by reducing the electrical resistance between the cathode and the current collector and by increasing the electrical conductivity of the cathode,10 though clearly this improvement has to be balanced against the very high cost of platinum.
A problem with LSM is its chemical compatibility with the zirconia electrolyte, which generally restricts sintering temperatures to below 1300 °C. Above these temperatures manganese can diffuse into the zirconia electrolyte, detrimentally affecting both the cathode and the electrolyte.29 However, the level of manganese diffusion, even at the highest likely operating temperatures of SOFCs, is negligible,29 and long term studies show that there is no degradation of the SOFC due to interaction of the LSM with the zirconia electrolyte.30 Various studies have shown that there is no reaction between LSM and zirconia at temperatures up to 1200 °C, but that reaction occurs above 1200 °C with the formation of La2Zr2O7, and also SrZrO3 at higher strontium levels. The conductivity of La2Zr2O7 is more than 100 times lower than that of zirconia.
Another perovskite material that has been extensively studied as a cathode material for SOFCs is doped lanthanum cobaltite, LaCoO3.10,31 LaCoO3, like LaMnO3, shows intrinsic p-type conductivity, and has a large oxygen deficiency at high temperatures.32 The conductivity can be increased by substituting a divalent cation on the lanthanum site. As with LaMnO3, strontium is generally used.33 Further improvements in performance have been found by substituting iron on the cobalt site to form:
La1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xSrxCo1 xSrxCo1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) yFeyO3 (LSCF) yFeyO3 (LSCF) |
LaCoO3 has a superior electrical conductivity to LaMnO3 under equivalent conditions.29,31 However, there are several disadvantages with LaCoO3 which generally preclude its use as the cathode in zirconia-based SOFCs. LaCoO3 shows much greater reactivity towards zirconia than LaMnO3 at high temperatures,10 and it is also much more susceptible to reduction at high temperatures than LaMnO3. In addition, the thermal expansion coefficient of LaCoO3 is considerably greater than that of LaMnO3, which is already higher than that of yttria-stabilised zirconia. LaCoO3 has been mixed with LaMnO3 in an attempt to improve the electrical conductivity of the cathode and to better match the thermal expansion coefficient to that of zirconia.
The higher electrical conductivity of LSCF means that it is the most commonly used cathode in intermediate temperature SOFCs, with gadolinia-doped ceria or lanthanum gallate electrolytes, since LSM cathodes do not perform well enough at these lower operating temperatures, and significantly limit the performance of the SOFC. There is currently much interest in developing cathode materials with improved performance at temperatures as low as 500 °C,34 with some promise being shown by the use of composite cathodes.35
These requirements severely restrict the choice of materials for the interconnect, especially at the higher operating temperatures of most zirconia-based SOFCs. The vast majority of zirconia-based SOFCs use lanthanum chromite, LaCrO3, as the interconnect. LaCrO3 has a perovskite structure and is a p-type conductor, and satisfies all the above criteria. It is refractory and is oxygen deficient under very reducing conditions, but otherwise is stoichiometric. The electrical conductivity of LaCrO3 can be enhanced by substituting the La3+ with a divalent cation, such as strontium, calcium or magnesium. Conduction is by the small polaron hopping mechanism:10,36,37
La3+1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xM2+xCr3+1 xM2+xCr3+1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) xCr4+xO3 xCr4+xO3 |
As noted above the SOFC interconnect is exposed to both oxidising and reducing atmospheres. The conductivity of LaCrO3 in hydrogen has been shown to be around 1000 times lower than that in air.10 Thus there is an electrical conductivity gradient across an LaCrO3 interconnect. However, at the elevated operating temperatures of the SOFC the conductivity of LaCrO3 overall is still sufficient for it not to limit the SOFC performance.10
The interconnect must be impermeable to prevent any cross leakage of the fuel and oxidant gases under operating conditions. LaCrO3 is difficult to sinter to high density, due to the volatisation of chromium oxides under oxidising conditions.10 Consequently to sinter LaCrO3 to high densities low oxygen partial pressures are generally employed with firing temperatures above 1600 °C being necessary.10 The ability to sinter LaCrO3 at lower firing temperatures in an oxidising atmosphere is highly desirable, and hence this has been extensively studied. Approaches include the use of dopants and sintering aids, use of different processing techniques, use of different synthesis routes to prepare more reactive powders, and the use of non-stoichiometric LaCrO3 deficient in chromium.10
For SOFCs operating in the intermediate temperature range, 500–750 °C, it becomes feasible to use certain ferritic stainless-steel composites which fulfil the necessary criteria for the SOFC interconnect.13 The use of a metallic interconnect offers very substantial cost benefits compared to LaCrO3, but their use is presently precluded in SOFCs operating at higher temperatures.
