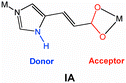
Crystal engineering toward intersecting channels in a interpenetrated diamondoid network based on a net-to-net H-bonding interaction
Yen-Hsiang
Liu
,
Huang-Chun
Wu
,
Hsiu-Mei
Lin
,
Wei-Hsien
Hou
and
Kuang-Lieh
Lu
*
Institute of Chemistry, Academia Sinica, Taipei 115, Taiwan. E-mail: lu@chem.sinica.edu.tw; Fax: +886-2-27831237
First published on 25th November 2002
Abstract
A thermally stable, four-fold interpenetrating diamondoid coordination network, Cd(imidazole-4-acrylate)2, with open intersecting channels within the interwoven nets, is strategically designed and synthesized on the basis of a spring-like net-to-net hydrogen-bonding interaction.
The benefits derived from diamondoid network topology such as the robustness, the porous architecture, and the favoring of acentric deposition have spanned the area of materials chemistry on the basis of modular assembled metal–organic diamondoid solids.1 Elegant studies have been demonstrated by an acentric solid Zn(INA)2 (INA = isonicotinate) by Lin and coworkers,2 which is three-times as NLO-active as KDP. The incorporation of counter ions and guest molecules toward the guest-included interpenetrating diamondoid framework has been demonstrated in Cu(1,4-dicyanobenzene)2(BF4) by Robson and coworkers,3 [Cu(bpe)2](BF4) (bpe =1,2-trans-(4-pyridyl)ethene) by Champness and Schroder and coworkers,4 and a highly intepenetrated diamondoid netowrk is reported by Ciani and coworkers.5 The replacement of tetrahedral nodes by more decorated moieties are exemplified by [{Mn(CO)3(μ3-OH)}4] by Zaworotko and coworkers,6 4,4′,4″-tricyanotriphenyletanol by Hosseini and coworkers,7 [Ge4Se10]4− by Kanatzidis and coworkers,8 and indium sulfide cluster by O’Keeffe and Yaghi and coworkers.9 These decorated diamondoid networks demonstrated modified robustness, degree of interpenetration, and porosity and may provide potential for guest inclusion, gas storage, and ion exchange.
In the design of diamondoid networks with large cavities, minimizing the degree of interpenetration is a formidable challenge. In addition to the strategies on the basis of the guest templates and the decorated cluster nodes toward porous diamondoid networks, we envision that the incorporation of a secondary interaction between the interwoven diamondoid nets could enhance the robustness of the interpenetrated networks. Therefore, large cavities are expected to be retained within the interwoven nets. Herein we wish to report the synthesis and X-ray single-crystal structure of a porous four-fold interpenetrating diamondoid network, [Cd(IA)2·1.7H2O]n (1) (IA = imidazole-4-acrylate) and its de-guest compound [Cd(IA)2]n (1a). Our strategy lies in the recognition that the IA ligand with a imidazole ring and a carboxylate group can serve as a strong N–H⋯O hydrogen bonding donor–acceptor site, and generate secondary net-to-net interaction in addition to the coordination and π–π interaction. In nature, the protein that is found in silk fibers epitomizes this strategy: the primary polypeptide chains are further linked by a secondary inter-chain hydrogen bonding interaction to generate a β-pleated sheet topology, which provides the flexibility yet very strong and resistant to stretching.10
Compound 1 is synthesized by dissolving Cd(NO3)2·4H2O (0.5 mmol) and imidazole-4-acrylate (IA) (0.5 mmol) in 5 mL of H2O–ethanol–DMF–ethylene glycol in a volume ratio of 0.5∶2∶2∶1. The mixture was sealed in a 23-mL Telfon-lined Parr acid digestion bomb and heated at 140 °C for 48 h. The final product contained colorless crystals of 1 in a yield of 58% based on IA ligand, and a trace amount of an unknown pale-yellow impurity.† An X-ray single-crystal structure determination reveals that the CdII centers in 1 adopts a pseudo-tetrahedral geometry,‡ which are in turn bound to four other CdII centers to result in a diamondoid network (Fig. 1).
