Monitoring solid phase synthesis reactions with electrochemical impedance spectroscopy (EIS)
Roger S.
Hutton
*a,
Joseph P.
Adams
b and
Harish S.
Trivedi
b
aGlaxoSmithKline, Technology Development, New Frontiers Science Park, Third Ave, Harlow, Essex, UK CM19 5AW. E-mail: Roger.S.Hutton@GSK.com; Fax: 01279 622749; Tel: 01279 622407
bGlaxoSmithKline, Medicines Research Centre, Gunnels Wood Road, Stevenage, Hertfordshire, UK SG1 2NY
First published on 6th December 2002
Abstract
This work describes the use of electrochemical impedance spectroscopy (EIS) as a means to monitor solid phase synthesis on resin beads. EIS was used to track changes during the swelling of beads in various solvents, during three typical reactions and throughout cleavage of the final product from the bead. The impedance response was investigated in a chemical reactor and was found to be faintly sensitive to the resin swelling and solvent flow. The position of the electrode within the reactor was found to be critical as polystyrene based beads float or sink dependent upon the solvent used. However, by choosing electrode position it was possible to monitor reaction progress on beads or within the bulk reactant/product mixture. Of the three typical chemical reactions studied impedance spectroscopy successfully followed two. Fitting of the impedance data to an equivalent electrical circuit provided an estimate as to the relative contribution of capacitive and resistive components to the overall response. Kinetic data from two reactions were also modelled, in both cases complex kinetics was observed, in close agreement with other studies.
Introduction
Solid phase methods, as used in synthetic combinatorial chemistry, have the potential to be employed on a large scale, such an approach negates the need for developing alternative synthetic strategies on scale-up. However in doing so reactions need to be optimised i.e. select high yielding, reliable and clean reactions and to limit undesirable side reactions. At present limited means of monitoring solid phase reactions in real time exist, UV-Vis and refractive index measurements have been used to monitor reactant mixtures with partial success.To our knowledge no work has been made on EIS monitoring of solid phase synthesis but EIS has found use monitoring a variety of other reactions. For example Walcarius and Bessiere1 used EIS to monitor Cu(II) fixation on silica gel. Measurements were performed in static and flowing solutions and used to construct adsorption isotherms. Compton et al.2,3 used impedance measurements at electrodes positioned down-stream of a flow cell, and monitored the dissolution and hydrolysis of weakly ionic species. Impedance spectroscopy has been widely used to monitor polymer reaction/curing processes. These measurements have correlated reaction status, degree of cross-linking, gelation, rheology, and presence of ionic impurities to the impedance response.4 Many studies have investigated adsorption processes at surfaces such as self-assembled monolayers. The interest here lies in the development of biosensors with applications in receptor and drug assays and investigating ion channels. In this case EIS provides information on the interaction of species at the surface.5,6 Other applications include: monitoring the activity and mobility of enzymes in organic solvents, which provides a measure of enzyme hydration.7 Markx and Bell estimated the viable biomass in a fermenting vessels at radio frequencies.8 Several investigations have considered the behaviour of particles, these include the use of the dielectric behaviour to actively sort particles by size.9 An alternating electric field has also been used to induce rotation instead of current flow. The rotation rate is determined by the dielectric properties of the particle and its coating. It has been shown that the motion of latex beads is sensitive to attached protein sequences.10 Impedance based tomography experiments could be used to monitor reaction uniformity.11 At present such tomography measurements have been used to image uniformity of composition within pipes, on filters, in bioreactor and stirred vessels. Thus far information relating to the chemical composition and reaction uniformity have been largely neglected.
In this work the use of EIS to monitor solid phase synthesis on resin beads is described. In principle EIS provides several advantages over other methods, not only does impedance spectroscopy provide an alternative detection method, but in contrast to optical methods EIS provides rapid feedback representative of part or the complete reaction chamber. EIS also allows monitoring of reactants, intermediates and products to be followed in real time. It is also believed the impedance response is also scalable and could be readily transferred as reactions are scaled-up.
Experimental
Synthetic chemistry
Three reactions were studied which represent typical chemistry of interest to the pharmaceutical industry (Schemes 1–3), further details of which are as follows. 1. Imine formation—performed in trimethylorthoformate (TMOF). Wang aldehyde was allowed to swell in TMOF for 20 min. Cyclohexylamine was added. Mixing was achieved by recirculating the solution via a peristaltic pump through the solution. After 3.5 h the solution was drained off and more TMOF and cyclohexylamine was added. After a further 4 h the solution was drained off and the resin was dried in a vacuum oven at 350 °C. 2. Amine formation—Wang imine was allowed to swell in DMF for 10–15 min, sodium triacetoxyborohydride(STAB) was dissolved in DMF and acetic acid, STAB solution was added to the reaction vessel and recirculation was continued. After mixing overnight the solution was drained off from the beads and then washed consecutively with DMF, water, DMF, CH2Cl2, methanol and CH2Cl2. Beads were then dried in a vacuum oven for 1 h. 3. Amide formation—Wang amine was allowed to swell in DMF for 20 min after which a solution of 1-hydroxybenzotriazole (HOBT) in DMF was added followed immediately by 2-dimethylaminoisopropylchloride hydrochloride (DIC) and AcOH. The reaction was performed overnight. Samples were analysed by NMR.Instrumentation
A Solartron Instruments frequency response analyser (FRA) was used for all measurements. The basis of impedance spectroscopy is that the solution or system of interest is excited by applying a small alternating voltage between electrodes and the resulting current is analysed as a function of its magnitude and phase relative to the original excitation. The measurement of relative phase and amplitude is made as a function of the excitation frequency. Often the behaviour of a system is compared to electrical equivalents, for example if a sample has the ability to store charge it is said to behave like a capacitor.The reaction vessel consisted of a glass cylinder fitted with a glass frit at the bottom of the cylinder which contains the solid phase resin and detection electrodes. Solution was circulated through chamber such that reactions occur on the resin beads. The beads occupied about half the cylinder volume and sunk or floated dependent upon the solvent used. Usually the state of the reaction is monitored by extracting a sample and subsequent analysis or by examination of the solution composition in the recirculation loop. In this study an eight electrode assembly in which each electrode could be individually addressed was inserted into the glass reactor (volume ≈150 ml). Each of the electrodes were made of platinum. Platinum was chosen as it is chemically inert to aid reactor cleaning, easy to incorporate into glass, relatively inert during impedance measurement, and physically robust such that the electrodes will not be deformed during bed packing. Usually in electrochemical measurements reference electrodes are used to measure voltage differences, the choice of which depends upon the solvent and pH. Reference electrodes are usually made from Ag/AgCl, calomel or other mercury salts. In this case we used platinum as a pseudo-reference electrode in order to remove the difficulties of positioning an electrode within the reactor and to cut out problems with contamination of chloride or other salts. A schematic of the electrode configuration and cell is shown in Fig. 1. In each experiment the small alternating current was measured over a 20 min period to construct an impedance spectrum. Data was collected continuously during the reaction. Unless otherwise stated reagents were peristaltically pumped at 80 ml min−1.
 | ||
| Fig. 1 Schematic of the experimental set up—typically four of the electrodes were embedded within the beads at any one time. | ||
Results
Throughout EIS monitoring of reactions on solid phase supports a number of variables could influence the response. For example the extent to which the electric field penetrates a bead is dependent upon the relative dielectric constant of the bead and the solvent. The penetration depth will also be dependent upon the measurement frequency, thus by tuning the measurement frequency it should be possible to ‘examine’ the beads rather than the solvent. However to fully understand the impedance response is not a straightfoward process and requires extensive experimentation. The aim therefore here is to ′fingerprint′ chemical reactions. In these experiments we are essentially looking for reproducible changes in the impedance response (Z) that result from the reaction. For the most part data is plotted as Bode diagrams in which the magnitude of the impedance (|Z|) and relative phase angle (θ) are plotted as a function of frequency. For a capacitor the current precedes the voltage by a 90° phase angle. A resistor has a phase lag of zero degrees.I. Bead swelling in solvents
Initial measurements were performed using all eight electrodes or individual pairs and followed the swelling of initially dry resin beads after the addition of solvent. Measurements were performed to determine suitable experimental conditions (amplitude, integration time, etc.). Fig. 2 shows a typical result obtained with dichloromethane. From Fig. 2 it can be seen that at intermediate frequencies (ca. 10–1000 Hz) the reactor behaves as a resistor and at frequency extremes it behaves as a capacitor. The impedance is greater at lower measurement frequencies. In each case the results were found to be reproducible. Movement of the electrodes within the mass of beads had practically no effect upon the response obtained. However, the impedance shifted slowly with time. This was attributed to the swelling of beads and changes in the dichloromethane level. This assumption was supported by the observation that in an early experiment a small leak was detected in the tubing that visibly affected the level in the reactor. Fixing the leak and addition of extra solvent allowed the response to return to its original level afterwhich the impedance continued to drift. Fitting of the data to a simple RC circuit demonstrated that changes in the effective capacitance were dominant and that the cell resistance was insensitive to the solvent level changes and swelling process. It is not unsurprising that the amount of solvent affects the response, some of these changes are likely to be due to movement of the bead mass around the electrodes. | ||
| Fig. 2 Typical impedance response observed for resin beads during swelling in CH2Cl2—measurement made with all eight electrodes after swelling for 2 h. | ||
All subsequent measurements reported were made on electrode pairs from deep within the resin beads or in the bulk solution. When the solvent is changed and the beads no longer sink but float, a different electrode pair was employed. Measurements were made between electrode pairs within the beads, near the bead mass/solvent edge and in the bulk solvent. The measurements obtained within the bulk dichloromethane were ‘noisy’ at low frequencies, presumably due to fluctuations in flow due to peristaltic pumping and the high impedance response. Interestingly the impedance was much smaller within the resin bed than in the bulk solution, this suggested that any conduction is via bead or bead surfaces as opposed to through the solvent. The influence of solvent flow was also investigated, the results of which are summarised in Fig. 3. Once more the impedance was modelled according to a simple RC circuit. The effective cell capacitance was hardly affected by the solution pumping, the majority of the effect is demonstrated through the cell resistance.
 | ||
| Fig. 3 Impedance data showing the effect of CH2Cl2 flow. Solid line: flow rate 80 μl min−1; dotted line: stagnant solution. | ||
Following the above measurements the CH2Cl2 was removed and replaced with DMF. Since the resin beads sink in DMF measurements were made at the lower two electrodes. Fig. 4 shows how the impedance response obtained as a function of time during swelling. DMF has different dielectric properties to CH2Cl2 and consequently the impedance was smaller by an order of magnitude. Once again the beads were observed to swell, this was manifested in the impedance which initially varied quickly and reached a steady state after approximately 2.5 h. As previously observed the solvent flow rate and position of electrodes were investigated and identical behaviour was observed.
 | ||
| Fig. 4 Impedance response as a function of time during bead swelling in DMF. | ||
Subsequently the solvent was again removed and the beads dried. TMOF was added and the beads sank to the bottom of the reactor. In TMOF the impedance response again differed and the impedance drifted slowly with time as the beads swelled. However on this occasion the impedance did not settle and equilibrium was not reached even after 6 h (Fig. 5).
 | ||
| Fig. 5 Impedance response during bead swelling over a six hour period in TMOF. | ||
II. Reaction studies
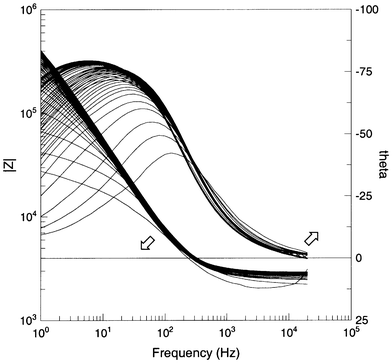 | ||
| Fig. 7 Impedance spectra obtained during amide formation as a function of time. | ||
The impedance response is obviously complex and controlled by many factors: these may include, surface charge, void volume, ions in solution etc. It is not realistic to develop a physical model for the reactor and assign electrical equivalents. However as an attempt to deconvolute the response the impedance was fitted to a five component equivalent circuit. Simplistically one may envisage that the impedances R1 and C1 represent processes at the bead surface, R2 and C2 are changes in the reagent/product molecular concentration near the surface (akin to an electrical double layer) and Rs represents the solution resistance. All the experimental data was fit to this five component equivalent circuit, a typical fit is shown in Fig. 8b. In each case values for the equivalent circuit change rapidly at first and level out reaching a steady state value after about 14 h (Fig. 9). The break observed in all plots coincides with a point at which data collection was momentarily stopped. Assuming that the measured capacitance C2 is linearly related to the concentration of the reactant/products the data was modelled according to standard rate equations. However such analysis indicated that the reactions did not adhere to any simple kinetic model. This is as expected for a complex heterogeneous reaction at a surface since, for example, in the first instance the rate determining step may be adsorption whereas at later times it may be a pseudo-first order reaction in solution. It is suffice to say the reaction was complete after 14 h. Ideally a method that is amenable to automation is required that will provide an easily identifiable point at which the reaction is said to be complete. Thus for all data representations the standard deviation of the last three consecutive measurements was plotted as a function of time (Fig. 9). Such an approach worked well enabling the reaction to be deemed complete after the standard deviation fell below a particular value.
