Ferromagnetic bonding in high-spin alkali-metal clusters. How does sodium compare to lithium?
Sam P.
de Visser
,
David
Danovich
and
Sason
Shaik
*
Department of Organic Chemistry and the Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University of Jerusalem, 91904, Jerusalem, Israel. E-mail: sason@yfaat.ch.huji.ac.il; Fax: +972-2-6584680
First published on 20th November 2002
Abstract
High-spin sodium clusters (n+1Nan; n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–12) are studied using a combined density functional (B3P86/cc-pVDZ) and UCCSD(T)/cc-pVDZ approach. In agreement with previous findings for high-spin lithium clusters, here too the minimum energy clusters are compact species with a high coordination number for each atom in the cluster. Generally, though, high-spin bonding in sodium clusters is much weaker than in the corresponding high-spin lithium clusters. Furthermore, the electronic configuration and the ordering of the molecular orbitals of high-spin sodium clusters differ from those in the high-spin lithium clusters. The electronic states of high-spin sodium clusters have much less p-character than corresponding high-spin lithium clusters. Molecular orbital and valence bond considerations show that the reduced p-participation in bonding is the root cause of the weak ferromagnetic bonding in sodium vs. lithium clusters.
2–12) are studied using a combined density functional (B3P86/cc-pVDZ) and UCCSD(T)/cc-pVDZ approach. In agreement with previous findings for high-spin lithium clusters, here too the minimum energy clusters are compact species with a high coordination number for each atom in the cluster. Generally, though, high-spin bonding in sodium clusters is much weaker than in the corresponding high-spin lithium clusters. Furthermore, the electronic configuration and the ordering of the molecular orbitals of high-spin sodium clusters differ from those in the high-spin lithium clusters. The electronic states of high-spin sodium clusters have much less p-character than corresponding high-spin lithium clusters. Molecular orbital and valence bond considerations show that the reduced p-participation in bonding is the root cause of the weak ferromagnetic bonding in sodium vs. lithium clusters.
Introduction
Ferromagnetic (FM) bonding is a special type of bonding in which the molecules or clusters are devoid of electron pairs.1–5 This peculiar bonding type, for instance, occurs in the triplet lithium dimer (3Li2),1–4 which in its lowest lying state has two singly occupied orbitals, namely the bonding and anti-bonding combination formed from the two atomic 2s orbitals on the two lithium centers. Although the covalent wave function which involves pure 2s orbital occupation produces a repulsive potential, the mixing in of covalent and ionic structures which involve 2p atomic orbital occupation into the 2s-only wave function provides the bonding interaction.2,4 For a series of high-spin lithium clusters (n+1Lin; n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–12), we showed4 that the FM-bonding energy rises steeply and reaches a value of as much as 12 kcal mol−1 per atom. Thus, FM-bonding can be very strong despite the absence of electron pairing. Furthermore, these ferromagnetic clusters were found to prefer highly symmetrical egg-shaped structures with high coordination numbers. Do other alkali atoms form ferromagnetically bound clusters? This question is the focus of the present study.
2–12), we showed4 that the FM-bonding energy rises steeply and reaches a value of as much as 12 kcal mol−1 per atom. Thus, FM-bonding can be very strong despite the absence of electron pairing. Furthermore, these ferromagnetic clusters were found to prefer highly symmetrical egg-shaped structures with high coordination numbers. Do other alkali atoms form ferromagnetically bound clusters? This question is the focus of the present study.
Despite the fact that alkali-metal clusters are widely studied, the amount of work done on the high-spin clusters is limited. Early theoretical work using the MCSCF technique was performed by Konowalow et al.6,7 who studied a series of low lying electronic states of the sodium dimer. The bond dissociation energy of the lowest triplet energy state (x 3Σ+u) was calculated to be 0.52 kcal mol−1 at an interatomic distance of 5.206 Å. Using an experimental Rydberg–Klein–Rees potential Friedman-Hill and Field8 determined a dissociation energy of 0.50 kcal mol−1 at an equilibrium bond length of 5.011 Å for the 3Σ+u state. This bond dissociation energy was reproduced by Ho et al.9 using the Reproducing Kernel Hilbert Space (RKHS) method and by Gutowski10 at the CCSD(T) level of theory. The optimized equilibrium bond lengths using these methods were 5.089 Å and 5.192 Å, respectively.
Higgins and coworkers have performed laser induced emission spectroscopy experiments of sodium dimers11 and trimers12–15 in helium droplets. In the case of the trimer, the emission spectrum for the 2 4E′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ←
←![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 4A2′ transition was studied. In addition, the vibrational spectra were calculated using the Reproducing Kernel Hilbert Space (RKHS) methodology and CCSD(T) calculations. The obtained spectra compared best with 4Na3 in D3h symmetry. The bond dissociation energy was found to be 2.43 kcal mol−1 with a bond distance of 4.406 Å. Other experimentally studied high-spin ferromagnetic clusters are the 3Rb2 and 3Cs2 dimers, which were also found to be weakly bound.16,17
1 4A2′ transition was studied. In addition, the vibrational spectra were calculated using the Reproducing Kernel Hilbert Space (RKHS) methodology and CCSD(T) calculations. The obtained spectra compared best with 4Na3 in D3h symmetry. The bond dissociation energy was found to be 2.43 kcal mol−1 with a bond distance of 4.406 Å. Other experimentally studied high-spin ferromagnetic clusters are the 3Rb2 and 3Cs2 dimers, which were also found to be weakly bound.16,17
Since our previous work on high-spin lithium clusters2–4 showed that these type of clusters can be strongly bound, we decided to investigate high-spin sodium clusters (n+1Nan; n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–12) using a density functional theoretic (DFT) approach. The aim of the study is to delineate the differences and similarities between FM-bonds in lithium vs. sodium clusters.
2–12) using a density functional theoretic (DFT) approach. The aim of the study is to delineate the differences and similarities between FM-bonds in lithium vs. sodium clusters.
Theoretical methods
All calculations presented here were performed with the Gaussian 98 program package.18 Following our experience with high-spin lithium clusters, the calculations were performed with B3P86 method using Becke's 3 parameter exchange functional19 in combination with the correlation functional due to Perdew (P86)20 and a cc-pVDZ basis set.21–23 In the case of the dimer, the correlation function of Perdew and Wang24 was also tested but found less suitable, based on the experimental data and the benchmark values obtained by the UCCSD(T) method (see below). Full geometry optimization followed by a complete frequency analyses were performed in all cases. Many different geometrical configurations were tested with a variety of symmetry groups but only the local minima are discussed here. In all situations, it was checked that the doubly occupied orbitals correspond to the core 1s, 2s and 2p orbitals only. For the smaller clusters additional UCCSD(T)/cc-pVDZ optimizations were performed as a benchmark for the DFT calculations. We emphasize that our studies of the lithium clusters demonstrated that the high-spin species invariably prefer different geometries than the low-spin ground state species. For example, while 5Li4 prefers a tetrahedral structure,2–4 the low-spin state 1Li4 assumes a rhombic (D2h) structure.25,26 This trend was found, during the present study, also for sodium clusters in a few test cases, 5,1Na4 and 12,2Na11. A rationale for the different geometries was given in ref. 2.Valence bond calculations of the covalent and ionic energy gaps (Fig. 4, see later) for square 5Li4vs.5Na4 were done with the Xiamen VB package.27
Results
Many different high-spin sodium clusters, n+1Nan in the range between n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–12, were studied with a variety of techniques ranging from DFT (B3P86 and B3PW91) to UCCSD(T). In the following, BDE stands for bond dissociation energy of the cluster of size n into n individual atoms in their ground states.
2–12, were studied with a variety of techniques ranging from DFT (B3P86 and B3PW91) to UCCSD(T). In the following, BDE stands for bond dissociation energy of the cluster of size n into n individual atoms in their ground states.
The triplet sodium dimer is optimized using the B3P86 and B3PW91 hybrid density functional methods as well as with UHF, UMP2 and UCCSD(T) ab initio methods and the results are summarized in Table 1. Similarly to the triplet lithium dimer, here too in the sodium dimer the lowest triplet state converged to a 3Σ+u state in all cases.
BDEs calculated with the density functional method B3P86 seem to be in excellent agreement with UCCSD(T) calculations. Bond lengths, however, are considerably underestimated by up to 10%. Changing the basis set from cc-pVDZ to cc-pVTZ does not improve the quality of the bond length. Notwithstanding the large discrepancy in bond lengths, the bond dissociation energy and the vibrational frequency are similar to the UCCSD(T)/cc-pVDZ result. Other density functional methods like B3PW91 give a slightly better bond distance but underestimate the bond dissociation energy. Since, the calculated bond dissociation energy and the vibrational frequency are of good quality with B3P86/cc-pVDZ we decided to continue with this method alone.
As a comparison, calculations on 3Na2 using UHF and UMP2 methods are added to Table 1. Bond lengths are even further off than the corresponding DFT obtained values. Furthermore, the energetics are extremely poor in comparison to the UCCSD(T) result. Therefore, the best cost-effective method for treating high-spin sodium clusters is the B3P86 density functional method in combination with a cc-pVDZ basis set.
Bond dissociation energies and geometric parameters of optimized 4Na3 and 5Na4 structures are written in Table 2. Geometry optimization of the minimum energy structures is repeated with the UCCSD(T)/cc-pVDZ method. Bond dissociation energies of 2.6 kcal mol−1 for the cyclic 4Na3 isomer and of 1.0 kcal mol−1 for the linear isomer are obtained. The B3P86 values (2.4 and 1.0 kcal mol−1) are in excellent agreement with the UCCSD(T) results. The bond distances, however, are underestimated with DFT, for the linear isomer, where we obtain r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.561 Å
(B3P86), vs.r
4.561 Å
(B3P86), vs.r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.051 Å with UCCSD(T). In the case of the triangle a much better agreement between B3P86 and UCCSD(T) is observed, namely 4.222 Å
vs. 4.351 Å, respectively. Our values compare very well with the calculated value of Higgins et al.14 who obtained a BDE
5.051 Å with UCCSD(T). In the case of the triangle a much better agreement between B3P86 and UCCSD(T) is observed, namely 4.222 Å
vs. 4.351 Å, respectively. Our values compare very well with the calculated value of Higgins et al.14 who obtained a BDE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 kcal mol−1 at an equilibrium distance of 4.406 Å for the D3h symmetrical sodium trimer for the 4A2′ state. Our calculations, however, gave a 4A1′ ground state. All our attempts to locate the 4A2′ state failed and resulted in 4A1′.
2.4 kcal mol−1 at an equilibrium distance of 4.406 Å for the D3h symmetrical sodium trimer for the 4A2′ state. Our calculations, however, gave a 4A1′ ground state. All our attempts to locate the 4A2′ state failed and resulted in 4A1′.
| Isomera | B3P86/cc-pVDZ | UCCSD(T)/cc-pVDZ | ||
|---|---|---|---|---|
| BDE/kcal mol−1 | Bond distance/Å | BDE/kcal mol−1 | Bond distance/Å | |
| a After each isomer is written the electronic state and the symmetry point group. All isomers correspond to local minima and had real frequencies only. | ||||
| 4Na3 | ||||
| Triangle 4A1′; D3h | +2.4 | 4.222 | +2.6 | 4.351 |
| Linear 4Σu; D∞h | +1.1 | 4.561 | +1.0 | 5.051 |
| 5Na4 | ||||
| Pyramid 5A1; Td | +8.3 | 3.854 | +10.2 | 3.947 |
| Linear 5Σg; D∞h | +1.9 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.508 4.508 |
+1.7 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.063 5.063 |
r
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.410 4.410 |
r
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.767 4.767 |
|||
Optimized 5Na4 geometries and energies calculated with B3P86/cc-pVDZ compare favorably with UCCSD(T)/cc-pVDZ data, as found for the smaller clusters. The UCCSD(T) calculated total BDEs are reproduced within 2 kcal mol−1 with the hybrid DFT method (B3P86). The bond length of the lowest lying quintet tetramer is reproduced reasonable well with B3P86, while the distances in the linear entity are underestimated by roughly 10%. In agreement with our previous results on the quintet lithium tetramer,3,4 the pyramidal configuration in Td symmetry is the lowest energy structure. The bond dissociation energy of the lithium tetramer, however, is substantially higher than the one obtained for the corresponding sodium cluster. At the UCCSD(T) level of theory BDEs of 29.6 kcal mol−1 (5Li4) and 10.2 kcal mol−1 (5Na4) were obtained.
The optimized high-spin sodium clusters in the range between the pentamer and the dodecamer are collected in Table 3. In agreement with our previous calculations on the sextet lithium pentamer,3,4 the linear and cyclic configurations are local minima also for 6Na5. The most stable 6Li5 cluster, however, was the pyramidal structure with C4v symmetry. In contrast, in the case of sodium the bipyramid in D3h symmetry is the most stable structure and is 3.7 kcal mol−1 lower in energy than the 6Na5 pyramid in C4v symmetry. The 6Na5 pyramid has one imaginary frequency of 32 cm−1 corresponding to a b2 vibrational mode which distorts the system toward C2v symmetry. A geometry optimization using the Cartesian coordinates of the vibrational mode added to the optimized pyramidal Cartesian led to a geometry identical in energy and geometrical parameters to the bipyramid. Consequently, the imaginary frequency of the pyramid leads to a distortion toward the bipyramid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–12) isomers calculated with the B3P86/cc-pVDZ method
5–12) isomers calculated with the B3P86/cc-pVDZ method
| Isomera | BDE/kcal mol−1 | Geometric parameters/Å |
|---|---|---|
| a After each isomer is written the electronic state and the symmetry point group. All isomers have real frequencies only. | ||
| 6Na5 | ||
| Bipyramid 6A2″; D3h | 9.8 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.789; r15 3.789; r15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.227 4.227 |
| Cyclo 6A1′; D5h | 5.2 |
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.010 4.010 |
| Linear 6Σg; D∞h | 2.7 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.481; r23 4.481; r23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.346; r34 4.346; r34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.268 4.268 |
| 7Na6 | ||
| Compact 7A; C1 | 12.1 | See Fig. 1. |
| Octahedral 7A1g; Oh | 7.1 |
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.543 4.543 |
| Linear 7Σu; D∞h | 3.6 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.462; r23 4.462; r23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.304; r34 4.304; r34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.268 4.268 |
| Cyclo 7B2u; D6h | 2.8 |
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.809 3.809 |
| 9Na8 | ||
| Compact 9A; C2 | 20.1 | See Fig. 1. |
| Linear 9Σg; D∞h | 5.5 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.429; r23 4.429; r23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.242; r34 4.242; r34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.167 4.167 |
r
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.145 4.145 |
||
| 10Na9 | ||
| Compact 10A2; C2v | 29.6 | See Fig. 1. |
| 11Na10 | ||
| Compact 11B2; D4d | 35.0 | See Fig. 1. |
| Linear 11Σu; D∞h | 7.7 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.382; r23 4.382; r23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.172; r34 4.172; r34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.082 4.082 |
r
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.041; r56 4.041; r56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.029 4.029 |
||
| 12Na11 | ||
| Compact 12A; C1 | 35.8 | See Fig. 1. |
| 13Na12 | ||
| Compact 13A; C1 | 39.1 | See Fig. 1. |
| Linear 13Σg; D∞h | 10.3 |
r
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.329; r23 4.329; r23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.105; r34 4.105; r34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.012 4.012 |
r
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.967; r56 3.967; r56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.947; r67 3.947; r67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.940 3.940 |
||
The difference between the pyramidal 6Li5 and 6Na5 systems is the ordering of the electronic states. In the pyramidal 6Li5 cluster4 the ground state is 6B1, whereas in 6Na5 this state is higher lying and the 6B2 state is the ground state. The highest singly occupied orbital of the 6B1 electronic state is a molecular orbital (with b1 symmetry) made up of the bonding combination of 3px and 3py atomic orbitals on the bottom plane of the pyramid containing four sodium atoms, i.e. the 3pσ orbital. The 6B2 electronic state, on the other hand, has singly occupied orbitals which involve only linear combination of the 3s atomic orbitals. In 6Na5 clusters the 3pσ bonding molecular orbital is higher in energy than the 3sσ* antibonding orbital. In the corresponding lithium clusters, the 2pσ orbital is lower in energy than the 2sσ*.4 Therefore, the 6B2 electronic state in sodium clusters is lower in energy than the 6B1 electronic state.
The most stable high-spin clusters of the sodium hexamer, octamer, nonamer decamer, undecamer and dodecamer are depicted in Fig. 1, while Table 3 contains some additional structures. The septuplet sodium hexamer has local minima in C1, Oh, D∞h and D6h point group symmetries. The 7Na6 minimum is a bicapped tetrahedron and close to being C2v symmetry, but all attempts to optimize this structure under the geometry constraint failed, leading to the C1 structure depicted in Fig. 1. In contrast to the lithium hexamer for which BDE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43.9 kcal mol−1, the 7Na6 octahedral system is a local minimum albeit with a considerably smaller BDE of 7.1 kcal mol−1, at the B3P86/cc-pVDZ level of theory. For high-spin sodium clusters from size n
43.9 kcal mol−1, the 7Na6 octahedral system is a local minimum albeit with a considerably smaller BDE of 7.1 kcal mol−1, at the B3P86/cc-pVDZ level of theory. For high-spin sodium clusters from size n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–12 we located two types of minima per cluster size, namely a linear structure and a structure with a maximum amount of intramolecular bonds, see Table 3.
8–12 we located two types of minima per cluster size, namely a linear structure and a structure with a maximum amount of intramolecular bonds, see Table 3.
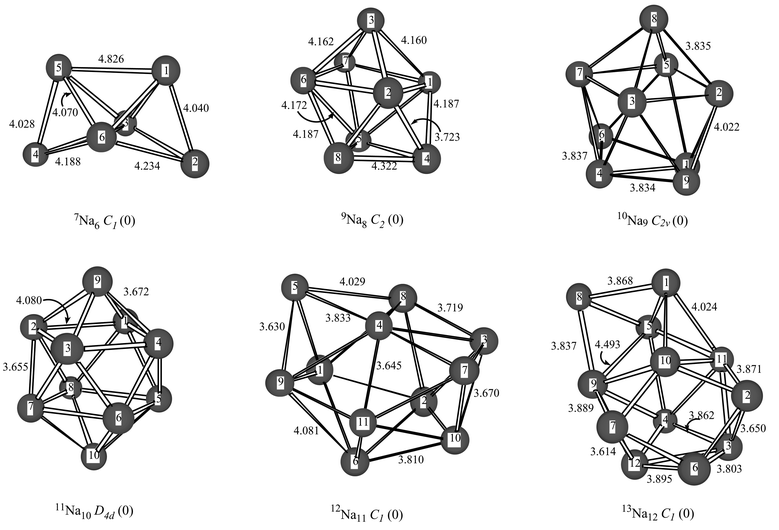 | ||
| Fig. 1 Optimized geometries of 7Na6 (C1 symmetry), 9Na8 (C2 symmetry), 10Na9 (C2v symmetry), 11Na10 (D4d symmetry), 12Na11 (C1 symmetry) and 13Na12 (C1 symmetry) at the B3P86/cc-pVDZ level of theory. Bond lengths are in Å. | ||
Discussion
In agreement with the high-spin lithium clusters the most stable high-spin sodium cluster is generally a compact system with a high and uniform coordination number. Fig. 2 shows the progression of the most stable clusters for the two atom systems. The similarities and differences are apparent. The most stable cluster gradually evolves to an egg-shaped structure with a high and uniform coordination number. However, the Li clusters are much more strongly bonded than their Na analogs.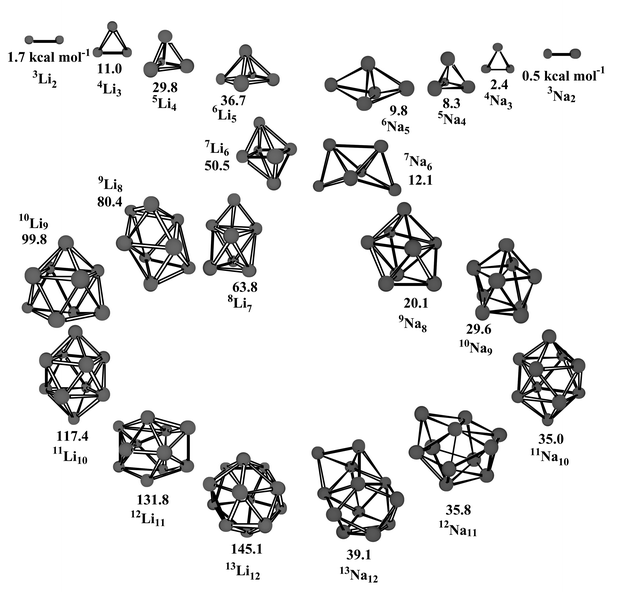 | ||
| Fig. 2 Evolution of the most stable n+1Linvs.n+1Nan clusters. BDEs (in kcal mol−1) are shown under each structure. | ||
In Fig. 3, the relative bond dissociation energy (BDE/n) is plotted as a function of cluster size for both the minimum energy sodium and lithium clusters. The relative bond dissociation energy is defined as the total bond dissociation energy divided by the number of atoms in the cluster. As can be seen from Fig. 3, the relative bond dissociation energies of the lithium and sodium clusters gradually increase and reach a flat region at around 10–12 atoms. In the case of sodium the maximum stabilization energy is around 3.3–3.5 kcal mol−1, the values obtained for the dodecamer and decamer, while in the case of lithium the value is approximately 12.0–12.1 kcal mol−1. In agreement with the high-spin lithium clusters, the relative bond dissociation energy for the tetrahedral sodium tetramer shows a small peak due to the relative short M–M distances and the high-symmetry.4
 | ||
Fig. 3 Relative bond dissociation energies (BDE/n) of minimum energy sodium and lithium clusters (n+1Lin and n+1Nan; n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–12) as a function of cluster size. The BDE/n values are indicated for the flat regions of the two curves. Energies calculated with B3P86/cc-pVDZ. 2–12) as a function of cluster size. The BDE/n values are indicated for the flat regions of the two curves. Energies calculated with B3P86/cc-pVDZ. | ||
In order to compare the differences in electronic properties of high-spin lithium and sodium clusters, we investigated several low lying states of the square 5Li4 and 5Na4 configurations. The square species have high symmetry (D4h symmetry) and therefore, the orbital shapes are easier to interpret than in three-dimensional structures like the pyramidal configuration. In the case of the quintet cyclic tetramer, three low lying electronic states were investigated. The relative ordering of the lowest electronic states in cyclic lithium and sodium tetramers are depicted on the left-hand-side of Fig. 4. The right-hand-side of this figure shows the relevant orbitals for the lithium clusters. The 3sσ, 3pσ and 3pπ orbitals on sodium show much similarity to the corresponding lithium 2sσ, 2pσ and 2pπ orbitals, and are therefore not drawn. All investigated electronic states have singly occupied sσ(a1g), eu and eu orbitals.
 | ||
| Fig. 4 Low-lying electronic states of 5Li4 and 5Na4 in D4h symmetry calculated with the UCCSD(T) and B3P86 (in brackets) methods. The shape of the orbitals of the lithium clusters are plotted on the right-hand-side. The 2pσ(b2g) and 2pσ(a1g) orbitals show only interstitial lobes. Relative energies are in kcal mol−1. | ||
The lowest lying electronic state in the lithium square has a highest singly occupied orbital that possesses electron densities in between the atoms5 and is a 2pσ orbital generated from the bonding combination of 2px and 2py atomic orbitals with b2g symmetry, 2pσ(b2g). An excited state corresponding to a b2g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a2u transition is 3.1 kcal mol−1
(UCCSD(T)) above the ground state and leads to filling of the 2pπ(a2u) bonding orbital made from a linear combination of 2pz atomic orbitals. Above this 2pπ(a2u) molecular orbital is another bonding orbital which is the result of a linear combination of 2px and 2py atomic orbitals and has a1g symmetry, 2pσ(a1g). We were not able to locate the corresponding electronic state (5A1g) with the UCCSD(T) method. However, using B3P86 this state was found to lie 9.5 kcal mol−1 above the lowest square conformation. Finally, the b1g molecular orbital is the result of the antibonding combination of 2s atomic orbitals and occupation of this particular orbital (2sσ*(b1g)) leads to a highly excited state, 16.0 kcal mol−1
(UCCSD(T)) above the 5B2g electronic state, see Fig. 4.
a2u transition is 3.1 kcal mol−1
(UCCSD(T)) above the ground state and leads to filling of the 2pπ(a2u) bonding orbital made from a linear combination of 2pz atomic orbitals. Above this 2pπ(a2u) molecular orbital is another bonding orbital which is the result of a linear combination of 2px and 2py atomic orbitals and has a1g symmetry, 2pσ(a1g). We were not able to locate the corresponding electronic state (5A1g) with the UCCSD(T) method. However, using B3P86 this state was found to lie 9.5 kcal mol−1 above the lowest square conformation. Finally, the b1g molecular orbital is the result of the antibonding combination of 2s atomic orbitals and occupation of this particular orbital (2sσ*(b1g)) leads to a highly excited state, 16.0 kcal mol−1
(UCCSD(T)) above the 5B2g electronic state, see Fig. 4.
In contrast to the lithium square, in the sodium square configuration the 5B1g state is the lowest electronic state in Fig. 4. Replacing the 3sσ*(b1g) orbital with either the 3pπ(a2u) or the 3pσ(b2g) orbitals gives excited states which are 3.7 and 4.7 kcal mol−1 higher lying. The difference in ordering of the molecular orbitals is due to the difference in splitting of 2s/2p atomic orbitals and 3s/3p atomic orbitals. This in turn derives from the interatomic distances, which are short in n+1Lin and considerably longer in n+1Nan.
Fig. 5 illustrates the splitting of 2s and 2p atomic orbitals into molecular orbitals for lithium vs. the 3s and 3p atomic orbitals of sodium, into the corresponding molecular orbitals. In lithium clusters with the shorter distances the splitting is large and the 2pσ and 2pπ bonding type orbitals drop below the 2sσ* anti-bonding orbital. In sodium clusters where the distances are long this is not the case and the 3pσ orbital remains well above the 3sσ*. Consequently, in lithium clusters the highest filled orbital is the bonding 2pσ orbital, while in the sodium cluster the highest filled orbital is 3sσ*. With occupation in the bonding 2pσ orbital, the net ferromagnetic bonding energy in 5Li4 is large, BDE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.8 kcal mol−1. In contrast, with 3sσ* occupancy in 5Na4 the ferromagnetic bonding diminishes to a mere 2.1 kcal mol−1.
19.8 kcal mol−1. In contrast, with 3sσ* occupancy in 5Na4 the ferromagnetic bonding diminishes to a mere 2.1 kcal mol−1.
 | ||
| Fig. 5 Orbital splitting in lithium (left) and sodium (right) clusters. | ||
The valence bond (VB) model in our previous papers2,4 offers a complementary insight into the diminished ferromagnetic bonding in n+1Nanvs.n+1Lin. Fig. 6 shows the main VB structures which participate in the ferromagnetic (FM) bonding, for the example of the square 5M4
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li, Na) species. The lowest configuration (Φcov) is the covalent all-s wave function where all the electrons occupy the s(2s, 3s) orbitals of the atoms and have the same spin. This configuration is repulsive and at a finite distance lies above the separate atoms.2,4 Above the covalent s-only there is a group of ionic configurations, Φion. As we showed before,4 it is enough to consider the mono ionic configurations. Each such ionic structure involves an excited 3M− atom with s1p1 occupancy. Still higher in energy are the excited covalent structures, Φcov* where adjacent pairs of atoms involve p1p1 occupancy due to excitations from s to p on these atoms. The FM-bonding arises due to mixing of the ionic and excited covalent configurations into the repulsive all-s structure. The ionic structures were shown to be crucial for attaining net bonding. The Φion–Φcov mixing is inversely proportional to the energy gap, ΔEcov–ion, between the corresponding configurations. The smaller the gap, the stronger the mixing and the more significant is the FM-bonding. An important factor that sets the gap, ΔEcov–ion, is the electrostatic stabilization of the ionic structures. Since the ionic radii of sodium (Na+ and 3Na−) are significantly larger than those of lithium, ionic structures of the sodium cluster will undergo a much lesser electrostatic stabilization compared with the lithium case. Indeed, the computed energy gaps show a much smaller ΔEcov–ion for the lithium tetramer. Consequently, the Φcov–Φion mixing in sodium will be much smaller than in lithium. The resulting ferromagnetic bond energy, BDE(FM) will accordingly be much smaller for sodium. The weakening in Φcov–Φion mixing for 5Na4 will in turn correspond to a diminished p-contribution to FM-bonding in 5Na4 and in general in high-spin sodium clusters, n+1Nan, compared with their lithium analogs, n+1Lin.
Li, Na) species. The lowest configuration (Φcov) is the covalent all-s wave function where all the electrons occupy the s(2s, 3s) orbitals of the atoms and have the same spin. This configuration is repulsive and at a finite distance lies above the separate atoms.2,4 Above the covalent s-only there is a group of ionic configurations, Φion. As we showed before,4 it is enough to consider the mono ionic configurations. Each such ionic structure involves an excited 3M− atom with s1p1 occupancy. Still higher in energy are the excited covalent structures, Φcov* where adjacent pairs of atoms involve p1p1 occupancy due to excitations from s to p on these atoms. The FM-bonding arises due to mixing of the ionic and excited covalent configurations into the repulsive all-s structure. The ionic structures were shown to be crucial for attaining net bonding. The Φion–Φcov mixing is inversely proportional to the energy gap, ΔEcov–ion, between the corresponding configurations. The smaller the gap, the stronger the mixing and the more significant is the FM-bonding. An important factor that sets the gap, ΔEcov–ion, is the electrostatic stabilization of the ionic structures. Since the ionic radii of sodium (Na+ and 3Na−) are significantly larger than those of lithium, ionic structures of the sodium cluster will undergo a much lesser electrostatic stabilization compared with the lithium case. Indeed, the computed energy gaps show a much smaller ΔEcov–ion for the lithium tetramer. Consequently, the Φcov–Φion mixing in sodium will be much smaller than in lithium. The resulting ferromagnetic bond energy, BDE(FM) will accordingly be much smaller for sodium. The weakening in Φcov–Φion mixing for 5Na4 will in turn correspond to a diminished p-contribution to FM-bonding in 5Na4 and in general in high-spin sodium clusters, n+1Nan, compared with their lithium analogs, n+1Lin.
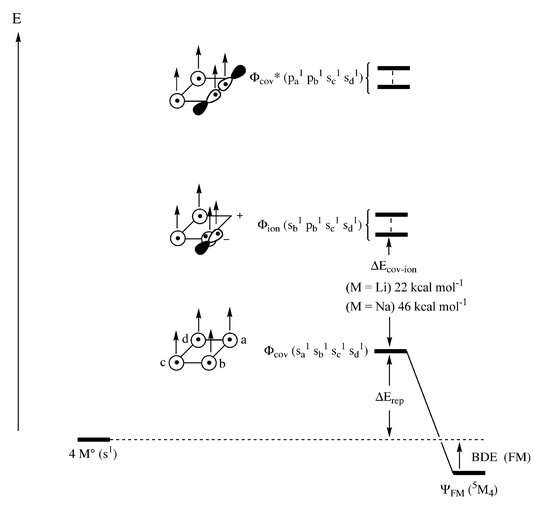 | ||
Fig. 6 The mixing of covalent (Φcov) structure with higher lying ionic (Φion) and excited covalent (Φcov*) structures to generate a bonding wave function (ΨFM) for 5M4
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li, Na). The occupancies of one Φion and Φcov configurations are shown explicitly. Li, Na). The occupancies of one Φion and Φcov configurations are shown explicitly. | ||
Conclusions
A DFT study of high-spin sodium clusters is performed for the first time, yielding an extended set of FM-bond energies and structures. In contrast to high-spin lithium clusters, the corresponding sodium clusters are weakly bound. The differences between high-spin lithium and sodium clusters are associated with the relative ordering of the molecular orbitals and derive from the longer Na–Na distances in comparison with Li–Li. Due to the shorter Li–Li distances the 2pσ orbitals cross below the 2sσ* type orbitals and become populated. This endows the high-spin lithium clusters with a significant FM-bonding energy. In high-spin sodium clusters, on the contrary, the distances are longer and the 3pσ orbitals remain higher than the 3sσ* orbitals. Therefore, the set of singly occupied orbitals in the high-spin clusters contain the same number of bonding as antibonding orbitals, hence causing the high-spin sodium clusters to be only marginally stable. Using a VB description2,4 the diminished p-contribution to the FM-bonding is associated with a smaller-extent of mixing of the ionic structures into the all s-covalent structure (Fig. 6).Except for the quantitative aspect, the high-spin sodium and lithium clusters exhibit the same general trends. In both cases, the FM-bond energy per atom increases with the cluster size and converges at clusters with 10–12 atoms. Furthermore, in both cases the clusters tend to maximize the coordination number of the constituent atoms in order to optimize FM-bonding. Although going from lithium to sodium caused weakening of ferromagnetic bonding, this may not necessarily be the case when going further down to potassium as nonregularities are often observed down a column in the periodic table.28 Important new factors such as 3d and 4f contractions, as well as relativistic effects may disturb the regularity. Therefore, further mapping of the occurrence and strength of ferromagnetic bonding will have to follow in order to explore FM-bonding trends in the periodic table.
Acknowledgements
The authors wish to thank the Ministry of Sciences of the Niedersachsen States and the Robert Szold Fund for support.References
- M. N. Glukhovtsev and P. von Ragué Schleyer, Isr. J. Chem., 1993, 33, 455 Search PubMed.
- D. Danovich, W. Wu and S. Shaik, J. Am. Chem Soc., 1999, 121, 3165 CrossRef CAS.
- S. P. de Visser, Y. Alpert, D. Danovich and S. Shaik, J. Phys. Chem. A, 2000, 104, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 CAS.
223 CAS. - S. P. de Visser, D. Danovich, W. Wu and S. Shaik, J. Phys. Chem. A, 2002, 106, 4961 CrossRef CAS.
- M. H. McAdon and W. A. Goddard III, J. Phys. Chem., 1988, 92, 1352 CrossRef CAS.
- D. D. Konowalow, M. E. Rosenkrantz and M. L. Olson, J. Chem. Phys., 1980, 72, 2612 CrossRef CAS.
- D. D. Konowalow and M. E. Rosenkrantz, J. Phys. Chem., 1982, 86, 1099 CAS.
- E. J. Friedman-Hill and R. W. Field, J. Chem. Phys., 1992, 96, 2444 CrossRef CAS.
- T.-S. Ho, H. Rabitz and G. Scoles, J. Chem. Phys., 2000, 112, 6218 CrossRef CAS.
- M. Gutowski, J. Chem. Phys., 1999, 110, 4695 CrossRef CAS.
- J. Higgins, C. Callegari, J. Reho, F. Stienkemeier, W. E. Ernst, M. Gutowski and G. Scoles, J. Phys. Chem. A, 1998, 102, 4952 CrossRef CAS.
- J. Higgins, C. Callegari, J. Reho, F. Stienkemeier, W. E. Ernst, K. K. Lehmann, M. Gutowski and G. Scoles, Science, 1996, 273, 629 CAS.
- J. Higgins, W. E. Ernst, C. Callegari, J. Reho, K. K. Lehmann and G. Scoles, Phys. Rev. Lett., 1996, 77, 4532 CrossRef CAS.
- J. Higgins, T. Hollebeek, J. Reho, T.-S. Ho, K. K. Lehmann, H. Rabitz, G. Scoles and M. Gutowski, J. Chem. Phys., 2000, 112, 5751 CrossRef CAS.
- J. H. Reho, J. Higgins, M. Nooijen, K. K. Lehmann and G. Scoles, J. Chem. Phys., 2001, 115, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 CrossRef CAS.
265 CrossRef CAS. - F. R. Brühl, R. A. Miron and W. E. Ernst, J. Chem. Phys., 2001, 115, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 CAS.
275 CAS. - A. Fiortti, D. Comparati, A. Crubelier, O. Dulieu, F. Mansou-Seeuws and P. Pillet, Phys. Rev. Lett., 1998, 80, 4402 CrossRef CAS.
- Gaussian 98, Revision A.9, Gaussian Inc., Pittsburgh PA, 1998.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B, 1986, 33, 8822 CrossRef.
- T. H. Dunning, Jr., J. Chem. Phys., 1989, 90, 1007 CrossRef CAS.
- D. E. Woon and T. H. Dunning, Jr., J. Chem. Phys., 1993, 98, 1358 CrossRef CAS.
- D. E. Woon, private communication.
- J. P. Perdew and Y. Wang, Phys. Rev. B, 1992, 45, 13
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 CrossRef.
244 CrossRef. - F. Gardet, F. Rogemond and H. Chermette, J. Chem. Phys., 1996, 105, 9933 CrossRef.
- V. Bonacic-Koutecky, P. Fantucci and J. Koutecky, Chem. Rev., 1991, 91, 1035 CrossRef CAS.
- W. Wu, L. Song, Y. Mo and Q. Zhang, XIAMEN—an ab initio spin-free valence bond program, Xiamen University, Xiamen, P. R. of China.
- P. Pyykkö, Int. J. Quantum Chem., 2001, 85, 18 CrossRef CAS.
| This journal is © the Owner Societies 2003 |