2.5 Fuels and fuel processing in SOFCs
As noted in section 2.3, the elevated operating temperature of SOFCs, combined with their ability to utilise carbon monoxide as a fuel and their greater resistance to poisoning by impurities in the fuel, means that it is possible to operate the cell directly on hydrocarbon fuels without the need for a separate complex external fuel processor to reform the hydrocarbon fuel into hydrogen and carbon dioxide, removing all traces of CO. Instead the fuel can be catalytically converted to H2 and CO within the SOFC stack, that is internally reformed. The ability to internally reform practical hydrocarbon fuels within the SOFC, together with the ability to utilise CO and increased tolerance to other impurities in the fuel, is a significant advantage of SOFCs over low temperature fuel cells, and is essential if SOFCs are to become economic, since it both significantly increases the system efficiency by recuperating waste heat from the stack into the fuel supply, whilst at the same time substantially reducing the complexity of the system, by elimination of the external reformer and associated heating arrangements and by reduction in the stack cooling air requirements and associated equipment. Thus internally reforming SOFCs offer significantly higher system efficiencies and reduced complexity compared to lower temperature fuel cell variants.The most common fuel for the SOFC, especially for stationary applications, is natural gas, which is cheap, abundant and readily available, with a supply infrastructure already in existence in many places. Natural gas can be internally reformed within the SOFC at temperatures as low as 600 °C, which means that even lower temperature SOFCs can be operated on natural gas without the need for a complex external fuel reformer. However, in certain applications, especially remote and small-scale, stand-alone ones, bottled gas, such as propane and butane, offers significant practical advantages. The SOFC represents a real viable alternative to conventional power generation methods in remote areas with no natural gas supply, where diesel is generally used, which is both inefficient and highly polluting.
The choice of fuel is partly governed by the operating temperature. For intermediate temperature SOFCs operating at temperatures as low as 500 °C, methanol is considered the most likely fuel. Intermediate temperature SOFCs operating directly on methanol offer some potential for transport applications, though the problems associated with slow start-up times and temperature cycling, discussed in sections 2.3 and 2.4, still have to be overcome. There is also considerable interest in the longer term in the possibility of using higher hydrocarbons, such as petrol and diesel, in internally reforming SOFCs.
Recently, the possibility of using waste biogas, generated from vegetable matter, and landfill gas, both totally renewable fuels, directly in SOFCs has been demonstrated.38 SOFCs can also be operated on the output from coal gasification systems. The sulfur content of these gases poisons the anode in particular, and the internal reforming catalyst, causing loss of performance and eventual cell deactivation. Thus the sulfur has to be removed from the gas prior to entering the SOFC, though this is comparatively straightforward. Although the efficiencies of SOFCs operating on biogas, landfill gas or the output from coal gasification systems are lower than for SOFCs operating on natural gas, they do offer significantly cleaner and more efficient power generation compared to alternative means of energy generation currently utilised for these gases.