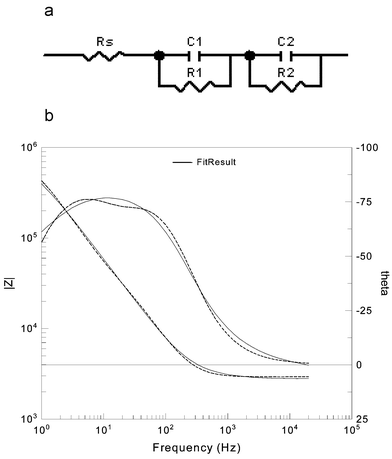 | ||
| Fig. 8 (a) 5 component equivalent circuit used to model data and (b) typical comparison of experimental and simulated data. | ||
 | ||
| Fig. 9 Following fitting of data shows during amide formation (a) the variation in solution resistance (b) capacitance C2 and (c) the standard deviation of a threepoint rolling average from the measured capacitance as a function of time. | ||
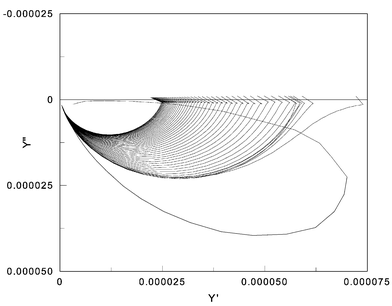 | ||
| Fig. 10 Complex admittance measured during amide formation. | ||
 | ||
| Fig. 11 Variation in impedance during acid cleavage of products from resin. | ||
Conclusions
It has been shown that by measuring the impedance response between different electrodes information on dielectric properties of the solution and bead mass can be obtained. In the case of amine and amide formation reactions the dielectric behaviour within the reactor is strongly related to the state of the chemical reaction. In the case of the amide formation the ability to follow the course of the reaction is very useful since this cannot be achieved by refractive index and UV-Visible absorption measurements. It follows that impedance spectroscopy provides a relatively simple technique to follow chemical reactions on solid phase supports.Clearly we have not considered the many factors that may influence the impedance response and cannot make any reasonable conclusions as to why the measurements work in some cases but not in others. Without a doubt the presence of static and mobile charge and the dielectric properties of media critically affect the measured resistance, capacitance and inductance. In systems where ions are present the ionic concentration, charge and mobility usually dominate the response. In the absence of ionic contributions more subtle dielectric effects dominate.
During solid phase synthesis the following are perceived as contributing factors. Bead properties—surface charge, surface wetting or void volume, particle size distribution, surface morphology and surface swelling. Surface composition—presence of charged functional or end groups. Dielectric shielding of the bead. pKa of species present. Solution composition—ionic contribution (e.g. presence of Sn salts, formation of complexes presence of H+ acetate, benzoate etc.), solvent composition (for example presence of water in hydroscopic solvent), presence of supporting electrolyte. Miscellaneous—presence of dissolved gases and bubbles.
References
- A. Walcarius and J. Bessiere, Anal Chim. Acta, 1998, 361, 273–283 CrossRef CAS.
- K. Y. Tam, J. P. Larsen, B. A. Coles and R. G. Compton, J. Electroanal. Chem., 1996, 407, 23–35 CrossRef CAS.
- R. G. Compton and J. Winkler, J. Phys. Chem., 1995, 99, 5029–5034 CrossRef CAS.
- J. Mijovic, J. Belucci and L. Nicolais, J. Electrochem. Soc., 1995, 142, 1176–1182 CAS.
- T. D. Osborn and P. Yager, Langmuir, 1995, 11, 8–12 CrossRef CAS.
- F. F. Zha, H. G. L. Coster and A. G. Fane, J. Membr. Sci., 1994, 93, 255–271 CrossRef CAS.
- P. Johann, P. Dennison, B. D. Moore and P. J. Halling, Biochim. Biophys. Acta, 1998, 1386, 79–89.
- G. H. Markx and D. B. Kell, Biotechnol. Prog., 1995, 11, 64–70 CrossRef CAS.
- W. G. Sisson, R. R. Brunson, T. C. Scott, M. T. Harris and J. L. Hook, Sep. Sci. Technol., 1995, 30, 1421–1434 Search PubMed.
- R. Pethig, Ann. Biol. Clin., 1996, 54, 253 Search PubMed.
- F. Dickin and M. Wang, Meas. Sci. Technol., 1996, 7, 247–260 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |