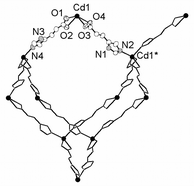 | ||
| Fig. 1 The coordination environments of the CdII centers. Atoms in the crystallographic asymmetric unit are represented at 40% ellipsoids. Hydrogen atoms are omitted for clarity. | ||
The Cd–IA–Cd separations are 10.83 and 11.02 Å, which results in large hexagonal apertures with edge-to-edge distances ranging from 17.4 to 18.6 Å. Usually, the adamantoid aperture of comparable size as in 1 is expected to compensate a maximum of four identical diamondoid nets (five-fold interpenetration),2 and potential guest accessible area is about 12% of unit cell volume.11 However, the crystal packing diagram of 1 shows that these adamantoid apertures are only filled with three other identical [Cd(IA)2]n nets (four-fold interpenetration) (Fig. 2). Consequently, void spaces (∼36.4% of unit cell volume) with diameters ranging from 2.1 to 7.7 Å appear within the four-fold interpenetrating diamondoid nets of 1, and generate intersecting channels that are occupied by disordered water molecules.11 The disordered water molecules can mostly be removed when a single crystal of 1 was treated under vacuum for 12 h to become [Cd(IA)2]n (1a) as indicated by crystallographic studies.‡
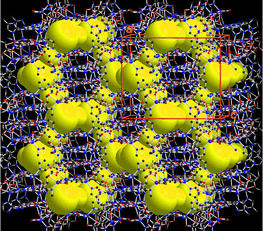 | ||
| Fig. 2 The crystal structure of 1, deliberately designed to have intersecting channels (represented by the yellow region) within the four-fold interpenetrating networks. Channel openings are observed along the crystallographic (100) and (010) directions. | ||
As expected from our design strategy, the strong N–H⋯O bonding serves as a ‘spring’ to form a net-to-net interaction force perpendicular to that of the coordinating ligands, and stabilizes the mutually interpenetrating diamondoid networks (Fig. 3(a)). In particular, the net-to-net H-bonding interaction leads to unusual mode of interpenetration for diamondoid networks: the four interpenetrating adamantane units are neither all equally spaced, nor positioned exactly along one single line (Fig. 3(b)).1c,1f,5
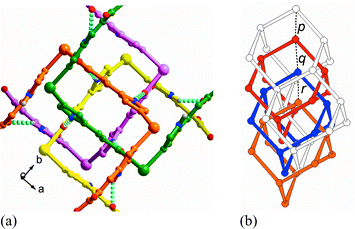 | ||
| Fig. 3 (a) The ‘spring-like’ N–H⋯O net-to-net hydrogen-bonding arrays (cyan broken line) ‘glue’ the diamondoid networks. (The N⋯O distances are 2.70(1) and 2.83(1) Å and N–H⋯O angles are 160.5(5) and 153.3(6)°.) For clarity, each independent network is in different color schemes. (b) The four-fold interpenetrating diamondoid nets. Two adamantane units of one of the nets fused together by two shared rods are represented here with open connections. Distances between the nodes: p = 7.748 Å, q = 8.529 Å, r = 7.748 Å. Key: node: CdII center, rod: IA ligand. | ||
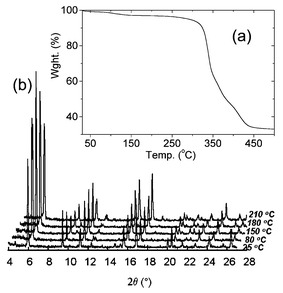
The crystalline sample of 1 was kept in a desiccator at room temperature for 20 h and was examined by the thermogravimetric analysis (TGA) and powder X-ray thermodiffractometry, as shown in Fig. 4.
TGA revealed a weight loss of 2.9% (∼0.65 mole of H2O) indicating that the host framework of 1 gradually releases guest molecules during the drying period (originally 1.7 mole of H2O) (Fig. 4(a)). TGA and powder X-ray thermodiffractometry showed that compound 1 is thermally stable up to ∼250 °C and the porous framework host remains intact.
We believe that the strategically designed net-to-net strong hydrogen-bonding interaction favors the formation of the resulting four-fold interpenetrated structure of 1 instead of reaching a maximum of five-fold interpenetration topology. It is also responsible for stabilizing the existing intersecting solvent accessible area within the network of 1 even in the absence of in situ included template guests.12
In conclusion, we have successfully demonstrated a feasible way in obtaining accessible free volume and permanent porosity in a diamondoid network by employing strong net-to-net hydrogen bonding interactions to enhance the framework rigidity. Further extending this novel approach to the synthesis of other porous polymeric networks is currently under progress.
We thank Academia Sinica and the National Science Council of the Republic of China for financial support.