A particular problem is carbon deposition resulting from hydrocarbon pyrolysis [eqn. (1)], especially on the nickel cermet anode, as well as on other active components within the SOFC, which leads to deactivation and poor durability.
| CH4 → C + 2 H2 | (1) |
| H2 + O2− → H2O + 2 e− | (2) |
| CO + O2− → CO2 + 2 e− | (3) |
Internal reforming of the fuel is achieved either indirectly using a separate catalyst within the SOFC, or directly on the nickel anode. Direct reforming of the fuel on the anode offers the simplest and most cost-effective solution, and in principle provides the greatest system efficiency with least loss of energy. In direct reforming the anode must fulfil three roles, firstly as a reforming catalyst, catalysing the conversion of hydrocarbons to hydrogen and CO, secondly as an electrocatalyst responsible for the electrochemical oxidation of H2 and CO to water and CO2, respectively, and finally as an electrically conducting electrode. High efficiency results from utilising the heat from the exothermic electrochemical reaction to reform the hydrocarbon fuel, this being a strongly endothermic reaction. However, one of the major problems with direct reforming is that it gives rise to a sharp cooling effect at the cell inlet, generating inhomogeneous temperature distributions and a steep temperature gradient along the length of the anode, which is very difficult to control and can result in cracking of the anode and electrolyte materials. Significant reductions in operating temperature of the SOFC due to the endothermic reforming reaction have been reported. Various approaches are being investigated to give greater control of the reforming reaction to minimise the temperature gradient. This includes the possibility of developing mass transfer controlled steam reforming catalysts with reduced activity.
Another particular problem with direct reforming is the susceptibility of the nickel anode to catalyse the pyrolysis of methane [eqn. (1)], which results in carbon deposition, and leads to rapid deactivation of the cell. As noted in section 2.4.2 the high metal content of the anode precludes the use of precious metals, such as rhodium or platinum, which are more resistant to carbon deposition. Much research is currently being carried out to develop nickel-based anodes which are active for hydrocarbon reforming but are more resistant to carbon deposition. Approaches include the incorporation of small amounts of dopants such as gold, molybdenum and copper into the nickel anode, and the addition of ceria to nickel/zirconia cermets.21 Another problem with reforming directly on the anode which has been reported is that it can cause sintering of the anode particles, resulting in a reduction in the catalytic activity of the anode and a loss of cell performance.
In indirect internal reforming a separate catalyst, which reforms the hydrocarbon fuel to synthesis gas, is integrated within the SOFC upstream of the anode. The heat from the exothermic fuel cell reaction is still utilised. Although indirect internal reforming is less efficient and less straightforward than direct reforming it is still significantly more efficient, simpler and more cost-effective than using an external reformer. The major advantage of indirect reforming over direct reforming is that it is much easier to manage and control from a thermodynamic standpoint. One approach involves the development of mass transfer controlled steam reforming catalysts with reduced activity. It is also easier to develop dispersed catalysts which do not promote carbon formation to the same extent as the nickel anode. Consequently the SOFCs currently being developed generally use a separate catalyst within the SOFC stack, upstream of the anode to indirectly reform the majority of the hydrocarbon fuel, with some residual reforming occurring directly on the anode.
The most common oxidant for reforming the hydrocarbon fuel is steam, which is added to the hydrocarbon feed, which is subsequently converted to CO and H2via steam reforming. This is shown in eqn. (4) for methane:
| CH4 + H2O → CO + 3 H2 | (4) |
| CO + H2O → CO2 + H2 | (5) |
However, as described above, in addition to the reforming reactions, there is also the possibility of hydrocarbon pyrolysis occurring [eqn. (1)], which leads to carbon deposition on either the internal reforming catalyst or the nickel anode. Nickel in particular is well known for its propensity to promote this reaction.21,39,40
Carbon deposition can also occur via the disproportionation of CO (the Boudouard reaction) [eqn. (6)], and by reduction of CO by H2[eqn. (7)].
| 2 CO → C + CO2 | (6) |
| CO + H2 → C + H2O | (7) |
As noted in section 2.5, natural gas is the most likely fuel for the SOFC. Natural gas, although predominantly methane, contains significant proportions of higher hydrocarbons. It is well known that higher hydrocarbons are more reactive and show a greater propensity towards carbon deposition than methane, and in reality it is the presence of these higher hydrocarbons in natural gas that represents the most likely source of deleterious carbon build-up in SOFCs.
In addition to containing higher hydrocarbons, sulfur-containing compounds, such as dimethyl sulfide, diethyl sulfide, ethyl mercaptan, tert-butyl mercaptan and tetrahydrothiophene, are added to natural gas as odorants at the level of ∼5 ppm. Although at the elevated operating temperature of SOFCs the nickel anodes, and any internal reforming catalyst, show some tolerance to sulfur, generally the majority of the sulfur is removed from the natural gas prior to entering the SOFC to prevent poisoning of the anode and reforming catalyst. The tolerance of the anode and reforming catalyst to sulfur becomes progressively less as the operating temperature of the SOFC is lowered.
Consequently there is much interest in developing optimised catalyst and anode formulations, and establishing appropriate operating conditions, for internally reforming SOFCs that avoid carbon deposition and which show some tolerance to sulfur. This is particularly important if some of the fuel processing is occurring directly on the anode.
As water is formed as the product of the electrochemical oxidation of hydrogen [eqn. (2)] at the anode, this water can be recirculated and re-introduced into the hydrocarbon fuel feed, rather than continuously adding water to the system. Carbon dioxide, formed by electrochemical oxidation of carbon monoxide [eqn. (3)], is present in the exit gas leaving the anode, so if the exit gas is recirculated, in addition to steam, CO2 will be present in the fuel supply at the cell inlet. It is well known that CO2 can act as an oxidant for hydrocarbons [dry reforming, eqn. (8)].41 Therefore, in addition to the steam, the CO2 can also reform the methane, though it also represents a possible source of carbon deposition.
| CH4 + CO2 → 2 CO + 2 H2 | (8) |
In certain applications, especially in small-scale devices being developed for stand-alone or remote applications, oxygen, or simply air in many cases, is used as the oxidant rather than steam, because of the cost and complexity associated with using large quantities of steam, which makes its use less favourable in small-scale applications. Using oxygen, or air, is much simpler and cheaper in terms of system configuration and manufacture. However, it does lead to an inherent efficiency due to the large energy loss in oxidising the hydrocarbon. Further, in order to maximise the power output from the SOFC it is necessary for the internal reforming catalyst or the anode to be selective for the partial oxidation of the hydrocarbon [eqn. (9)].
| CH4 + ½ O2 → CO + 2 H2 | (9) |
| CH4 + 2 O2 → CO2 + 2 H2O | (10) |
Although for most SOFCs under normal operation steam (and CO2) will be used to internally reform the natural gas, self-sustained internal reforming is precluded during start-up from cold and operation at low power levels because of the strongly endothermic nature of steam (and CO2) reforming. Partial oxidation of hydrocarbons, being exothermic, offers the potential for start-up and self-sustaining operation of internally reforming SOFCs running on natural gas or other hydrocarbon fuels at low power. Thus it is likely that a combination of partial oxidation and steam reforming (autothermal reforming) will be used as the basis for operation from zero power through low power loads to operation at full load—at zero and self-sustaining low power operation partial oxidation will be used, and at high load, i.e. normal operation, exclusively steam (and CO2) reforming will be used.
| CH4 + O2− → CO + 2 H2 + 2 e− | (11) |
| CH4 + 4 O2− → CO2 + 2 H2O + 8 e− | (12) |
The concept of using an SOFC for chemical cogeneration has attracted much interest,43 offering the possibility of achieving higher product selectivity using electrochemically pumped oxygen ions compared to gas phase oxygen, whilst at the same time using air rather than pure oxygen as the oxidant, which would bring a significant cost benefit. In addition to synthesis gas, it has also been shown that oxidative coupling of methane to ethene and ethane can be carried out in an SOFC electrocatalytic reactor [eqns. (13)–(15)].
| 2 CH4 + O2− → C2H6 + H2O + 2 e− | (13) |
| 2 CH4 + 2 O2− → C2H4 + 2 H2O + 4 e− | (14) |
| C2H6 → C2H4 + H2 | (15) |
3 Concluding remarks
Solid oxide fuel cells offer tremendous potential for clean, efficient and economic energy generation, especially for combined heat and power and small-scale stand-alone and remote applications. The ability to operate SOFCs directly on a range of practical hydrocarbon fuels, internally reforming the fuel and utilising the high quality heat by-product, to give high system efficiencies and reduced system complexity, coupled to their ability to utilise carbon monoxide and their greater tolerance to impurities in the fuel, represent significant advantages of the SOFC over low temperature fuel cells.Significant advances in the preparation and processing of inorganic materials over the last two decades, together with the development of new advanced materials with superior structural, electrical and catalytic properties, mean that SOFCs can be expected to become a commercial reality within the next few years. Such SOFCs will most commonly be operated on natural gas, but with bottled gas being the fuel of choice in certain applications, internally reforming the fuel within the fuel cell. Initial applications are most likely to be combined heat and power systems in the range 1–100 kW, small-scale and remote, stand-alone applications, and applications where there is a requirement for high quality, uninterrupted power supplies, such as in information technology companies, hospitals and airports, and thus a premium for the guarantee of uninterrupted power. Eventually it is hoped that SOFCs will break through into large-scale distributed power generation.
Future challenges involve the development of direct reforming SOFCs, operating on hydrocarbon feeds, without the need for a separate reforming catalyst upstream of the anode. In this context developing anodes capable of stable direct reforming of methane under operating conditions is essential. Another challenge is the development of SOFCs operating at lower temperatures, either by the development of ultra-thin dense, impermeable zirconia electrolyte films, or by the use of alternative solid electrolytes to yttria-stabilised zirconia, such as gadolinia-doped ceria or lanthanum gallate based structures. Lowering the operating temperatures of the SOFC would bring very significant cost benefits in terms of the scope of interconnect, manifold and sealing materials which can be used.
A particularly challenging objective is the development of SOFCs operating on pure hydrocarbon feeds without any oxidant being added to the fuel inlet, instead directly oxidising the hydrocarbon at the anode using electrochemically pumped oxygen ions formed at the cathode. The major challenge here is the development of anodes which are stable towards carbon formation from hydrocarbon pyrolysis under these conditions. Recent studies have shown considerable promise in this area, including the use of electrically conducting oxide materials in place of conventional nickel based anodes. Such an SOFC is an extremely attractive proposition, especially if partial oxidation of the hydrocarbon to synthesis gas can be coupled to electricity production, using the SOFC as an electrocatalytic reactor.
Another intriguing possibility, which has recently been demonstrated, is utilising renewable fuels such as biogas, generated from vegetable matter, and landfill gas, directly in SOFCs.
4 Acknowledgements
The UK Engineering and Physical Sciences Research Council is gratefully acknowledged for the award of an Advanced Research Fellowship to the author.5 References
- R. Kingston, Chem. Br., 2000, 24 Search PubMed.
- C. K. Dyer, Sci. Am., 1999, 281, 70 Search PubMed.
- W. R. Grove, Phil. Mag., Ser. 3, 1839, 14, 127 Search PubMed.
- W. R. Grove, Philos. Mag., Ser. 3, 1843, 21, 417 Search PubMed; W. R. Grove, Proc. R. Soc. London, 1843, 4, 463 Search PubMed.
- L. Mond and C. Langer, Proc. R. Soc. London, 1889, 46, 296 Search PubMed.
- D. Hart, Chem. Ind., 1998, 344 Search PubMed.
- W. Nernst, Z. Electrochem., 1899, 6, 41 Search PubMed.
- H.-H. Möbius, J. Solid State. Electrochem., 1997, 1, 2 CrossRef CAS , and refs. therein.
- C. B. Choudhary, H. S. Maiti and E. C. Subbarao, in, Solid Electrolytes and their Applications, ed. E. C. Subbarao, Plenum Press, New York, 1980, p.1 Search PubMed.
- N. Q. Minh, J. Am. Ceram. Soc., 1993, 76, 563 Search PubMed , and refs. therein.
- M. Mogensen, N. M. Sammes and G. A. Tompsett, Solid State Ionics, 2000, 129, 63 Search PubMed , and refs. therein.
- B. C. H. Steele, Solid State Ionics, 2000, 129, 95 Search PubMed , and refs. therein.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345 CrossRef CAS.
- E. D. Wachsmann, P. Jayaweera, N. Jiang, D. M. Lowe and B. G. Pound, J. Electrochem. Soc., 1997, 144, 1.
- H. Yahiro, Y. Baba, K. Eguchi and H. Arai, J. Electrochem. Soc., 1988, 135, 2077 CAS.
- A. V. Virkar, J. Electrochem. Soc., 1991, 138, 1481 CAS.
- C. M. Finnerty and R. M. Ormerod, J. Power Sources, 2000, 86, 390 CrossRef CAS.
- S. Majumdar, T. Claar and B. Flandermeyer, J. Am. Ceram. Soc., 1986, 69, 628 Search PubMed.
- H. Uchida, H. Suzuki and M. Watanabe, J. Electrochem. Soc., 1998, 145, 615 CAS.
- T. Tsai and S. A. Barnett, J. Electrochem. Soc., 1998, 145, 1696 CAS.
- R. M. Ormerod, Stud. Surf. Sci. Catal., 1999, 122, 35 Search PubMed , and refs. therein.
- E. S. Putna, J. Stubenrauch, J. M. Vohs and R. J. Gorte, Langmuir, 1995, 11, 4832 CrossRef CAS.
- J. T. S. Irvine, D. P. Fagg, J. Labrincha and F. M. B. Marques, Catal. Today, 1997, 38, 467 CrossRef CAS.
- P. Han and W. L. Worrell, J. Electrochem. Soc., 1995, 142, 4235 CAS.
- E. P. Murray and T. Tsai, Nature, 1999, 400, 649 CrossRef.
- S. Park, R. Craciun, V. Radu and R. J. Gorte, J. Electrochem. Soc., 1999, 146, 3603 CrossRef CAS.
- S. S. Liou and W. L. Worrell, Appl. Phys. A, 1989, 49, 25 CrossRef.
- M. Kertesz, I. Riess, D. S. Tannhauser, R. Langpape and F. J. Rohr, J. Sol. State Chem., 1982, 42, 125 Search PubMed.
- O. Yamamoto, Y. Takeda, R. Kanno and M. Noda, Solid State Ionics, 1987, 22, 241 Search PubMed.
- H. Taimatsu, K. Wada and H. Kaneko, J. Am. Ceram. Soc., 1992, 75, 401 Search PubMed.
- Y. Ohno, S. Nagata and H. Sato, Solid State Ionics, 1981, 3, 439 Search PubMed.
- J. Mizusaki, Y. Mima, S. Yamauchi, K. Fueki and H. Tagawa, J. Solid State Chem., 1989, 80, 102 CrossRef CAS.
- J. Mizusaki, J. Tabuchi, T. Matsuura, S. Yamauchi and K. Fueki, J. Electrochem. Soc., 1989, 136, 2082 CAS.
- J. M. Ralph, A. C. Schoeler and M. Krumpelt, J. Mater. Sci., 2001, 36, 1161 CrossRef CAS.
- R. Doshi, V. L. Richards, J. D. Carter, X. P. Wang and M. Krumpelt, J. Electrochem. Soc., 1999, 146, 1273 CrossRef CAS.
- D. P. Karim and A. T. Aldred, Phys. Rev. B, 1979, 20, 2255 CrossRef CAS.
- W. J. Weber, C. W. Griffin and J. B. Bates, J. Am. Ceram. Soc., 1987, 70, 265 Search PubMed.
- J. Staniforth and R. M. Ormerod, Green Chem., 2001, 3, G61 RSC.
- J. R. Rostrup-Nielson and L. J. Christiansen, J. Catal., 1995, 126, 381.
- C. H. Bartholomew, Catal. Rev. Sci. Eng., 1982, 24, 67 Search PubMed , and refs. therein.
- J. R. Rostrup-Nielson, Catal. Today, 1993, 18, 305 CrossRef.
- S. C. Tsang, J. B. Claridge and M. L. H. Green, Catal. Today, 1995, 23, 3 CrossRef CAS , and refs. therein.
- V. V. Galvita, V. D. Belyaev, V. N. Parmon and V. A. Sobyanin, Catal. Lett., 1996, 39, 209 Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |
