Analysis of copolymers of styrene and methyl methacrylate using size exclusion chromatography with multiple detection
Ricardo
Medrano
,
M. Teresa R.
Laguna
,
Enrique
Saiz
and
M. Pilar
Tarazona
Departamento de Química Física, Universidad de Alcala, 28871 Alcalá de Henares, Spain. E-mail: enrique.saiz@uah.es; Fax: +34 91 8854763
First published on 26th November 2002
Abstract
Size exclusion chromatography (SEC) with differential refractive index (RI), multiangle light scattering (MALS) and UV detectors has been used to obtain information about copolymers of styrene (S) and methyl methacrylate (MMA). Homopolymers PS and PMMA having broad molecular weight distributions, mixtures of PS and PMMA homopolymers, and copolymers containing different weight fractions of S and MMA units were studied. Molecular weight distributions, molecular dimensions and scaling laws are reported for these systems. The behavior of the random copolymers is very different from that exhibited by the heterogeneous mixtures of homopolymers. Thus, the results obtained for the copolymers can be explained by assuming that these molecules have homogeneous distributions of S and MMA units along their chains. On the contrary, the mixtures produce the results that would be expected from heterogeneous combinations of different molecules. The SEC technique offers the possibility of determining the composition of random copolymers and discriminating among random copolymers (having homogeneous distributions of units) and block copolymers.
Introduction
Many polymer properties are influenced by their molecular weight or more precisely by their molecular weight distribution (MWD), since most synthetic polymers are polydisperse. Size exclusion chromatography (SEC) is the best way to obtain information about the molecular weight distribution. In this technique, molecules are separated according to their size, yielding a molar size distribution. Since the size of the polymer depends not only on its molecular weight but also on the polymer–solvent interactions, a calibration with narrow polydispersity index standards of the same polymer is required in order to obtain the true molecular weight distribution. Unfortunately, suitable monodisperse standards are scarce and other approaches are used to overcome this lack of standards. Universal calibration1 can be applied if the Mark–Houwink constants for the polymer in the conditions of the SEC experiment, solvent and temperature, are known. Numerical procedures, using broad polydisperse samples can also be used.2 Nevertheless, the best approach consists in using molecular weight sensitive detectors coupled with the customary concentration detector.3–5 The use of multiangle light scattering6,7 as the detection method for SEC offers the possibility of analyzing a polymer sample in terms of the distribution of both molecular weight and molecular dimensions, represented by the mean square radius of gyration,6,8 〈s2〉. Both magnitudes are determined in an absolute way and, for broad molecular weight distribution polymer samples, the dependence of the radius of gyration on molecular weight affords information about the solution properties of the polymer.9The use of SEC with several detectors for providing quantitative information on copolymer composition and molecular weight distribution has been studied during the last decades.10–13 However, this use is complicated by the fact that the heterogeneity of the copolymer may influence the relationship of dimensions and molecular weight. In this sense, the light scattering detector which affords both dimensions and molecular weights simultaneously, seemed a promising technique and it has been studied intensively in recent years.14–18 Unfortunately, the heterogeneity in the chemical composition of the copolymer can also affect the value of the refractive index increment along the chromatogram and therefore may introduce errors in the magnitudes obtained with both differential refractive index and light scattering detectors.19
In this paper we describe a modification of the technique consisting in the addition of a new concentration sensitive detector, i.e. employing differential refractive index (RI), multiangle light scattering (MALS) and ultraviolet (UV) detectors. This combination allows the study of systems containing two different kinds of repeating units, such as copolymers with different sequences or mixtures of homopolymers, and provides magnitudes of interest from the physicochemical point of view such as molecular weight distributions and averages, chain shape and dimensions, scaling law coefficients, etc. for these systems. Three different kinds of systems have been studied, namely: (a) polystyrene (PS) and poly(methyl methacrylate) (PMMA) homopolymers with broad molecular weight distributions, (b) random styrene–methyl methacrylate copolymers containing different weight fractions of both monomers and (c) Mixtures of PS and PMMA homopolymers. Thus, the influence of the chemical homogeneity on the measured magnitudes can be studied since the mixtures of homopolymers are heterogeneous while the random copolymers are supposedly homogeneous. In fact, the copolymerization of styrene and methacrylate has been widely studied and it is used frequently as a model of free radical polymerization.20–23
Experimental procedures
Materials
Styrene (STY) and methyl methacrylate (MMA) (Acros) were vacuum distilled and refrigerated until required. 2,2′-azobis(isobutyronitrile) (AIBN) (Across) and HPLC grade Toluene (Scharlau) were used as received. THF used as eluent for SEC was HPLC grade (Scharlau). PS was obtained by thermal bulk polymerization of styrene at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 14 h, while PMMA was bought from Aldrich (ref. 18, 226-5).
°C for 14 h, while PMMA was bought from Aldrich (ref. 18, 226-5).
Copolymers were prepared according to the following procedure: 20 mg of AIBN were added to four large Pyrex tubes capped with serum stoppers. The tubes were evacuated and flushed with dry nitrogen. The following monomer amounts were injected with a syringe: 4 ml MMA and 16 ml STY (Polymer A); 8 ml MMA and 12 ml STY (Polymer B); 12 ml MMA and 8 ml STY (Polymer C); 16 ml MMA and 4 ml STY (Polymer D). The tubes were shaken and placed in a constant temperature bath at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2.5 h. Then the polymers were precipitated into methanol and dried in a vacuum oven. The reactivity ratios for radical copolymerization of styrene and methyl methacrylate are24rS
°C for 2.5 h. Then the polymers were precipitated into methanol and dried in a vacuum oven. The reactivity ratios for radical copolymerization of styrene and methyl methacrylate are24rS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.48, rMMA
0.48, rMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.46. Application of the copolymer equation with these values of r allows the evaluation of the composition of the copolymer formed at the first stages of the reaction (i.e. at low conversion) as function of the composition of the reaction mixtures. The composition calculated in this fashion for samples A through D are given as weight fraction of styrene in the second column of Table 1.
0.46. Application of the copolymer equation with these values of r allows the evaluation of the composition of the copolymer formed at the first stages of the reaction (i.e. at low conversion) as function of the composition of the reaction mixtures. The composition calculated in this fashion for samples A through D are given as weight fraction of styrene in the second column of Table 1.
| System | w S (Ec. copolymer) | w S (1H NMR) | (dn/dc)/ml g−1 | w S (eqn. (3)) | M i RI/MiUV (eqn. (14)) | w S (eqn. (18)) |
|---|---|---|---|---|---|---|
| A | 0.73 | 0.63 | 0.152 | 0.68 | 0.79 | 0.79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
| B | 0.57 | 0.51 | 0.145 | 0.61 | 0.63 | 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| C | 0.44 | 0.41 | 0.130 | 0.45 | 0.49 | 0.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| D | 0.38 | 0.35 | 0.124 | 0.39 | 0.36 | 0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| PMMA | 0.087 | 0 | ||||
| PS | 0.182 | 1 | ||||
| M1 | 0.166 | 0.83 | ||||
| M2 | 0.138 | 0.54 |
Two mixtures of PS and PMMA homopolymers were prepared from weighted amounts of PS and PMMA. Their weight fraction of styrene were wS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.83 (mixture M1) and wS
0.83 (mixture M1) and wS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.54 (mixture M2).
0.54 (mixture M2).
Measurements
SEC measurements were carried out using a Waters Associates equipment with a model 510 pump, a U6K injector, a differential refractive index detector model 410 (RI) and a UV detector model 490E (UV) working at λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 262 nm where styrene presents a strong absorption while methyl methacrylate does not absorb so that this detector only sees styrene units. A Dawn-DSP-F multiangle laser light scattering photometer (MALS) operating at 632.8 nm from Wyatt Technology Corp. was the molecular weight and size detector used. Calibration of the Dawn was done with toluene and the normalization of the detectors was performed with standard monodisperse polystyrene of low molecular weight that did not show angular dependence on the light scattering signal. Standard monodisperse polystyrene was also used to determine the interdetector volume.7 Two columns PLgel mixed B (Polymer Laboratories) in series completed the equipment. THF, freshly distilled from sodium and benzophenone, filtered through a 0.2 μm Fluoropore filter and degassed, was used as eluent at a flow rate of 1.0 ml min−1. The software used, ASTRA 4.2 from Wyatt Technology Corp., allowed on-line data collection of molecular weight and radius of gyration as well as calculation of the distributions and averages.
262 nm where styrene presents a strong absorption while methyl methacrylate does not absorb so that this detector only sees styrene units. A Dawn-DSP-F multiangle laser light scattering photometer (MALS) operating at 632.8 nm from Wyatt Technology Corp. was the molecular weight and size detector used. Calibration of the Dawn was done with toluene and the normalization of the detectors was performed with standard monodisperse polystyrene of low molecular weight that did not show angular dependence on the light scattering signal. Standard monodisperse polystyrene was also used to determine the interdetector volume.7 Two columns PLgel mixed B (Polymer Laboratories) in series completed the equipment. THF, freshly distilled from sodium and benzophenone, filtered through a 0.2 μm Fluoropore filter and degassed, was used as eluent at a flow rate of 1.0 ml min−1. The software used, ASTRA 4.2 from Wyatt Technology Corp., allowed on-line data collection of molecular weight and radius of gyration as well as calculation of the distributions and averages.
The differential refractive index increments for the polymers in THF solution were measured with a Brice–Phoenix differential refractometer at 436 and 546 nm, at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, and extrapolated to 632.8 nm using the Cauchy relationship.6
°C, and extrapolated to 632.8 nm using the Cauchy relationship.6
The 1H NMR spectra of the copolymers were obtained with a UNITY 300-MHz. The composition was determined in two ways. The molar fraction of styrene units can be obtained by multiplying the integral of aromatic protons by a factor of 8/5 and dividing this quantity by the sum of the integrals for all of the protons.20 Also, the molar fraction of styrene can be determined from the relationship between the aromatic protons of the styrene units and the methoxy protons of the methyl methacrylate units, which appear between δ values of 2.2 and 3.5 ppm.22 Both procedures gave the same results that are presented in the third column of Table 1 as weight fraction of styrene units.
Fig. 1 shows the raw signals from the detectors versus elution volume for both PS and PMMA homopolymers. In the case of PMMA only the RI signal is given because the UV detector does not see this polymer. These raw data were converted into true molecular weight distributions (MWD) by transforming detector signals into weight fractions and measuring molar masses at each elution volume with the MALS detector. The MWD of both polymers are shown in Fig. 2. The molecular weight averages obtained for PMMA are: Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (3.4
(3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2)
0.2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105, Mw
105, Mw![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (6.3
(6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2)
0.2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 g mol−1, polydispersity ratio r
105 g mol−1, polydispersity ratio r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw/Mn
Mw/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9.
1.9.
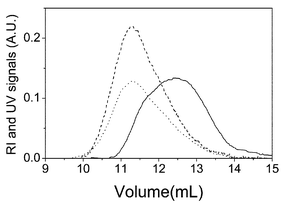 | ||
| Fig. 1 Raw signal from the detectors as function of elution volume for PMMA (solid line: RI detector) and PS (dash line: UV and dot line: RI detectors) homopolymers. | ||
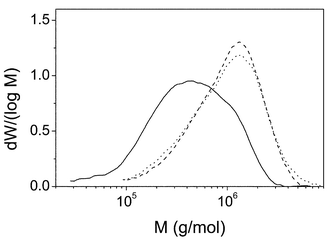 | ||
| Fig. 2 Molecular weight distributions for PMMA (solid line, obtained from RI detector) and PS (dash line from UV and dot line from RI) homopolymers. | ||
Both detectors see the PS, and the data obtained from UV are shown as dash lines in Figs. 1 and 2 while data from RI are represented by dot lines. The UV signal for a homopolymer solution having mass concentration c is given by:
SUV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kUVc kUVc | (1) |
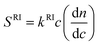 | (2) |
However, the normalization required to transform raw signals into weight fractions eliminates the k response factors of both detectors and the refractive index increment of the RI detector, so that both kinds of signals should provide the same MWD distribution, as the comparison of dot and dash lines of Fig. 2 indicates. Molecular weight averages obtained for the PS homopolymer from both detectors agree within experimental errors providing: Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (8.1
(8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5)105, Mw
0.5)105, Mw![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (13.0
(13.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4)
0.4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 g mol−1, r
105 g mol−1, r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw/Mn
Mw/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6.
1.6.
Theory
Refractive index increment
The refractive index increment, dn/dc, of a copolymer may be expressed as a function of the corresponding values of the homopolymers as:6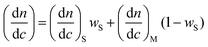 | (3) |
Light scattering detector
The basic light scattering equation is:6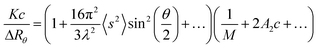 | (4) |
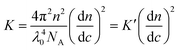 | (5) |
When a light scattering detector is employed in SEC, the polymer concentration used is very small so that the 2A2c and higher terms are negligible and thus eqn. (4) can be expressed for slice i of the chromatogram as:
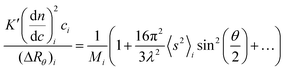 | (6) |
The MALS detector measures simultaneously the excess Rayleigh ratio at different θ angles for each slice i which is assumed to be monodisperse both in composition and molecular weight.4,6 Thus, a plot of K′(dn/dc)2ici/(ΔRθ)iversus sin2(θ/2), affords to calculate the molecular weight Mi from the intercept and the radius of gyration, 〈s2〉i, from the slope for each slice of the chromatogram.
In the case of a copolymer the value of the refractive index increment of a given slice (dn/dc)i depends on chemical composition according to eqn. (3). This magnitude coincides with the mean refractive index increment of the whole sample dn/dc only if the chemical composition is homogeneous throughout the chromatogram. If the composition of eluted copolymer varies as a function of the volume of elution, the refractive index increment (dn/dc)i will change. When the refractive index increment of the whole sample is used instead the true (dn/dc)i for each slice, indeterminations are introduced and apparent molecular weights can be obtained.11,15,16
Concentration detectors
The signals of both the refractive index detector (RI) and the UV detector are proportional to the mass concentration of polymer. However, the response factor of the RI detector is independent of the chemical nature of the eluted sample and therefore eqn. (2) may be applied to the ith slice of the chromatogram produced by a copolymer or a mixture of polymers with the only modification of particularizing the mass concentration and refractive index increment to the values appropriated for that particular slice: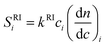 | (7) |
When the SRIi signal is transformed into a mass concentration datum, a mean value dn/dc of the refractive index increment is employed to integrate the peak of the whole sample. The mass concentration ci thus obtained will be the true concentration if the (dn/dc)i coincides with the mean value dn/dc. If this condition is not fulfilled, an apparent concentration16 will be obtained:
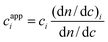 | (8) |
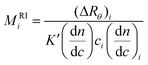 | (9) |
Unlike RI, the response factor of the UV detector depends on the chemical nature of the sample. Thus, the signal produced by the ith slice of a chromatogram obtained from a sample containing different chemical species would be:
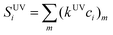 | (10) |
In the present case, we have two different repeating units namely styrene and methyl methacrylate. However, at λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 262 nm employed by the UV detector, (kUV)MMA
262 nm employed by the UV detector, (kUV)MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 while (kUV)S
0 while (kUV)S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≠
≠![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 so that eqn. (10) only contains the term corresponding to S units:
0 so that eqn. (10) only contains the term corresponding to S units:
SUVi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kUVS(ci)S kUVS(ci)S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kUVSci(wi)S kUVSci(wi)S | (11) |
The apparent molecular weight calculated with this SUVi using eqn. (6) with the value of dn/dc for the whole sample and extrapolated to zero angle will then be:
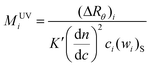 | (12) |
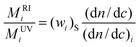 | (13) |
In the case of a perfectly homogeneous system, for instance a PS homopolymer, neither the composition (wi)S, nor refractive index increment do depend on molecular weight, so that local and mean values of these magnitude will coincide and eqn. (13) reduces to:
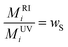 | (14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the same value of molecular weight should be obtained with both detectors.
1 and the same value of molecular weight should be obtained with both detectors.
The situation would be very similar for a perfectly homogeneous styrene–methyl methacrylate copolymer. Both the composition and the refractive index increment would again be independent of the slice studied and eqn. (14) will hold providing a constant value of wS, which of course would be smaller than unity, throughout the whole chromatogram.
On the contrary, an heterogeneous sample such a mixture of PS and PMMA homopolymers or a block copolymer of those two units would show molecular weight dependence on both the composition and refractive index increment and therefore the ratio MRIi/MUVi will be different from one slice to another.
Thus, the ratio between the apparent molecular weights obtained with these two detectors offers a simple way of analyzing random copolymers of S and MMA units allowing the determination of the composition and, what may be more interesting, deciding whether or not the distribution of monomer units along the copolymer chain is really random.
On the other hand, the ratio of responses of UV and RI detectors at the same elution volume can yield information about the composition of the copolymer.10 This ratio can be expressed combining eqns. (11), (7) and (3) as:
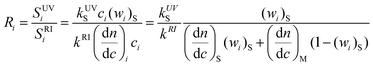 | (15) |
That can be solved for the styrene weight fraction at elution point i:
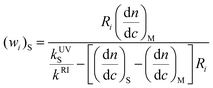 | (16) |
Application of eqns. (1) and (2) to the PS homopolymer and division among them allows the evaluation of the ratio kUVS/kRI between the response factors of the detectors as function of the ratio RSi among the signals obtained for PS:
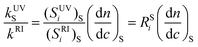 | (17) |
Substitution of eqn. (17) into eqn. (16) gives:
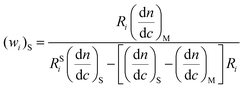 | (18) |
This equation holds only if the UV response factor of styrene units, through the absorption coefficient, is the same independently of their position in the homopolymer, copolymers, and mixtures. However, assuming that this condition is fulfilled, eqn. (18) provides another way of determining the composition of the copolymer and deciding whether or not this composition is homogeneous along the chains.
Results and discussion
Molecular weights
Fig. 3 shows the signals of the RI and UV detectors and one of the MALS detectors (at 90° scattering angle) in the region of the polymer peak of the SEC chromatograms for the PS homopolymers in THF. The light scattering signal is proportional to the product of M and c and consequently, has a different shape than that of RI and UV signals which are proportional to c. Moreover, comparison of the relative errors of the signals shows that the light scattering signal loses sensitivity at low molecular weights whereas, the UV and RI signals present lower sensitivity at the high molecular weight side of the chromatogram. The values of the ratio of UV/RI signals for PS, RSi, are also plotted in Fig. 3. According to eqn. (17), this magnitude for PS should not depend on the slice of the chromatogram and should be a constant for the whole PS peak. As has been reported10 and can be seen in the Figure, RSi is constant in the central part of the chromatogram whereas, it varies at the chromatogram head and tail. Several recent works on the use of SEC with multiple detectors for the analysis of homopolymers also show that several factors such as interdetector delay, baseline selection, non-linearity of the detector response25–28 can increase the error in the polymer analysis. Thus we have used only the central values (10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≤
≤![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ve
ve![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≤
≤![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ml) for obtaining an average value RSi
12 ml) for obtaining an average value RSi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.70
1.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 that will be used in eqn. 18.
0.04 that will be used in eqn. 18.
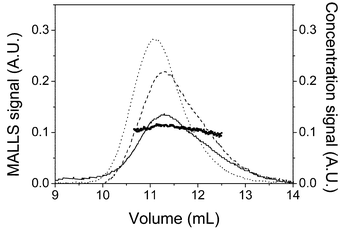 | ||
| Fig. 3 Chromatogram for polystyrene showing the RI signal (solid line) UV signal (dashed line) and the MALS signal at 90° (dotted line). Solid circles are the values of RSi used in eqns. (17) and (18). | ||
Fig. 4 shows the molecular weight of all the systems studied, calculated from the scattered light, plotted versus the elution volume. The signals from the differential refractive index detector have also been plotted in the same figure. The plots of molecular weight versusve are linear and the uncertainties in the tails or heads of the molecular weight distributions are much easily discernable from the scattering in data points of the calibration curve than from the raw chromatograms. For the homopolymers PS and PMMA, these plots are their respective absolute calibration curves for SEC whereas for the copolymers and mixtures the molecular weight can be an apparent molecular weight if the (dn/dc)i of each slice is different than the dn/dc of the whole sample according to eqn. (6). Since the values of the Mark–Houwink parameters in THF for both PS and PMMA are comparable,29 the values of the absolute calibration curves for all polymers are very similar. The data for copolymers, A through D, is more scattered than that of homopolymers, PS and PMMA, and mixtures, M1 and M2. This is because the molecular weights of the copolymers are considerably lower than the other polymers and are thus susceptible to higher experimental errors. Although the solution properties of a random copolymer are not strictly the weighted averaged of the homopolymer properties, it has been shown that the average approximation is valid for the calibration curve for random copolymers of styrene and methyl methacrylate in THF.20 This result is in agreement with the fact that their calibration curves are alike as explained above.
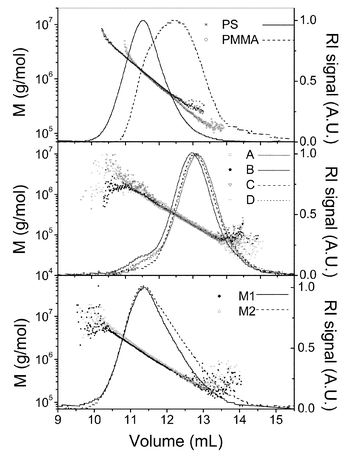 | ||
| Fig. 4 Logarithm of molecular weight versus elution volume for all the systems studied. The corresponding RI signals are also shown. | ||
The first difference between the copolymers (samples A, B, C and D) and the mixtures (samples M1 and M2) can be qualitatively observed in Fig. 4. The RI signals of the different copolymers have almost identical shape and the main differences are displacements along the volume axis due to their distinct molecular weight produced because of the differences in the polymerization process. However, in the mixtures, the signal for M1, that has a larger MMA content, becomes noticeably higher that that of M1 at elution volumes beyond 11.5 ml. Moreover, the slope of the calibration curve is larger for M2 than for M1. These facts support the previous assumptions about the different homogeneity of copolymers and mixtures. If copolymers A through D are random, then they should be much more homogeneous than the prepared mixtures that are completely heterogeneous. These qualitative arguments will be supported by further quantitative results presented below.
Solid lines in Figs. 5 and 6 present the absolute molecular weight distributions of the copolymers (Fig. 5) and the mixtures (Fig. 6) calculated from the combined measurements of molecular weight (MALS detector) and concentration (RI detector). The dots on these figures show the styrene weight fractions (wi)S calculated using eqn. (18), for each slice of the central part of the chromatogram. At the head and tail parts of the chromatograms the values of the styrene weight fraction are very disperse due to the errors in the ratio of the signals of UV and RI and are no represented in the figure. The differences in the values of (wi)S for copolymers and mixtures due to the different heterogeneity of the sample are clearly appreciable in both figures. Thus, the calculated values of (wi)S for copolymers are constant within experimental error whereas (wi)S for mixtures increases as the molecular weight grows (elution volume diminishes). This is a consequence of the different molecular weights of the two homopolymers used to prepare the samples. Thus, as the top panel on Fig. 4 shows, PS chains are, in average, larger than PMMA's. Consequently, when the mixture elutes through the column, the first portions (i.e. low elution volume and high molecular weight) will be richer in PS than the last ones. Thus, the behavior of (wi)S through the chromatogram provides information about the heterogeneity of the system allowing discrimination between homogeneous and heterogeneous copolymers. The average values of wS obtained for the copolymers A through D are presented in the seventh column of Table 1. The agreement of these values of wS and those obtained by dn/dc (fifth column) or NMR (third column) is qualitatively good and the quantitative differences can be attributed to several features. First, errors in the analysis of the chromatograms, especially in the determination of the base lines, can significantly affect the value of the ratio of the UV/RI signals ratio for PS, RSi and thus, the final results.22 Second, in the deduction of eqn. (18), it has been assumed that the UV response factor of styrene does not depend on the micro heterogeneity of the polymer.
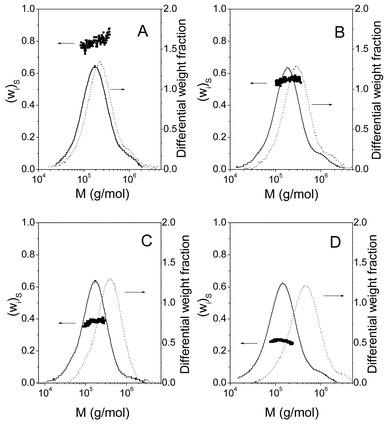 | ||
| Fig. 5 Molecular weight distributions for copolymers A through D calculated from MALS and RI detector (solid lines) or MALS and UV detector (broken line). Solid circles represent the values of styrene weight fraction calculated according to eqn. (18). | ||
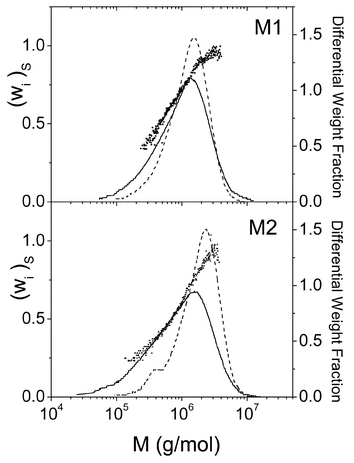 | ||
| Fig. 6 Apparent molecular weight distributions and styrene weight fraction for each slice of mixtures M1 and M2. See legend of Fig. 3. | ||
Broken lines in Figs. 5 and 6 represent the MWDs calculated with concentrations obtained by the UV detector instead of the RI detector. It is interesting to compare the apparent MWD of each sample (copolymer or mixture) obtained using RI or UV as the concentration detectors. According to eqn. (8), the concentration seen by the RI detector will be an apparent concentration if the (dn/dc)i does not coincide with the dn/dc of the whole sample. On the other hand, the concentration that sees the UV detector is proportional to the concentration of the styrene units (eqn. (11)) of the copolymer. As can be seen in Fig. 5, the MWDs obtained with both detectors for each copolymer are similar in form although they are displaced along the molecular weight axe. The similarity in the form supports the random composition of the copolymers A through D, whereas the displacement is a consequence of the differences in the molecular weights calculated according eqns. (9) and (12). On the contrary, the MWDs of each mixture (Fig. 6) obtained using RI and UV detectors are different since they are heterogeneous samples. The sixth column on Table 1 presents the averaged values of the ratio MRIi/MUVi for copolymers A through D. According to eqn. (14), this ratio is the polymer composition expressed as weight fraction of styrene units.
Scaling laws
The use of a multiangle light scattering detector enables the evaluation of both the weight average molecular weight Mw and the mean square radius of gyration, 〈s2〉, for each slice across a sample peak of the size exclusion chromatogram. Thus, the dependence of molecular dimensions on molecular weight,30,31i.e. the scaling law 〈s2〉1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) QMq, can be obtained with just one sample of a polydisperse polymer.9Fig. 7 shows the log–log plot of the radius of gyration versus molecular weight, obtained for the polymer samples. The slope of the log–log plot of the radius of gyration versus molecular weight provides the q coefficient from which information about the shape of the chain can be deduced. Values of 0.5
QMq, can be obtained with just one sample of a polydisperse polymer.9Fig. 7 shows the log–log plot of the radius of gyration versus molecular weight, obtained for the polymer samples. The slope of the log–log plot of the radius of gyration versus molecular weight provides the q coefficient from which information about the shape of the chain can be deduced. Values of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) q
q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⩽
⩽![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 are predicted for random-coil chains, from polymers in theta conditions (q
0.6 are predicted for random-coil chains, from polymers in theta conditions (q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) to polymers in very good solvents (q
0.5) to polymers in very good solvents (q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6). The values of parameters q and Q for all the samples studied are shown in Table 2. The values of q indicate that, as expected THF is a very good solvent for these polymers. Since the copolymers A through D have much lower molecular weights than the homopolymers the scaling law presents a higher dispersion of the points (see Fig. 7) and therefore, the values of q and Q are obtained with larger errors (see Table 2).
0.6). The values of parameters q and Q for all the samples studied are shown in Table 2. The values of q indicate that, as expected THF is a very good solvent for these polymers. Since the copolymers A through D have much lower molecular weights than the homopolymers the scaling law presents a higher dispersion of the points (see Fig. 7) and therefore, the values of q and Q are obtained with larger errors (see Table 2).
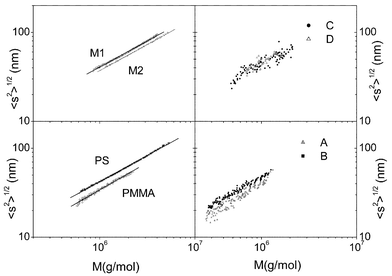 | ||
| Fig. 7 Log–log plot of root mean squared radius of gyration versus molecular weight for the polymers. | ||
| Polymer | 10−5Mn | 10−5Mw | 103Q | q |
|---|---|---|---|---|
| PS | 8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
13.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
12.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
0.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| PMMA | 3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
7.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
0.61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| A | 1.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09 0.09 |
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 0.04 |
| B | 1.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09 0.09 |
2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 0.02 |
| C | 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0. 1 0. 1 |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
0.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
| D | 1.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 0.06 |
2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
0.58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
| M1 | 7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 0.04 |
15.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
12.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
0.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
| M2 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
14.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
11.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
0.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
Conclusion
It is well known that SEC technique combining two detectors, one of them sensitive to mass and the second one sensitive to concentration, allows the evaluation of several molecular magnitudes of homopolymers. The present work shows that the addition of a second concentration sensitive detector increases the capabilities of the technique which can then be applied to study systems containing two different kinds of repeating units such as copolymers with different sequences or mixtures of homopolymers. In such systems, the procedure presented here allows a full physico chemical characterisation of the sample since it provides molecular weight distributions and averages, chains shape and dimensions, scaling law coefficients, etc. Furthermore, the technique provides some analytical information since it discriminates among random copolymers, block copolymers and mixtures of homopolymers and could also be used to evaluate the composition of homogeneous systems.Acknowledgements
Financial support from DGICYT through projects PB97-0778 and BQU2001-1158 are gratefully acknowledged.References
- Z. Grubisic, P. Rempp and H. Benoit, J. Polym. Sci. Part B, 1967, 5, 753 Search PubMed.
- J. Bravo, M. P. Tarazona and E. Saiz, Macromolecules, 1991, 24, 4089 CrossRef CAS.
- B. Trathnigg, Prog. Polym. Sci., 1995, 20, 615 CrossRef CAS.
- Strategies in Size Exclusion Chromatography, ed. M. Potschka and P. L. Dubin, American Chemical Society, Washington DC, 1996 Search PubMed.
- J. S. Lindner and S. S. Huang, in Modern Methods of Polymer Characterization, ed. H. G. Barth and J. W. Mays, John Wiley and Sons, New York, 1991, ch. 9, pp. 317–375 Search PubMed.
- Light Scattering from Polymer Solutions, ed. M. B. Huglin, ch. 7, Academic Press, London, 1972 Search PubMed.
- P. J. Wyatt, Anal. Chim. Acta, 1993, 272, 1 CrossRef CAS.
- W. L. Mattice and U. W. Suter, Conformational Theory of Large Molecules, Wiley, New York, 1994 Search PubMed.
- J. Búrdalo, R. Medrano, E. Saiz and M. P. Tarazona, Polymer, 2000, 41, 1615 CrossRef CAS.
- S. Mori and T. J. Suzuki, Liq. Chromatogr., 1981, 4, 1685 Search PubMed.
- L. H. Garcia Rubio, J. F. McGregor and A. E. Hamielec, in Polymer Characterization, ed. C. D. Craver, Advances in Chemistry Series, American Chemical Society, Washington DC, 1983, ch. 13 Search PubMed.
- L. H. Garcia Rubio, Detection and Data Analysis in SEC, ACS Symposium Series, ed. T. Provder, American Chemical Society, Washington DC, 1987, ch. 13 Search PubMed.
- A. J. Revillon, Liq. Chromatogr., 1980, 3, 1137 Search PubMed.
- P. M. Cotts and R. Siemens, Polymer, 1991, 32, 3052 CrossRef CAS.
- W. Radke, P. F. W. Simon and A. H. E. Müller, Macromolecules, 1996, 29, 4926 CrossRef CAS.
- M. Netopilík, M. Bohdanecký and P. Kratochvíl, Macromolecules, 1996, 29, 6023 CrossRef CAS.
- M. L. Coote, M. D. Zammit, T. P. Davis and G. D. Willet, Macromolecules, 1997, 30, 8182 CrossRef CAS.
- L. Mrkvicková, Macromolecules, 1997, 30, 5175 CrossRef CAS.
- W. Radke and A. H. E. Müller, in Strategies in Size Exclusion Chromatography, eds. M. Potschka and P. L. Dubin, American Chemical Society, Washington DC, 1996, ch. 1 Search PubMed.
- M. L. Coote, L. P. M. Johnston and T. P. Davis, Macromolecules, 1997, 30, 8191 CrossRef CAS.
- P. A. Clay, R. G. Gilbert and G. T. Rusell, Macromolecules, 1997, 30, 1935 CrossRef CAS.
- J. P. A. Heuts, D. Kukul, D. J. Forsters and T. P. Davis, Macromolecules, 1998, 31, 2894 CrossRef CAS.
- T. Fukuda, M. Nagata and H. Inagaki, Macromolecules, 1984, 17, 548 CrossRef CAS.
- I. A. Maxwell, A. M. Aerdts and A. L. German, Macromolecules, 1993, 26, 1956 CrossRef CAS.
- S. D. Cook and V. S. Sible, Eur. Polym. J., 1997, 33, 163 CrossRef CAS.
- C. Jackson, Y. J. Chen and J. W. Mays, J. Appl. Polymer Sci., 1996, 61, 865 Search PubMed.
- M. D. Zammit and T. P. Davis, Polymer, 1997, 38, 865 CrossRef.
- W. F. Reed, in Strategies in Size Exclusion Chromatography, ed. M. Potschka and P. L. Dubin, American Chemical Society, Washington DC, 1996, ch. 2 Search PubMed.
- Polymer Handbook, ed. J. Brandrup, E. H. Immergut and E. A. Grulke, John Wiley, New York, 4th edn., 1999 Search PubMed.
- P. G. De Gennes, Scaling Concepts in Polymer Physics, Cornell University Press, Ithaca, NY, 1979 Search PubMed.
- P. J. Flory, Statistical Mechanics of Chain Molecules, Wiley, New York, 1969 Search PubMed.
| This journal is © the Owner Societies 2003 |