Notes and references
- (a) M. J. Zaworotko, Chem. Soc. Rev., 1994, 283 RSC; (b) D. S. Reddy, D. C. Craig and G. R. Desiraju, J. Am. Chem. Soc., 1996, 118, 4090 CrossRef CAS; (c) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef; (d) S. J. Clarke, P. R. Chalker, J. Holman, C. W. Michie, M. Puyet and M. J. Rosseinsky, J. Am. Chem. Soc., 2002, 124, 3337 CrossRef CAS; (e) O. R. Evans and W. Lin, Chem. Mater., 2001, 13, 2705 CrossRef CAS; (f) S. R. Batten, CrystEngComm., 2001, 18, 1 Search PubMed; (g) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef; (h) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS; (i) Y.-H. Liu, C.-S. Lin, S.-Y. Chen, H.-L. Tsai, C.-H. Ueng and K.-L. Lu, J. Solid State Chem., 2001, 157, 166 CrossRef CAS; (j) D. S. Reddy, T. Dewa, K. Endo and Y. Aoyama, Angew. Chem., Int. Ed., 2000, 39, 4266 CrossRef CAS; (k) G. Férey, Chem. Mater., 2001, 13, 3084 CrossRef CAS.
- O. R. Evans, R.-G. Xiong, Z. Wang, G. K. Wong and W. Lin, Angew. Chem., Int. Ed., 1999, 38, 536 CrossRef CAS.
- R. Robson, B. F. Abrahams, S. R. Batten, R. W. Gable, B. F. Hoskins and J. Liu, in Supramolecular Architecture: Synthetic Control in Thin Films and Solids, ed. T. Bein, American Chemical Society, Washington DC, 1992, pp. 256–273 Search PubMed.
- A. J. Blake, N. R. Champness, S. S. M. Chung, W.-S. Li and M. Schröder, Chem. Commun., 1997, 1005 RSC.
- L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, Chem. Eur. J., 2002, 8, 1519 CrossRef CAS.
- S. B. Copp, K. T. Holman, J. O. S. Sangster, S. Subramanian and M. J. Zaworotko, J. Chem. Soc., Dalton Trans., 1995, 2233 RSC.
- S. Ferlay, S. Koenig, M. W. Hosseini, J. Pansanel, A. De Cian and N. Kyritsakas, Chem. Commun., 2002, 218 RSC.
- M. Wachhold, K. K. Rangan, S. J. L. Billinge, V. Petkov, J. Heising and M. G. Kanatzidis, Adv. Mater., 2000, 12, 85 CrossRef CAS.
- H. Li, M. Eddaoudi, A. Laine, M. O’Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 1999, 121, 6096 CrossRef CAS.
- G. A. Jeffrey and W. Saenger, Hydrogen Bonding in Biological Structures, Springer-Verlag, Heidelberg, 1994 Search PubMed.
- The calculation is based on the program PLATON: P. van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A, 1990, 46, 194 Search PubMed.
- J. Zhang, W. Lin, Z.-F. Chen, R.-G. Xiong, B. F. Abrahams and H.-K. Fun, J. Chem. Soc., Dalton Trans., 2001, 1806 RSC.
Footnotes |
| † Elemental analysis for colourless crystals of Cd(C6H5N2O2)2·1.7H2O 1. Found: C, 34.72; H, 3.76; N, 12.87. Calc.: C, 34.54; H, 3.24; N, 13.43%. |
| ‡ A single crystal of 1 that was dried in a desiccator at room temperature for 15 h was selected for crystallographic analysis. Crystal data for 1: Cd(C6H5N2O2)2·0.5H2O, M = 395.65, tetragonal, space group I41/a, a = 18.569(3), c = 23.042(2) Å, U = 7945(2) Å3, Z = 16, μ(Mo-Kα) = 1.118 mm−1, 21094 reflections measured, 4485 unique (Rint = 0.0518). Final R[I > 2σ(I)] = 0.0796. Largest diff. peak and hole: 1.015/−0.692 e Å−3. The guest water molecules were disordered, and no attempt was made to assign the H atoms of the water molecules.Compund 1a was obtained by treating single crystals of 1 under vacuum. Its colorless appearance was retained, and was sealed for crystallographic analysis. Crystal data for 1a: Cd(C6H5N2O2)2, M = 386.64, tetragonal, space group I41/a, a = 18.569(3), c = 23.042(2) Å, U = 7945(2) Å3, Z = 16, μ(Mo-Kα) = 1.114 mm−1, 3546 reflections measured, 3331 unique (Rint = 0.0172). Final R[I > 2σ(I)] = 0.0588. Largest diff. peak and hole: 1.058/−0.465 e Å−3.Removal of the coordinates of water molecules from the refined structure of 1 followed by the treatment of the crystal data with the SQUEEZE11 routine suggests a largest cavity residual electron density of 4.19 e Å−3. Similar analysis of the data from 1a yields the largest cavity residual electron density of only 1.69 e Å−3 indicatting the release of guest molecules under vacuum.CCDC reference numbers 192958 and 192959. See http://www.rsc.org/suppdata/cc/b2/b208683b/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |