1,4-Dibutoxy-2,3-di(4-pyridyl)-8,11,15,18,22,25-hexakis(hexyl)phthalocyaninato zinc, a self-assembled coordination polymer in the solid state
Shaya Y.
Al-Raqa
,
Michael J.
Cook
* and
David L.
Hughes
School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich, UK NR4 7TJ. E-mail: m.cook@uea.ac.uk; Fax: 44 (0)1603 507773; Tel: 44 (0)1603 593135
First published on 26th November 2002
Abstract
The title compound forms intermolecular zinc–nitrogen coordinated species in solution and self-assembles to form a coordination polymer in the solid state, the X-ray structure for which shows that the unit cell contains eight macrocycle units in two chains comprising both enantiomeric forms as ABBA/BAAB sequences.
Phthalocyanines show a propensity to form co-facial or near co-facial molecular assemblies. This type of packing is well documented for the crystal state1 but is also evident among aggregated molecules in solution2 and in the long columnar stacks of liquid crystal phases exhibited by derivatives bearing long alkyl chains.3 In an earlier study we proposed4 that a hexa-octyl pyridino[3,4]tribenzoporphyrazine metallated with zinc(II) forms ‘edge-to-face’ rather than non-cofacial aggregates in the solution phase, resulting from the pyridyl N atom of one molecule complexing with the zinc centre of a second. We have now explored this type of self-assembled structure further using a phthalocyanine bearing two pyridyl substituents 1. A crystal structure determination, which we believe to be the first of a non-uniformly substituted phthalocyanine, establishes that the compound indeed self-assembles edge-to-face to form a ‘zigzag’ polymer in the solid state.
Compound 1 was prepared according to Scheme 1. The 4,5-dibromo-3,6-dibutoxyphthalonitrile precursor5 was reacted with pyridine-4-boronic acid under Suzuki conditions to give 4,5-dipyridyl-3,6-dibutoxyphthalonitrile along with a smaller amount of 4-pyridyl-3,6-dibutoxyphthalonitrile. The former was cross-cyclotetramerised with 3,6-dihexylphthalonitrile to form the metal-free phthalocyanine 2. Compound 2 was metallated using zinc acetate to form 1.
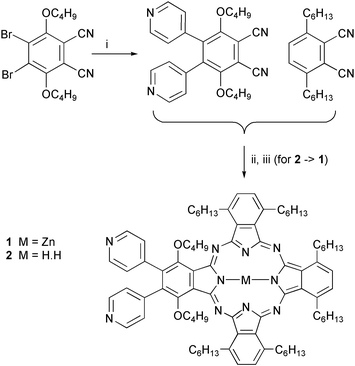 | ||
| Scheme 1 Reagents and conditions: i, pyridine-4-boronic acid, (PPh3)4Pd, CsF, dry DME, reflux under N2, 45%; ii, Li, BuOH, reflux, AcOH, 10%. iii, Zn(OAc)2·H2O, BuOH, reflux, 85%. | ||
Recrystallisation from THF/MeOH afforded shiny dark green prisms, mp >290 °C, the X-ray crystal structure for which is shown in Fig. 1.† There are four independent phthalocyanine moieties in the unit cell, (1)-1, (1)-2, (1)-3 and (1)-4, centred on Zn(1)–Zn(4), respectively. Each zinc has square pyramidal coordination and the apical Zn–pyridine bonds link the molecules in an infinite polymer chain. Such a chain is reminiscent of that reported earlier for zinc 5,10,15-triphenyl-20-pyridylporphyrin.7 Unlike the latter, 1, though possessing a plane of symmetry in the monomeric state, generates a chiral complex upon coordination. Fig. 1 shows that the unit (1)-1, (1)-2, (1)-3 and (1)-4 contains the two enantiomers, (A and B) in the sequence ABBA. This unit packs with chains of the racemic sequence, BAAB, i.e. (1)-1″, (1)-2″, (1)-3″ and (1)-4″, related by a centre of symmetry. There is partial overlap of near neighbour macrocyclic rings of the two polymer chains, e.g. (1)-3 and (1)-3″.
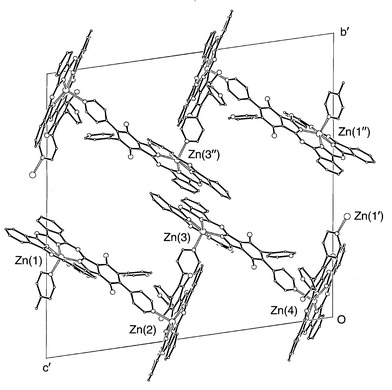 | ||
Fig. 1 The linking of molecules in the crystal of 1. The substituent hexyl and butyl groups have been omitted for clarity. The primed suffices indicate symmetry operations: ′x, y, z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; ″1 1; ″1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, 1 x, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y, 1 y, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z. z. | ||
Fig. 2 shows the structure of one of the individual molecules, (1)-1, with the six hexyl groups included. As expected the zinc atom is out of the plane of the four coordinating ring N atoms, by 0.438(7) Å. Its bond to the pyridine N is ca. 0.13 Å longer than the mean length of the bonds to the macrocycle’s four coordinating N atoms. Key bond lengths and angles for all four variants of 1 are shown in Table 1. These show very similar patterns in the four residues but considerable variation in the out-of-base-plane displacement of the zinc atoms towards the neighbouring pyridine ligand.
