The molecular basis for sound velocity in n-alkanes, 1-alcohols and dimethylsiloxanes
Malcolm J. W. Povey*a, Scott A. Hindlea, John D. Kennedya, Zoe Steca and Richard G. Taylorb
aUniversity of Leeds, Leeds, UK LS2 9JT. E-mail: m.j.w.povey@leeds.ac.uk; Fax: 44 (0) 1132332982; Tel: 44 (0) 1132332963
bDow Corning, Central Research (BA104) Cardiff Road, Barry, South Glamorgan, Wales CF63 2YL. E-mail: r.taylor@dowcorning.com; Fax: 44 (01446) 747944; Tel: (01446) 723841
First published on 19th November 2002
Abstract
The velocity of sound has been determined in pure liquid n-alkanes (pentane to hexadecane), 1-alcohols (methanol to 1-dodecanol) and dimethylsiloxanes (200 fluid (L2, 0.65 cSt) to 200 fluid (5000 cSt)) at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Corresponding density data have been taken from the literature and the adiabatic compressibility determined. The measured adiabatic compressibility has been compared with two molecular models of sound velocity, the Schaaffs model and a development of the Urick equation. The Urick equation approach is based on a determination of the compressibility of the methylene or siloxane repeat units which make up the chains in these linear molecules. We show that the Urick equation approach accurately predicts sound velocity and compressibility for the higher members of each series, whilst the Schaaffs approach fails for the 1-alcohols. We suggest this is because of the influence of the hydroxyl end group on nearby molecules through hydrogen bonding. The Schaaffs approach does not take into account interactions between the end units and the chain repeat units. This interaction modifies the derived compressibility of the methylene groups reducing their compressibility as compared to their compressibillity in the n-alkanes. The technique described provides valuable new insights into end-group, inter-molecular and intra-molecular interactions in liquid linear-chain molecules. We suggest that it provides a powerful new method for characterising polymeric fluid materials.
°C. Corresponding density data have been taken from the literature and the adiabatic compressibility determined. The measured adiabatic compressibility has been compared with two molecular models of sound velocity, the Schaaffs model and a development of the Urick equation. The Urick equation approach is based on a determination of the compressibility of the methylene or siloxane repeat units which make up the chains in these linear molecules. We show that the Urick equation approach accurately predicts sound velocity and compressibility for the higher members of each series, whilst the Schaaffs approach fails for the 1-alcohols. We suggest this is because of the influence of the hydroxyl end group on nearby molecules through hydrogen bonding. The Schaaffs approach does not take into account interactions between the end units and the chain repeat units. This interaction modifies the derived compressibility of the methylene groups reducing their compressibility as compared to their compressibillity in the n-alkanes. The technique described provides valuable new insights into end-group, inter-molecular and intra-molecular interactions in liquid linear-chain molecules. We suggest that it provides a powerful new method for characterising polymeric fluid materials.
Introduction
Sound-velocity measurement is commonly used to determine the adiabatic compressibility of molecules.1–20 We have shown elsewhere that in mixtures of molecules, thermal scattering significantly affects the sound velocity, making the determination of molar sound velocity in the mixture more complicated.3 For the sake of simplicity, here we have determined the sound velocity in an extensive range of pure, liquid n-alkanes, 1-alkoxyalkanes (i.e. 1-alcohols) and polydimethylsiloxanes (pmds). The sound velocity has been compared with the Schaaffs model4,5 of molecular sound velocity and with a new model based upon the Urick equation.6 Our purpose is to produce a molecular model that accurately predicts the molecular sound velocity. Comparison between this molecular model and the measured sound velocity may reveal inter-molecular contributions to the compressibility and interesting features in the physical chemistry of the molecule, hitherto absent from current molecular models. Physical properties such as melting point variation with chain length in the n-alkanes21 are affected by variations in crystal packing. Since all our samples are liquid and well above their melting point, we would not expect crystal packing to be important here, although there will, of course, be some more general parallels with short-range ordering in the liquid state. A further reason for refining molecular models of sound velocity is for the monitoring of polymerisation reactions for changes in chain length, in molecular weight, and also in chemistry such as in a change of polymer oligomers from the cyclic to linear.Theory
Molecular compressibility was determined from the Laplace equation:1,2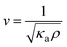 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−13 Pa−1. The major source of uncertainty in our data was the purity of the samples.
10−13 Pa−1. The major source of uncertainty in our data was the purity of the samples.A semi-phenomenological molecular model of sound velocity was devised in 1941 by Schaaffs4,5 as shown in eqn. (2).
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WρB/M WρB/M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W/(1 W/(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B/β) B/β) | (2) |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S1(2n S1(2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S2n S2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S3 S3 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06; S2
1.06; S2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.06 and S3
3.06 and S3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.59. The value of B ranges from 28.02 cm3 for pentane and 85 cm3 for hexadecane, while β is 0.2 cm3 for all alkanes.
4.59. The value of B ranges from 28.02 cm3 for pentane and 85 cm3 for hexadecane, while β is 0.2 cm3 for all alkanes.The Schaaffs approach requires that the product (V·v) of molar volume (V) and sound velocity be plotted against ‘molecular’ volume B, the slope giving the coefficient W. The value of W is usually between 4000–4500 m s−1, indicating the velocity of sound within a molecule, where frequency tends to zero. In general the three coefficients in eqn. (3) must be predetermined and these are tabulated elsewhere.4,5 So this approach requires the determination of four separate coefficients.
In our alternative approach, we assign a compressibility and density to each repeat unit in the series. The repeat unit is a {CH2} (methylene) unit in both the n-alkane and the 1-alcohol series and a {SiMe2O} (dimethlysiloxane) unit in the pmds series. We assign a separate compressibility and density to the terminating groups: both are {CH3} (methyl) in the n-alkanes, one is methyl and one is {OH} (hydroxy) in the 1-alcohols, and both are {SiMe3} (trimethylsilyl) in the pmds series.
The compressibility and density are combined using the Urick equation7 which is based on an idea suggested by Wood.8 Simply put, in a multicomponent system the compressibility and density in the Laplace eqn. (1) are replaced by their volume average values (eqns. (4) and (5)).
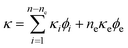 | (4) |
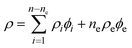 | (5) |
κ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (n (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ne)κrϕr ne)κrϕr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) neκeϕe neκeϕe | (6) |
ρ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (n (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ne)ρrϕr ne)ρrϕr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) neρeϕe neρeϕe | (7) |
Later we will see that it is not so straightforward thus to identify and account for the end units, since the effect of the end units on the compressibility of the repeat units extends quite far down the chain. On the other hand, we shall see that the molar volume of the repeat units is hardly affected. Initially, we chose ne![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to exclude repeat units attached to end units in our determination of repeat unit properties. However, the application of the Urick model to experimental results showed that the final value for ne varied and so in practice ne was chosen to minimise the standard error of the regression (see Table 2). It was thence found that ne
(which appears in Table 2 as nregression) varied from zero, in the case of the fit of molar volume to carbon/silicon number, to six in the case of the compressibility in the n-alkanes.
4 to exclude repeat units attached to end units in our determination of repeat unit properties. However, the application of the Urick model to experimental results showed that the final value for ne varied and so in practice ne was chosen to minimise the standard error of the regression (see Table 2). It was thence found that ne
(which appears in Table 2 as nregression) varied from zero, in the case of the fit of molar volume to carbon/silicon number, to six in the case of the compressibility in the n-alkanes.
We can see already a merit of the new approach in comparison to the previous Schaaffs method, in that ordinary measureable thermodynamic properties are assignable to individual chemical moieties.
Data analysis proceeds by first determining the molar volume as a function of chain length: linear regression of density against chain length gives the molar volume of each repeat unit and hence the fractional molar volume ϕr of the chain occupied by repeat units (eqn. (8)) and the fractional molar volume ϕe occupied by end units (eqn. (9)).
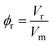 | (8) |
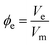 | (9) |
The compressibility of the repeat unit is then determined by linear regression of measured compressibility against the volume fraction of the chain occupied by repeat units. Hence the new method gives, explicitly, the adiabatic compressibility of the repeat unit and its molar volume. Examination of eqns. (6) and (7) indicates the physical basis for the Schaaffs method. If the contribution of the end units is ignored, then substitution of eqns. (6) and (7) into eqn. (1) gives:
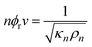 | (10) |
In other words, we expect a plot of the product of sound velocity and molar volume against the sound velocity of the repeat unit to yield a straight line whose slope is characteristic of the chemical repeat moiety. A corrollary of this would be that there would be a constant intercept contribution for a given molecule type that would vary according to the chemistry of the end units. Interactions between end units and neighbouring molecules are not explicitly accounted for in the Schaaffs model and the coefficients S1, S2 and S3 are a semi-phenomenological attempt to make the model fit the data.
In this regard our new method differs from the old, in that the effects of the molar volume and compressibility of the repeat and end units are explicitly accounted for. As will be seen this gives a more accurate model whose physical basis is more easily understood. This provides a firmer basis for comparison with other molecular models.
Experimental
Ultrasound measurements
The sound velocity was measured using a Cygnus UVM1 ultrasound velocity meter.6 The measurement cell consists of a cylindrical sample cell 35 mm in diameter and 50 mm deep. Using a PZT transducer 10 mm in diameter, a 2.25 MHz pulse of sound is transmitted across the cell reflected from the far wall of the cylinder, and then detected by the same transducer that produced the pulse. The time of flight of the pulse is measured to within 10 ns and the sound velocity is obtained by calibration of the cell with doubly distilled water.6 The sound velocity is determined ten times a second to within ±1 m s−1. The cell also contains a four-wire platinum resistance thermometer accurate to ±0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The sample is stirred with a small magnetic flea to ensure temperature uniformity throughout the sample. Temperature and sound velocity data are automatically logged to a spreadsheet. All other physical data have been taken from the literature. Samples were also checked for dispersion (frequency-dependent velocity) and its absence from our samples strongly suggests that relaxation effects were not significant at the measurement frequency.
°C. The sample is stirred with a small magnetic flea to ensure temperature uniformity throughout the sample. Temperature and sound velocity data are automatically logged to a spreadsheet. All other physical data have been taken from the literature. Samples were also checked for dispersion (frequency-dependent velocity) and its absence from our samples strongly suggests that relaxation effects were not significant at the measurement frequency.Materials
See Table 1.| Sample | Silicon/carbon number | Measured velocity of sound/m s−1 | Density/g m−3 | Compressibility/Pa−1 | Molecular wt./g | Molar volume, V/cm3 | D/nm | (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ne)·ϕr ne)·ϕr | B/cm3 |
|---|---|---|---|---|---|---|---|---|---|
| 200 fluid (L2, 0.65 cs) | 2 | 912.5 | 761![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.58E-09 | 162 | 212.9 | 1.24 | 0 | 12.48 |
| Decamethyltetrasiloxane | 4 | 952.2 | 854![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.29E-09 | 310 | 363.0 | 2.49 | 0 | 22.84 |
| Dodecamethylpentasiloxane | 5 | 963.3 | 875![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.23E-09 | 384 | 438.9 | 3.11 | 0.17 | 28.02 |
| L6 PDMS (Me3SiO(Me2SiO)4SiMe3 | 6 | 971.8 | 887![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.19E-09 | 458 | 516.3 | 3.73 | 0.29 | 33.2 |
| DMS-T03 (3.0 cSt) | 7 | 976.6 | 898![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.17E-09 | 550 | 612.5 | 4.51 | 0.40 | 39.64 |
| DMS-T05 (5.0 cSt) | 10 | 985.0 | 918![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.12E-09 | 770 | 838.8 | 6.36 | 0.56 | 55.04 |
| DMS-T07 (7.0 cSt) | 13 | 989.3 | 930![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.10E-09 | 950 | 1022 | 7.87 | 0.64 | 67.64 |
| DMS-T11 (10 cSt) | 17 | 996.9 | 935![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.08E-09 | 1250 | 1337 | 10.39 | 0.72 | 88.64 |
| DMS-T12 (20 cSt) | 27 | 1006.9 | 950![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.04E-09 | 2000 | 2105 | 16.70 | 0.82 | 141.1 |
| DMS-T50 (50 cSt) | 51 | 1012.5 | 960![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.02E-09 | 3780 | 3938 | 31.67 | 0.91 | 265.7 |
| DMS-T21 (100 cSt) | 80 | 1015.2 | 966![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.00E-09 | 5970 | 6180 | 50.09 | 0.94 | 419.0 |
| DMS-T22 (200 cSt) | 131 | 1017.0 | 968![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 9.99E-10 | 9730 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 052 052 | 81.71 | 0.96 | 682.2 |
| DMS-T23 (350 cSt) | 184 | 1017.6 | 970![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 9.96E-10 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072 072 | 114.7 | 0.97 | 956.6 |
| DMS-T25 (500 cSt) | 233 | 1018.0 | 971![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 9.94E-10 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765 765 | 145.0 | 0.98 | 1208.6 |
| DMS-T31 (1000 cSt) | 378 | 1017.6 | 971![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 9.95E-10 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 | 235.4 | 0.99 | 1961 |
| DMS-T35 (5000 cSt) | 667 | 1019.5 | 973![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 9.89E-10 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719 719 | 414.9 | 0.99 | 3455 |
| Pentane | 5 | 1034.7 | 626![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 | 1.49E-09 | 72 | 115.0 | 1.86 | 0.14 | 28.02 |
| Hexane | 6 | 1103.6 | 659![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 | 1.25E-09 | 86 | 130.4 | 2.23 | 0.25 | 33.2 |
| Heptane | 7 | 1155.0 | 683![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 | 1.10E-09 | 100 | 146.3 | 2.60 | 0.33 | 38.38 |
| Octane | 8 | 1196.0 | 702![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 | 9.95E-10 | 114 | 162.2 | 2.97 | 0.40 | 43.56 |
| Nonane | 9 | 1233.1 | 717![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 | 9.16E-10 | 128 | 178.3 | 3.34 | 0.45 | 48.74 |
| Decane | 10 | 1256.2 | 730![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 | 8.68E-10 | 142 | 194.5 | 3.71 | 0.50 | 53.92 |
| Undecane | 11 | 1279.2 | 740![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 | 8.26E-10 | 156 | 210.8 | 4.08 | 0.54 | 59.1 |
| Dodecane | 12 | 1297.8 | 748![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 | 7.93E-10 | 170 | 227.0 | 4.46 | 0.57 | 64.28 |
| Tetradecane | 14 | 1332.1 | 762![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 | 7.39E-10 | 198 | 259.7 | 5.20 | 0.62 | 74.64 |
| Hexadecane | 16 | 1358.4 | 773![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 | 7.01E-10 | 226 | 292.2 | 5.94 | 0.66 | 85 |
| Methanol | 1 | 1124.8 | 791![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 | 9.99E-10 | 32 | 40.4 | 0.37 | 0 | 11.83 |
| Ethanol | 2 | 1164.9 | 789![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 | 9.33E-10 | 46 | 58.3 | 0.75 | 0 | 17.01 |
| 1-Propanol | 3 | 1225.0 | 803![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 | 8.29E-10 | 60 | 74.7 | 1.12 | 0 | 22.19 |
| 1-Butanol | 4 | 1257.9 | 809![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 | 7.81E-10 | 74 | 91.4 | 1.50 | 0 | 27.37 |
| 1-Pentanol | 5 | 1293.3 | 814![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 | 7.34E-10 | 88 | 108.0 | 1.87 | 0.15 | 32.55 |
| 1-Hexanol | 6 | 1319.9 | 819![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 | 7.00E-10 | 102 | 124.4 | 2.25 | 0.27 | 37.73 |
| 1-Heptanol | 7 | 1344.6 | 822![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 | 6.73E-10 | 116 | 141.1 | 2.62 | 0.35 | 42.91 |
| 1-Octanol | 8 | 1364.6 | 825![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 | 6.50E-10 | 130 | 157.4 | 2.99 | 0.42 | 48.09 |
| 1-Nonanol | 9 | 1382.7 | 828![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 6.32E-10 | 144 | 173.9 | 3.37 | 0.48 | 53.27 |
| 1-Decanol | 10 | 1397.7 | 829![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 | 6.17E-10 | 158 | 190.4 | 3.74 | 0.52 | 58.45 |
| 1-Undecanol | 11 | 1408.7 | 832![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 | 6.05E-10 | 172 | 206.6 | 4.12 | 0.56 | 63.63 |
| 1-Dodecanol | 12 | 1433.2 | 834![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 | 5.84E-10 | 186 | 222.9 | 4.49 | 0.59 | 68.81 |
The n-alkanes
The following were used (C5 through C16): n-pentane (Aldrich (Dorset, UK), purity 99+%), n-hexane (Aldrich, purity 99+%), n-heptane (Aldrich, purity 99+%), n-octane (Aldrich, purity 98%), n-nonane (Aldrich, purity 99%), n-decane (Sigma (Dorset, UK), purity 99+%), n-undecane (Aldrich, purity 99+%), n-dodecane (Aldrich, purity 99+%), n-tetradecane (Sigma, purity min. 99%) and n-hexadecane (Sigma, purity min. 99%). n-tridecane and n-pentadecane were solid at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and so it was not possible to use them.
°C and so it was not possible to use them.The 1-alcohols
The following were used (C1 through C12): methanol (Aldrich, purity 99+%), ethanol (Aldrich, purity 99%), 1-propanol (Aldrich, purity 99+%), 1-butanol (Aldrich, purity 99%), 1-pentanol (Aldrich, purity 99+%), 1-hexanol (Aldrich purity 99+%), 1-heptanol (Aldrich, purity 98%), 1-octanol (Aldrich, purity 99+%), 1-nonanol (Aldrich, purity 98%), 1-decanol (Aldrich, purity 99%), 1-undecanol (Aldrich, purity 99%) and 1-dodecanol (Aldrich, purity 98%).Polydimethylsiloxanes
Except for silicon numbers 2, 4, 5 and 6 (purchased from Sigma, purity min. 97%) the silicon numbers for the trimethylsiloxyl terminated samples of pmds were estimated from molecular weight using gel permeation chromatography or by 29Si NMR spectroscopy. The remaining samples were purchased from ABCR Ltd. (Manchester, UK) (viscosity in cSt (accuracy ±10%)): DMS-T03, DMS-T05, DMS-T07, DMS-T11, DMS-T12, DMS-T50, DMS-T21, DMS-T22, DMS-T23, DMS-T25, DMS-T31, DMS-T35.Accuracy
We estimate the accuracy of our compressibility determination to be ±7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−13 Pa−1. This estimate is based on an error analysis using many different measurements in our apparatus and includes known systematic errors and random errors. Where we have repeated measurements on samples from different sources, for example in the case of methanol, it is apparent that the greatest error (ca. 5
10−13 Pa−1. This estimate is based on an error analysis using many different measurements in our apparatus and includes known systematic errors and random errors. Where we have repeated measurements on samples from different sources, for example in the case of methanol, it is apparent that the greatest error (ca. 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−12) in our measurements comes from variations in sample purity, despite our having purchased commercially high purity chemicals. This corresponds approximately to the standard error of the regression shown in Table 2.
10−12) in our measurements comes from variations in sample purity, despite our having purchased commercially high purity chemicals. This corresponds approximately to the standard error of the regression shown in Table 2.
| Symbol | Name | Material | |||
|---|---|---|---|---|---|
| n-Alkanes | 1-Alcohols | pmds | Units | ||
| a se - standard error of the regression. | |||||
| n | Number of members in series | 10 | 12 | 16 | |
| End unit data | |||||
| Chemistry | (CH3)...(CH3) | CH3...OH | SiO(CH3)3...Si(CH3)3 | ||
| Ve | Partial molar volume | 33.4 | 24.9 | 64.3 | cm3 |
| sea | 0.4 | 0.3 | 2.9 | cm3 | |
| nregression | 10 | 12 | 16 | ||
| ke | Compressibility | 1.370 | 0.793 | 1.293 | 10−9 Pa−1 |
| sea | 0.010 | 0.006 | 0.003 | 10−9 Pa−1 | |
| nregression | 5 | 6 | 12 | ||
| Repeat unit data | |||||
| Chemistry | CH2 | CH2 | SiO(CH3)2 | ||
| Vr | Partial molar volume | 16.14 | 16.54 | 76.00 | cm3 |
| sea | 0.04 | 0.04 | 0.01 | cm3 | |
| nregression | 10 | 12 | 16 | ||
| kr | Compressibility | −1.01 | −0.34 | −0.31 | 10−9 Pa−1 |
| sea | 0.016 | 0.013 | 0.003 | 10−9 Pa−1 | |
| nregression | 5 | 6 | 12 | ||
| Schaaffs data | |||||
| W | Ideal sound velocity | 4566 | 4496 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964 964 | m s−1 |
| sea | 34 | 44 | 1 | m s−1 | |
| nregression | 10 | 12 | 16 | ||
| S1 | Schaaffs coefficients | 1.06 | 1.06 | 1.06 | m3 |
| S2 | 3.06 | 3.06 | 3.06 | m3 | |
| S3 | 4.59 | 4.53 | 0 | m3 | |
Results and discussion
Measured data are summarised in Table 1 and the results for the compressibility are summarised in Fig. 1. The difference between the measured compressibility and that predicted from eqns. (1)–(7) (the residual error) is plotted in Fig. 2. The comparison for Schaaffs has been omitted from Fig. 2 because Schaaffs is always less accurate than Urick. The estimated chain-length in Fig. 2 is determined from the partial molar volume of the repeat unit (Table 2) where it is assumed that each repeat unit has a length equivalent to the diameter of a sphere whose volume is equal to the partial molar volume of the repeat unit, divided by Avogadro's number, and where it is assumed that the end-group unit approximates in size to a repeat unit. An indication of the influence of end unit on compressibility can be obtained from Table 2 by comparing nregression, the number of observations included in the regression, with n, the total number of observations. Whilst nregression![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n in the case of the fit for the determination of the molar volume for the repeat units, this is certainly not the case for the compressibility. In the alcohols in particular, the methylene unit next to a hydroxy end group is clearly to be treated differently to methylene units sandwiched between methylene units. Moreover, Table 2 and Fig. 2 indicate that the derived influence of the end-groups extends much further down the chain than the end unit and its adjacent methylene group. The fit between compressibility data and theory was tested by varying the number of the members of each series (starting with the lowest carbon or silicon number) omitted from the regression. (n
n in the case of the fit for the determination of the molar volume for the repeat units, this is certainly not the case for the compressibility. In the alcohols in particular, the methylene unit next to a hydroxy end group is clearly to be treated differently to methylene units sandwiched between methylene units. Moreover, Table 2 and Fig. 2 indicate that the derived influence of the end-groups extends much further down the chain than the end unit and its adjacent methylene group. The fit between compressibility data and theory was tested by varying the number of the members of each series (starting with the lowest carbon or silicon number) omitted from the regression. (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nregression), until the standard error of the regression ceased to reduce significantly. In this way the influence of end-groups on repeat unit compressibility was derived. This issue is discussed later in connection with Figs. 2 and 3.
nregression), until the standard error of the regression ceased to reduce significantly. In this way the influence of end-groups on repeat unit compressibility was derived. This issue is discussed later in connection with Figs. 2 and 3.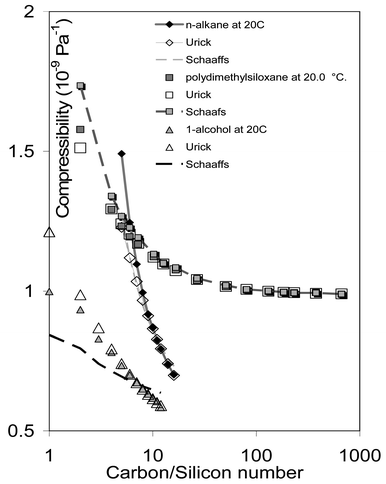 | ||
| Fig. 1 Measured and predicted compressibilities for n-alkanes, 1-alcohols and polydimethylsiloxanes. | ||
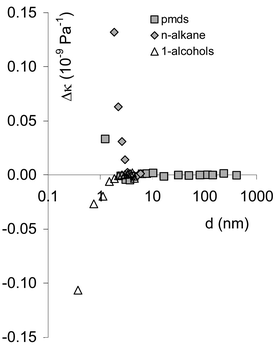 | ||
| Fig. 2 Difference between the measured compressibility and that determined by the Urick equation, plotted against estimated half chain length. | ||
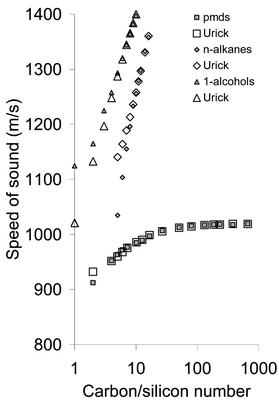 | ||
| Fig. 3 Measured and predicted velocity of sound plotted against carbon/silicon number. | ||
Examination of Fig. 1 indicates that the Urick approach is accurate for the higher numbers in all three series, whilst the Schaaffs approach works well for pmds and n-alkanes but not for 1-alcohols. Urick is consistently more accurate than Schaaffs but fails, not unexpectedly, for the first few members of each series. In terms of intramolecular geometrical structure, the 1-alcohols are identical to the n-alkanes apart from the hydroxyl group which replaces one hydrogen atom on one methyl end group. There will, however, be considerable differences in intermolecular structure, particularly for smaller carbon numbers. In the alcohols, the hydrogen-bonding ability of the hydroxyl group will generate inter-hydroxy intermolecular association with the terminal hydroxy groups of adjacent molecules, effectively binding molecules together to increase effective molecular chain-length. Purely statistically, effective dilution of the hydroxy groups to ensure that none are adjacent will not occur until the carbon number exceeds eleven, although lone-pair orientation requirements for strong O–H–O interaction may effectively reduce this to six or seven. The low carbon-number deviations from the Schaaffs approach are in apparent agreement with this last. This hydrogen-bonding effect is obviously not a factor in the other two comparator series.
In sum, since the Schaaffs approach does not account for the effect of the end-groups on compressibility, it is least accurate for the 1-alcohols, especially at lower carbon numbers. On the other hand, it is clear that if it were possible to measure the 1-alcohols up to sufficiently high carbon numbers, then the effect of the end-groups should be greatly reduced by effective dilution, as is apparent for pmds in Fig. 2. It is apparent from Fig. 2 that, for the extent of chain-length examined, neither the 1-alcohol series nor the n-alkanes have sufficient methylene group content for the effects of the end-groups to become negligible. In the case of the n-alkanes and 1-alcohols, it would be necessary to repeat the measurements at higher temperatures (e.g. 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) in order to include the members with higher carbon numbers in the series that are solid at room temperature. The derived values for the compressibilities of the repeat units in the cases of the 1-alcohols and n-alkanes are therefore strongly influenced by the end units. This is especially so in the case of the 1-alcohols, where hydrogen bonding and other dipolar effects22 between adjacent chains in the 1-alcohols may have a strong influence on the derived compressibility of the repeat {CH2} unit. This may arise from a number of factors. In general we expect the bulk compressibility to be influenced by interactions with nearest-neighbour molecules. This will be particularly so when there is chemical bonding between neighbours—for example, hydrogen bonding can have values of up to ca. 30 kJ mol−1 or so, and compressibility transmitted through such a bond will clearly be different to that between molecules and to that within more strongly covalently bonded molecules. Intermolecular interactions involving the {CH2} repeat units may also have some significance; here there will be intermolecular Van der Waals attractions of up to 5 kJ mol−1 or so between pairs of methyl and/or methylene groups, but this would be expected to be approximately constant and identical, per repeat unit, for a standard assembly of such repeat units. Interaction of methylene and methyl hydogen atoms with the hydroxy group, which may have a stronger dipolar compenents and also some OH–HC dihydrogen-bonding character, could on the other hand have a much more significant influence on the derived methylene-unit compressiblities.
°C) in order to include the members with higher carbon numbers in the series that are solid at room temperature. The derived values for the compressibilities of the repeat units in the cases of the 1-alcohols and n-alkanes are therefore strongly influenced by the end units. This is especially so in the case of the 1-alcohols, where hydrogen bonding and other dipolar effects22 between adjacent chains in the 1-alcohols may have a strong influence on the derived compressibility of the repeat {CH2} unit. This may arise from a number of factors. In general we expect the bulk compressibility to be influenced by interactions with nearest-neighbour molecules. This will be particularly so when there is chemical bonding between neighbours—for example, hydrogen bonding can have values of up to ca. 30 kJ mol−1 or so, and compressibility transmitted through such a bond will clearly be different to that between molecules and to that within more strongly covalently bonded molecules. Intermolecular interactions involving the {CH2} repeat units may also have some significance; here there will be intermolecular Van der Waals attractions of up to 5 kJ mol−1 or so between pairs of methyl and/or methylene groups, but this would be expected to be approximately constant and identical, per repeat unit, for a standard assembly of such repeat units. Interaction of methylene and methyl hydogen atoms with the hydroxy group, which may have a stronger dipolar compenents and also some OH–HC dihydrogen-bonding character, could on the other hand have a much more significant influence on the derived methylene-unit compressiblities.
It is noteworthy that the effects of the end-groups disappear completely at approximately 10 nm into the chain in the case of pmds. This also appears to be the case in the n-alkanes and 1-alcohols, although we await further measurements to confirm that this is so. This value of 10 nm appears not to be a function of the number of repeat units, corresponding as it does to carbon/silicon numbers of six (pmds), eleven (n-alkanes) and six (1-alcohols), see also Fig. 3. It may perhaps be more closely related to the number of chemical bonds along a chain, which has some independence from chemical composition. For example, each silicon number represents a {SiMe2O} unit that is two-bonds long, whereas one {CH2} unit is one-bond long. The lower alkanes may be anomalous in this regard because they are closer to their boiling points at the temperatures of the measurements reported here, and therefore have a looser liquid-state ensemble.
From the present experiments, the value determined for the derived compressibility of the repeat units can only be regarded as a constant in the case of pmds. The derived values for the 1-alcohol and n-alkane series will change if more, higher, members of these series could be included. This is despite the apparent quality of the fit of the model to the experimental data. It is important to recognise that in no case can this derived compressibility value be regarded as intrinsic to the chain, affected as it is by end-group and nearest-neighbour interactions. For example, the methylene repeat units in the n-alkane and 1-alcohol series engender completely different derived compressibilities, despite being chemically identical. This is at first sight counter-intuitive. However, these derived compressibilities are not true methylene compressibilities, as the values will also constitute error-sinks for assumptions in the various models. As pointed out earlier, this will be partly due to hydrogen bonding between adjacent molecules in the case of the 1-alcohols, and other dipolar effects may also be expected.22 As a consequence, we cannot extrapolate from the behaviour of the short chain 1-alcohols and n-alkanes to the long chain behaviour, because we cannot be sure that the end effects have been correctly accounted for in our mathematical model. In addition, at chain numbers above around 100 in n-alkanes, chain folding may have significant effects.23 The partial molar volume, on the other hand, is nowhere nearly as sensitive to these chain–chain interactions. Whilst the Urick model suggests that the velocity will flatten out at high carbon numbers for both the n-alkanes and 1-alcohols just as the dimethylsiloxanes do (Fig. 3), we cannot predict precisely how this will occur for the reasons given above. We can, however, say that the effects of compressibility are much more significant in the chain-length dependence of all three series than they are in the density dependence, the latter being hardly affected by end-group effects. This is reasonable on the basis that the density can be regarded essentially as a geometrical packing phenomenon, whereas the compressibility will have two components, intramolecular, associated with the flexibility of the covalent bonding chain, and intermolecular, associated with the flexibilities of a variety of intermolecular interactions.
In summary, these data provide a unique and valuable insight into chain–chain and chain–end-group interactions in liquids, an insight which has hitherto been unavailable.
Conclusions
A model of sound velocity in linear chain hydrocarbons and silicones accurately predicts the measured sound velocity and adiabatic compressibility for the higher members of the series. Data are summarised in Table 2. End-group effects dominate compressibility at low carbon or silicon numbers, whilst they have little impact on the partial molar volume. Despite the fact that the determined compressibility of the repeat unit is affected by interchain and intermolecular effects, and by end-group effects, these data give a unique insight into the molecule and its interactions.We have already used these data to determine molecular weight on-line during polymerisation interactions and have shown that the technique can easily detect the change from cyclic siloxane to linear polymer in ring-opening polymerisation to cylic polymerisation during the polymerisations of silicones. We intend to extend this work to higher members of the 1-alcohol series by working at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, to confirm that the effect of the hydroxyl end-group diminishes as carbon number increases. We also intend to compare these data with molecular-modelling predictions of intrinsic repeat unit compressibility in an attempt to deduce the magnitude of the intermolecular effects.
°C, to confirm that the effect of the hydroxyl end-group diminishes as carbon number increases. We also intend to compare these data with molecular-modelling predictions of intrinsic repeat unit compressibility in an attempt to deduce the magnitude of the intermolecular effects.
There are very few methods available which are sensitive to the interactions in fluids between end units, chain repeat units and their nearest neighbours and we envisage that the ultrasound velocity method, with its ease of use and in-line capability, can provide a powerful new method of characterising polymeric fluids, as well as contributing to the understanding of fluid compressibility and of the propagation of sound in terms of specific intermolecular and intramolecular physico-chemical behavior. The weaker molecular forces involved are difficult to probe by other methods and we hope that this method will offer new perspectives on the molecular basis (molecularics) of the liquid state.
We find that the compressibility of the methylene unit in the n-alkanes is −1.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 10−9 Pa−1, in 1-alcohol −0.34
0.01 10−9 Pa−1, in 1-alcohol −0.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 10−9 Pa−1 and in pmds the compressibility of the siloxane unit is −0.31
0.01 10−9 Pa−1 and in pmds the compressibility of the siloxane unit is −0.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.003 10−9 Pa−1.
0.003 10−9 Pa−1.
Acknowledgements
The authors acknowledge support from EPSRC grant GR/N04812, EPSRC grant GR/N17058, BBSRC grant 24/D11911, Dow Corning and Cygnus Instruments. We also wish to acknowledge valuable discussions with Dr Rammile Ettelaie.References
- J. W. Strutt and Baron Rayleigh, The Theory of Sound, 3rd edn., Macmillan, London, 1896 Search PubMed.
- P. S. Laplace, Ann. Chim., 1816, 3, 238 Search PubMed.
- V. J. Pinfield and M. J. W. Povey, J. Phys. Chem. B, 1997, 101, 1110–1112 CrossRef CAS.
- W. Schaaffs, Landolt-Börnstein New Series 2, ed. K.-H. Hellwege and A. M. Hellwege, Springer-Verlag, New York, 1967, pp. 79–109 Search PubMed.
- L. Bergmann, Der Ultraschall, Hirzel Verlag, Stuttgart, 1954, pp. 391–399 Search PubMed.
- M. J. W. Povey, Ultrasonic Techniques for Fluids Characterization, Academic Press, San Diego, 1997, pp. 34–45 Search PubMed.
- R. J. Urick, J. Appl. Phys., 1947, 18, 983–987.
- A. B. Wood, A Textbook of Sound, 3rd edn., Bell and Sons, London, 1964 Search PubMed.
- E. Aicart, M. K. Kumaran, C. J. Halpin and G. C. Benson, J. Chem. Thermodyn., 1983, 15, 919–925 CrossRef CAS.
- M. K. Kumaran, G. C. Benson and C. J. Halpin, J. Chem. Eng. Data, 1983, 28, 66–70 CrossRef CAS.
- J. Antosiewicz and D. Shugar, J. Solution Chem., 1984, 13, 493–503 Search PubMed.
- G. C. Benson, C. J. Halpin and M. K. Kumaran, J. Chem. Thermodyn., 1986, 18, 1147–1152 CrossRef CAS.
- G. C. Benson and C. J. Halpin, Can. J. Chem., 1987, 65, 322–325 CAS.
- T. Ramanjappa and E. Rajagopal, Can. J. Chem., 1988, 66, 371–373 CAS.
- C. S. Adgaonkar, V. D. Gokhale, D. A. Ghosle and N. N. Bhagwat, Indian J. Pure Appl. Phys., 1988, 26, 577–578 Search PubMed.
- V. Mushran, J. Indian Chem. Soc., 1990, 67, 130–133 Search PubMed.
- E. Aicart, M. Costas, E. Junquera and G. Tardajos, J. Chem. Thermodyn., 1990, 22, 1153–1158 CrossRef CAS.
- R. Paladhi and R. P. Singh, Eur. Polym. J., 1990, 26, 441–444 CrossRef CAS.
- S. Y. Ye, B. Lagourette, J. Alliez, H. Saintguirons, P. Xans and P. Montel, Fluid Phase Equilib., 1992, 74, 177–202 CrossRef CAS.
- T. M. Aminabhavi, M. I. Aralaguppi, S. B. Harogoppad and R. H. Balundgi, Indian. J. Technol., 1993, 31, 27–31 Search PubMed.
- V. R. Thalladi and R. Boese, New J. Chem., 2000, 24, 579–581 RSC.
- D. González, C. A. Cerdeiriña, L. Romani and E. Carballo, J. Phys, Chem. B., 2000, 104, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275–11
275–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282 CAS.
282 CAS. - G. Ungar and X. Zeng, Chem. Rev., 2001, 101, 4157–4188 CrossRef CAS.
| This journal is © the Owner Societies 2003 |
