The use of 4-substituted pyridines to afford amphiphilic, pegylated cadmium selenide nanoparticles†
Habib
Skaff
and
Todd
Emrick
*
Department of Polymer Science and Engineering, 120 Governors Drive, University of Massachusetts, Amherst, MA 01003, USA. E-mail: tsemrick@mail.pse.umass.edu; Fax: 413 545 0082; Tel: 413 577 1613
First published on 21st November 2002
Abstract
Amphiphilic cadmium selenide (CdSe) nanoparticles were prepared by surface functionalization with novel ligands 1 and 2, composed of pyridine moieties substituted in the 4-position with polyethylene glycol (PEG) chains.
Colloidal nanoparticles, especially those with active electronic and luminescent properties, have attracted a great deal of interest for their fundamental properties based on quantum confinement,1 as well as for use as components in electronically active device applications,2 and in exploratory biotechnology research as fluorescent tags.3 Semiconducting nanoparticles, or quantum dots (QDs), possess significant advantages over conventional organic fluorophores in terms of narrow luminescence emission profiles (ca. 20–30 nm fwhm), resistance to photobleaching, and continuous absorption above the bandgap.1a These unique properties allow multiple nanoparticles of various emission wavelengths to be excited simultaneously by a single light source for extended periods of time.
As nanoparticle solubilization depends critically on the ligand environment, ready access to a wide range of solubilities and miscibilities is desired, including materials that are hydrophobic, hydrophilic, neutral, charged, and conductive. In the case of CdSe nanoparticles, conventional synthetic procedures afford hydrophobic material (e.g., tri-n-octylphosphine oxide (TOPO)-covered CdSe),4 and ligand exchange processes are typically used to obtain hydrophilic, water soluble samples, through the use of α,ω-thiocarboxylic acid ligands.5 The most stable water-soluble CdSe samples are prepared by inorganic encapsulation (overcoats of ZnSe followed by silica), an effective but synthetically involved procedure for producing water-soluble CdSe nanoparticles.6 New procedures that offer simple, effective, and stable ligand environments continue to present important targets in the field.
We are interested in developing novel polymeric encapsulantion strategies for nanoparticles7 that enable the preparation of a diverse range of materials in a fashion amenable to scale-up. Encapsulation of nanoparticles with poly(ethylene glycol) (PEG) would significantly diversify properties and potential applications, as this ligand environment carries the potential to satisfy multiple requirements, including organic solubility, water solubility, and biocompatibility.8 However, PEG itself (i.e., HO(CH2CH2O)nOH) does not solubilize CdSe nanoparticles, as the hydroxy chain-ends do not coordinate sufficiently to the CdSe surface. Thus, we set out to provide a rapid route to amphiphilic CdSe nanoparticles, using a new set of encapsulating molecules, ligands 1 and 2, composed of pyridine moieties that serve as the surface active ligands, and covalently attached PEG tails that provide the amphiphilic environment.
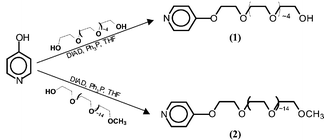
Ligands 1 and 2 were synthesized by Mitsunobu coupling of 4-hydroxypyridine with hexaethylene glycol and hexadecylethylene glycol monomethylether, respectively.‡ These reactions were performed at room temperature in tetrahydrofuran, using diisopropylazodicarboxylate (DIAD) as the coupling agent, as depicted in Scheme 1. Note that 1 and 2 are not single molecules, rather they possess an average number of ethylene glycol repeat units dictated by the inherent (albeit low) polydispersity of commercial poly(ethylene glycol).
 | ||
| Scheme 1 | ||
In the case of mono-substituted hexaethylene glycol 1, an excess of the PEG-diol was used in the coupling reaction, in order to minimize the formation of α,ω-dipyridine; when kept to a minimum, the dipyridine was removed easily from the desired product by column chromatography on silica gel. Ligands 1 and 2 were prepared in multigram quantities, and typically isolated in pure form in 50 and 80% yield, respectively. Distinctive features in the 1H NMR spectra of 1 and 2 include triplets at δ 4.1 and 3.8, assigned to the two PEG methylene groups nearest the aromatic ring; these are shifted downfield from the terminal methylene groups of the starting unsubstituted PEGs. The 13C NMR spectra of 1 and 2 showed the expected aromatic and aliphatic resonances, while the spectrum of 1 includes a resonance at δ 61.4 for the CH2OH chain-end, absent in the carbon spectrum of monomethyl ether 2, and indicative of complete end-group substitution.
The affinity of para-substituted pyridines for CdSe nanoparticles first attracted our attention when, upon addition of N,N-dimethylaminopyridine (DMAP) to THF solutions of TOPO-covered CdSe nanoparticles, we observed complete displacement of TOPO by DMAP (as judged by solubility changes and subsequently confirmed by 1H and 31P NMR). This rapid exchange is not seen when pyridine is used similarly; indeed, a large excess of refluxing pyridine is required to achieve near complete displacement of TOPO from the nanoparticle surface. We attribute the observed affinity of DMAP for the CdSe surface to its enhanced basicity relative to pyridine,9 due to the electron donating effect of the para-dialkylamine substituent. As 4-alkoxy-substituted pyridines exhibit basicity on the order of DMAP, our finding should be extendable to a number of pyridine derivatives containing electron-donating substituents in the para position.
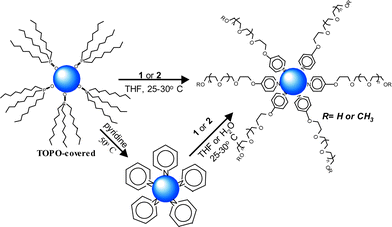
We found that pegylated pyridines 1 and 2, containing p-ether substituents, can be utilized effectively in a number of ligand exchange scenarios for attachment to the CdSe surface, as depicted in Scheme 2. For example, displacement of TOPO by conventional pyridine stripping, followed by adsorption of 1 or 2 to the nanoparticles from THF solutions, provided an effective pegylation method. Alternatively, the exchange process could be performed directly in water by addition of aqueous solutions of 1 or 2 to a suspension of pyridine-covered CdSe, whereupon the suspension became homogenous instantly. An even more convenient ligand exchange and aqueous solubilization was accomplished by simply adding 1 or 2 to a solution of TOPO-covered nanoparticles in THF, and allowing the mixture to stir at room temperature overnight. The observed changes in solubility in conjunction with NMR evaluation confirmed the success of this ligand exchange/pegylation strategy.
 | ||
| Scheme 2 | ||
Dissolution of 1- and 2-covered CdSe nanoparticles in either organic or aqueous media affords optically clear solutions, with similar absorption and emission profiles in each environment. Fig. 1 shows an example of approximately 3 nm CdSe nanoparticles covered with 1, where the first exciton absorption peak is found at ca. 520 nm, and emission maximum at ca. 530 nm, demonstrating that the optical properties of the same sample are maintained in either environment. These pegylated nanoparticles have shown excellent stability to date (about 3 months) with no sign of precipitation when stored in organic solvents, under ambient conditions (i.e., exposed to air and light). The stability of nanoparticles solubilized by ligands 1 and 2 proved superior to 3-mercatopropionic acid in aqueous media, under ambient conditions. Under conditions of irradiation,10 these PEG-covered nanoparticles showed superior stability relative to the thiol-covered nanoparticles, which precipitated quickly. We attribute this enhanced photostability to the fact that the pyridine-covered nanoparticles do not suffer from the disulfide formation that plagues nanoparticle coverage in thiol-based systems. In addition, aqueous samples of these PEG-covered nanoparticles stored in the dark and under nitrogen continue showed good stability/solubility, similar to that of the thiol-covered particles.11 Importantly, transmission electron micrographs of 1 and 2-covered nanoparticles show excellent dispersion whether cast from organic or aqueous solution (TEM images provided as ESI†).
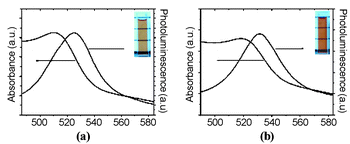 | ||
| Fig. 1 Absorbance and photoluminescence spectra of 1-covered CdSe nanoparticle solutions in (a) dichloromethane and (b) water. The horizontal lines in the figure aid visualization of the transparency of the solutions. | ||
In summary, we have demonstrated a rapid preparation of amphiphilic CdSe nanoparticles covered with poly(ethylene glycol) via attachment through a pyridine end-group, where the PEG-nanoparticle products show good solubility in both organic and aqueous media. In each environment, the inherent optical properties of the original nanoparticles are maintained. This represents the first use of para-substituted pyridines for nanoparticle passivation, and we expect such methodology to grow in its usefulness due to the nature of the 4-alkoxypyridine and 4-aminopyridine moieties, and the diverse range of substituents that can be attached at the 4-position. While there have been recent reports that use PEG as a component of the encapsulation method,12 this is the first extension to long PEG chains, and the first report where PEG, used exclusively as the encapsulating matrix, provides amphiphilic nanoparticles. This new ligand architecture gives rapid access to both water and organic soluble CdSe nanoparticles, where neither ionization nor the use of thiols are required to give water solubility. We believe the simplicity of this technique, and extensions thereof in the future, will further stimulate the integration of nanoparticles into both materials science and biology.
The authors gratefully acknowledge the financial support of the University of Massachusetts, Amherst and the NSF-sponsored Materials Research Science and Engineering Center (MRSEC) at UMASS. The Eastman Kodak Company (Rochester, N.Y.) is acknowledged for providing unrestricted funds. We thank Dr Stefan Hecht (Freie Universitat Berlin, Institute of Organic Chemistry) for valuable discussions.
Notes and references
- (a) C. D. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater., 2000, 30, 545 Search PubMed; (b) M. Nirmal and L. Brus, Acc. Chem. Res., 1999, 32, 407 CrossRef CAS; (c) A. P. Alivisatos, Science, 1996, 271, 933 CAS.
- (a) W. U. Huynh, J. J. Dittmer and A. P. Alivisatos, Science, 2002, 295, 2425 CrossRef CAS; (b) M. T. Harrison, S. V. Kershaw, M. G. Burt, A. L. Rogach, A. Kornowski, A. Eychmuller and H. Weller, Pure Appl. Chem., 2000, 72, 295 Search PubMed; (c) J. Lee, V. C. Sundar, J. R. Heine, M. G. Bawendi and K. F. Jensen, Adv. Mater., 2000, 12, 1102 CrossRef CAS.
- (a) M. Bruchez, M. Maronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013 CrossRef CAS; (b) M. Han, X Gao, J. Z. Su and S. Nie, Nat. Biotechnology, 2001, 19, 631 Search PubMed; (c) H. Mattoussi, J. M. Mauro, E. R. Goldman, G. P. Anderson, V. C. Sundar, F. V. Mikulec and M. G. Bawendi, J. Am. Chem. Soc., 2000, 122, 12142–12150 CrossRef CAS; (d) S. J. Rosenthal, I. Tomlinson, E. M. Adkins, S. Schroeter, S. Adams, L. Swafford, J. McBride, Y. Wang, L. J. DeFelice and R. D. Blakely, J. Am. Chem. Soc., 2002, 124, 4586 CrossRef CAS.
- (a) C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS; (b) J. E. Bowen Katari, V. L. Colvin and A. P. Alivisatos, J. Phys. Chem., 1994, 98, 4190.
- (a) S. Pathak, S. Choi, N. Arnheim and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4103 CrossRef CAS; (b) L. Qi, H. Colfen and M. Antonietti, Nano Lett., 2001, 1, 61 CrossRef CAS.
- D. Gerion, F. Pinaud, S. C. Williams, W. J. Parak, D. Zanchet, S. Weiss and A. P. Alivisatos, J. Phys. Chem. B., 2001, 105, 8861 CrossRef CAS.
- For core–shell materials with polymer encapsulation see: (a) S. Blomberg, S. Ostberg, E. Harth, A. W. Bosman, B. Van Horn and C. J. Hawker, J. Polym. Sci.: Part A: Polym. Chem., 2002, 40, 1309 Search PubMed; (b) K. L. Wooley, J. Polym. Sci.: Part A: Polym. Chem., 2000, 38, 1397 Search PubMed; (c) S. C. Farmer and T. E. Patten, Chem. Mater., 2001, 13, 3920 CrossRef CAS; (d) M. J. Lui, K. Kono and J. M. J. Fréchet, J. Controlled Release, 2000, 65, 121 CrossRef; (e) K. J. Watson, J. Zhu, S. T. Nguyen and C. A. Mirkin, J. Am. Chem. Soc., 1999, 121, 462 CrossRef CAS; (f) H. Skaff, M. F. Ilker, E. B. Coughlin and T. Emrick, J. Am. Chem. Soc., 2002, 124, 5729–5733 CrossRef CAS.
- J. M. Harris, Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications, Plenum Press, New York, 1992 Search PubMed.
- The pKa values of the conjugate acids of DMAP and pyridine are approximately 9.0 and 5.2, respectively.
- Dilute solutions of PEG-pyridine covered and thiol-covered nanoparticles were irradiated side-by-side with a 150 W lamp in the open air. Under these conditions, the thiol-covered particles precipitated completely within 1–2 h, while slight precipitation of the PEG-pyridine covered particles was observed after 5–6 days, and substantial precipitation after 9–10 days (see ESI†).
- J. Aldana, A. Wang and X. Peng, J. Am. Chem. Soc., 2001, 123, 8844 CrossRef CAS.
- W. J. Parak, D. Gerion, D. Zanchet, A. S. Woerz, T. Pellegrino, C. Michael, S. C. Williams, M. Seitz, R. E. Bruehl, Z. Bryant, C. Bustamante, C. R. Bertozzi and A. P. Alivisatos, Chem. Mater., 2002, 14, 2113 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: TEM images and absorbance profiles. See http://www.rsc.org/suppdata/cc/b2/b208718a/ |
‡ General procedure for 1 and 2: 4-hydroxypyridine (1.25 equiv.), triphenylphosphine (1.25 equiv.) and diisopropyl azodicarboxylate (1.25 equiv.) in THF were stirred at room temperature under nitrogen for 30 min. Poly(ethylene glycol) (1 equiv. for monomethyl ether, 3 equiv. for diol) was added, and the mixture was stirred for 12 h. THF was removed under vacuum, and the residue was purified by column chromatography eluting with chloroform: acetone: methanol mixtures to yield 1 and 2 in 56 and 78% yield, respectively: 1H NMR (300 MHz, CDCl3), δ 8.36 (d, aromatic), 6.78 (d, aromatic), 4.12 (t, pyr-OCH2CH2), 3.82 (t, pyr-OCH2CH2), 3.59 (br, CH2); 13C NMR (75 MHz, CDCl3), δ 164.7, 150.8, 110.2, 72.4, 70.7, 70.44, 70.38, 70.36, 70.2, 69.1, 67.1, 61.4; HRMS, calc. m/z 359.1942; found M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H 360.2022. H 360.2022. |
| This journal is © The Royal Society of Chemistry 2003 |