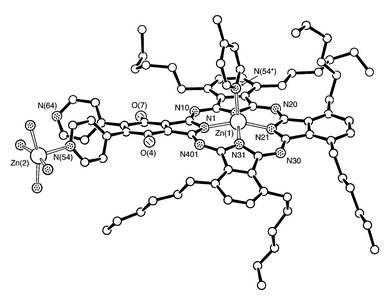 | ||
| Fig. 2 View of (1)-1, with its adjoining pyridyl ligand, coordinated through N(54*), and, bonded to N(54), the coordination sphere of Zn(2). The butyl groups (attached to the O atoms) and hydrogen atoms have been omitted for clarity. | ||
| Parameters for residues (1)-n | (1)-1 | (1)-2 | (1)-3 | (1)-4 |
|---|---|---|---|---|
| a N(base) refers to the four N atoms of the phthalocyanine core. b Mean values. | ||||
| Zn(n)–N(1) | 2.043(12) | 2.067(16) | 2.059(11) | 2.035(14) |
| Zn(n)–N(11) | 1.955(12) | 2.021(14) | 2.028(13) | 2.039(13) |
| Zn(n)–N(21) | 2.033(11) | 1.994(14) | 2.005(12) | 1.992(12) |
| Zn(n)–N(31) | 2.036(13) | 2.064(14) | 2.028(15) | 2.049(13) |
| Zn(n)–N(54*) | 2.143(12) | 2.156(12) | 2.163(14) | 2.173(11) |
| Mean Zn–N(base)a | 2.02(2) | 2.04(2) | 2.030(11) | 2.029(13) |
| N(11)–Zn(n)–N(1) | 86.4(6) | 87.8(7) | 87.3(5) | 88.0(6) |
| N(21)–Zn(n)–N(1) | 154.9(5) | 164.6(4) | 155.0(5) | 160.9(4) |
| N(31)–Zn(n)–N(1) | 86.7(6) | 87.0(8) | 86.9(6) | 88.4(6) |
| N(11)–Zn(n)–N(21) | 88.4(5) | 91.5(6) | 87.0(6) | 88.4(5) |
| N(11)–Zn(n)–N(31) | 154.9(5) | 163.8(4) | 157.9(6) | 162.7(4) |
| N(21)–Zn(n)–N(31) | 87.6(5) | 89.5(7) | 89.3(6) | 89.5(6) |
| N(1)–Zn(n)–N(54*) | 95.8(4) | 91.5(4) | 95.5(4) | 90.9(4) |
| N(11)–Zn(n)–N(54*) | 98.3(4) | 99.6(4) | 100.7(4) | 101.4(4) |
| N(21)–Zn(n)–N(54*) | 109.2(3) | 103.7(4) | 109.5(4) | 108.2(4) |
| N(31)–Zn(n)–N(54*) | 106.4(4) | 95.9(4) | 101.1(4) | 95.5(4) |
| N–Zn–N(cis, base)b | 87.3(4) | 88.9(10) | 87.6(6) | 88.6(3) |
| N–Zn–N(trans, base)b | 154.91(3) | 164.2(4) | 156.4(14) | 161.8(10) |
| N–Zn–N(from apex)b | 102(3) | 98(3) | 102(3) | 99(4) |
|
Displacement of Zn from
N4 base mean-plane |
0.438(7) | 0.276(6) | 0.415(7) | 0.320(5) |
The visible and near-IR spectrum of the polymeric structure was measured of a burnished film, i.e. one in which crystals are gently smeared onto a glass slide. The multi-band absorption envelope is shown as Fig. 3(a). A spin coated film of 1 obtained from a solution in THF gives rise to a closely similar spectrum, Fig. 3(b), implying that the film contains a comparable molecular assembly.
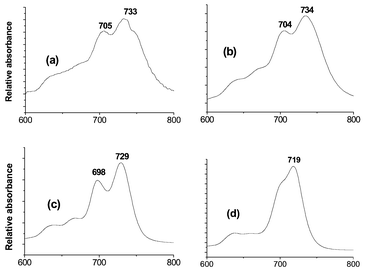 | ||
| Fig. 3 Visible and near-IR spectrum (600–800 nm) of 1 as (a), a burnished film; (b), a spin coated film; (c) as a solution in CH2Cl2, and (d), as (c) but after addition of one drop of pyridine. | ||
The behaviour of 1 in solution is solvent dependent. A similar electronic absorption envelope to that of the polymer is observed in the spectrum of the compound in CH2Cl2 solution, Fig. 3(c). However, the bands in the latter show a small hypsochromic shift, which may be attributed to the presence of lower aggregates. Addition of one drop of pyridine to the solution evidently breaks down these aggregates, giving a new spectrum, Fig. 3(d), which is superimposable upon that obtained for 1 in THF solution. Disruption of the solution phase aggregates is also observed by 1H NMR spectroscopy. The spectrum of 1 in CDCl3 shows no signals for pyridyl protons at chemical shifts downfield of those for phthalocyanine ring protons. This is consistent with an equilibrium which, on time average, contains a significant amount of edge-to-face aggregates. Addition of pyridine-d5 to the solution changes the spectrum to show pyridinoid proton signals at 8.62 and 7.40 ppm for uncomplexed pyridine rings.
We thank the King Abdulaziz University of Saudi Arabia for financial support (for S. Y. Al-Raqa) and EPSRC’s mass spectroscopy service, Swansea, for MS data.
Notes and references
- M. K. Engel, Kawamura Rikagaku Kenkyusho Hokoku (English), 1996, 11 ( Chem. Abstr. , 1997 , 127 , 313213 ) Search PubMed.
- A. N. Cammidge and R. J. Bushby, in, Handbook of Liquid Crystals: Vol 2B Low molecular weight liquid crystals II, ed. D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, New York, Chichester, p. 1998, 693 Search PubMed.
- M. J. Cook, in Spectroscopy of New Materials (Advances in Spectroscopy, vol. 22), ed. R. J. K. Clark and R. E. Hester, Wiley, Chichester, 1993, p. 87 Search PubMed.
- M. J. Cook and A. Jafari-Fini, J. Mater. Chem., 1997, 7, 2327 RSC.
- M. J. Cook and M. J. Heeney, Chem. Eur. J, 2000, 6, 3958 CrossRef CAS.
- N. B. McKeown, M. J. Cook and I. Chambrier, J. Chem. Soc., Perkin Trans. 1, 1990, 1169 RSC.
- E. B. Fleischer and A. M. Shachter, Inorg. Chem., 1991, 30, 3763 CrossRef CAS.
Footnote |
† Crystal data: C86H110N10O2Zn, M = 1381.2, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 19.979(4), b = 27.995(9), c = 28.253(8) Å, α = 98.52(2), β = 90.19(1), γ = 92.27(2)°, V = 15 615(7) Å3. Z = 8, Dc = 1.175 g cm−3, F(000) = 5936, T = 140(1) K, μ(Mo-Kα) = 3.7 cm−1, λ(Mo-Kα) = 0.71069 Å. Diffraction intensities measured on a Rigaku R-Axis IIc image plate diffractometer with rotating anode X-ray source. Total no. of reflections recorded, to θmax = 20° (limit of useful data in this poorly diffracting sample), was 45 (no. 2), a = 19.979(4), b = 27.995(9), c = 28.253(8) Å, α = 98.52(2), β = 90.19(1), γ = 92.27(2)°, V = 15 615(7) Å3. Z = 8, Dc = 1.175 g cm−3, F(000) = 5936, T = 140(1) K, μ(Mo-Kα) = 3.7 cm−1, λ(Mo-Kα) = 0.71069 Å. Diffraction intensities measured on a Rigaku R-Axis IIc image plate diffractometer with rotating anode X-ray source. Total no. of reflections recorded, to θmax = 20° (limit of useful data in this poorly diffracting sample), was 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 of which 26 798 of which 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 unique (Rint = 0.119); 14 641 unique (Rint = 0.119); 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 ‘observed’ with I > 2σI. Structure determination by direct methods shows four independent Zn–phthalocyanine complex molecules; refinement by full-matrix least-squares methods. The non-hydrogen atoms (except in disordered side-chains) refined with anisotropic thermal parameters. Hydrogen atoms (except in disordered sections) included in idealised positions and ‘riding’. Refinement with the low quality data gave rather unreliable displacement parameters for some atoms which were then refined isotropically. Final wR2 = 0.346 and R1 = 0.216 for all 26 910 ‘observed’ with I > 2σI. Structure determination by direct methods shows four independent Zn–phthalocyanine complex molecules; refinement by full-matrix least-squares methods. The non-hydrogen atoms (except in disordered side-chains) refined with anisotropic thermal parameters. Hydrogen atoms (except in disordered sections) included in idealised positions and ‘riding’. Refinement with the low quality data gave rather unreliable displacement parameters for some atoms which were then refined isotropically. Final wR2 = 0.346 and R1 = 0.216 for all 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 reflections; for the ‘observed’ data only, R1 = 0.146. Highest difference peaks (to ca. 1.2 e Å−3) close to zinc atoms. CCDC 191667. See http://www.rsc.org/suppdata/cc/b2/b207873m/ for crystallographic data in CIF or other electronic format. 641 reflections; for the ‘observed’ data only, R1 = 0.146. Highest difference peaks (to ca. 1.2 e Å−3) close to zinc atoms. CCDC 191667. See http://www.rsc.org/suppdata/cc/b2/b207873m/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
