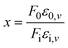Molecular sensors using a resonance Raman template
Andrew P.
Hard
,
Upali A.
Jayasooriya
* and
Andrew N.
Cammidge
School of Chemical Sciences, University of East Anglia, Norwich, UK NR4 7TJ. E-mail: u.jayasooriya@uea.ac.uk
First published on 9th December 2002
Abstract
The potential use of resonance Raman spectroscopy as a molecular sensing tool is illustrated using a metalloporphyrin template and pyridine as an analyte. The equilibrium binding constant for the axial binding of pyridine to zinc tetraphenylporphyrin has been measured using resonance Raman spectroscopy. Although no new peaks are observed and the porphyrin peaks do not shift position, the quantification is made possible by the selective resonance enhancement of the template vibrations. The value for log k was determined by resonance Raman to be 3.65 ± 0.32, which compares well with previously published values estimated using absorption data. Values for log k were determined for a series of related compounds, the picolines, and these also compare favourably with those previously reported.
Introduction
‘Normal’ Raman spectroscopy is not a trace analytical technique. However the variants of this technique such as resonance Raman scattering (RR), surface enhanced Raman scattering (SERS) and surface enhanced resonance Raman scattering (SERRS) can lend it exceptional sensitivity.1 For example, there are claims of single molecule detection using SERRS under special conditions.2–4 The need for such special conditions has prevented wider use of Raman spectroscopy as a useful sensing technique. Surface enhancement suffers from reproducibility problems due to a strong dependence on surface morphology. Resonance enhancement on the other hand requires the analyte to have an appropriate and easily accessible electronic transition. This means that even when the conditions are satisfied, each analyte will require a specific laser frequency for the scattering to be enhanced. This gives resonance Raman spectroscopy the advantage of being specific when such electronic transitions are accessible but puts it at a disadvantage when a more versatile sensor is needed. Here we report a method of overcoming this problem by using a fixed resonance Raman template for sensing different analyte molecules.A suitable template molecule should have the following properties. It should, (a) show strong resonance enhancement, (b) strongly and preferably reversibly bind the target analyte and (c) be robust and stable under a range of chemical and physical conditions. Metalloporphyrins are a family of compounds that satisfy all these conditions and were chosen for this investigation.
Relatively inexpensive and reliable blue diodes are being commercially manufactured at present for data storage purposes. The fact that the metalloporphyrins show resonance Raman enhancement with blue excitation provides an added advantage of the design of a really miniature RR sensor.
Metalloporphyrins have been studied extensively by a wide range of analytical techniques and are found to be of particular interest because they serve as effective model compounds for the heme centre of many metalloproteins.
Zinc(II) meso-tetraphenylporphyrin (ZnTPP, Fig. 1) and other zinc porphyrins bind strongly with nitrogen bases such as pyridine and related molecules, such as the methyl pyridines (picolines).5 The ligand binds to the zinc in an axial position and it has been shown that mono-pyridine complexes only are formed. This is presumed to be because the action of the ligand binding draws the zinc out of the porphyrin plane6 and is in contrast to Ni(II), Fe(II) and Mg(II)–porphyrins which are able to form bis-pyridine complexes.7–10
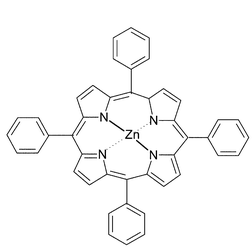 | ||
| Fig. 1 Schematic of the structure of zinc(II) meso-tetraphenylporphyrin, ZnTPP. | ||
There are numerous studies in the literature which have recorded thermodynamic data and equilibrium constants for the uptake of various N-containing ligands by ZnTPP at different temperatures.5,11–14 Absorption spectroscopy is the main technique used for these studies as metalloporphyrins are highly absorbent in the visible region and the spectra undergo significant red shifts upon axial ligand binding.
The degree of resonance enhancement of the Raman signal will change with the intensity of absorption at that wavelength—thus the nearer the laser wavelength is to λmax, the greater the enhancement. Indeed, this is the principle behind the measurement of RR excitation profiles.15–17 However, it should be noted that if the excitation wavelength is shorter than λmax, the Raman scattering will tend to be self-absorbed by the sample. Hence it is always best practice to use a laser wavelength on the longer wavelength side of the absorption maximum.
There is one report in the literature where Proniewicz et al.18 have reported a change in the RR spectrum of cobalt ‘picket fence’ porphyrins with the uptake of oxygen. The present investigation uses the ligation in the solution state for ‘proof of principle’ of the possibility of using a RR template to measure analytes quantitatively.
Experimental
Equipment
Absorption spectra were measured in 1 cm pathlength quartz cuvettes using a Hitachi U-3000 spectrometer. The RR spectra were recorded using a Spex 500M single monochromator equipped with a liquid nitrogen cooled CCD detector. Rayleigh scattering was removed with a holographic super-notch filter (Kaiser Optical Systems Inc.). A Liconix He–Cd laser provided excitation at 441.6 nm with maximum power of ca. 70 mW. The system was calibrated using CCl4, indene and toluene/acetonitrile. Samples were held in quartz NMR tubes and the scattering was measured at 90° to the incident radiation.Results
Visible absorption spectroscopy
Fig. 2 shows the typical changes to the ZnTPP absorption spectrum as pyridine is added. The equilibrium constant may be extracted from such data.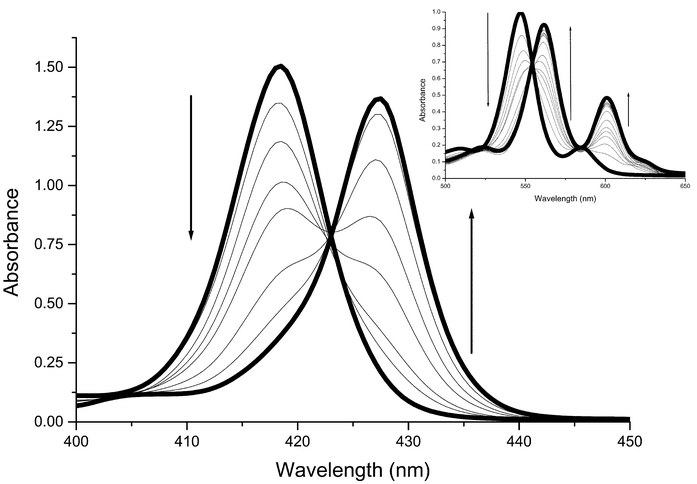 | ||
| Fig. 2 Changes in the Soret and Q (inset) band regions of the absorption spectrum of ZnTPP in dichloromethane solution during the titration with pyridine. Soret band region: 3.12 × 10−6 M ZnTPP; pyridine concentrations in the range 0 M to 1.04 × 10−3 M (0 M, 1.04 × 10−5 M, 2.48 × 10−5 M, 4.96 × 10−5 M, 1.04 × 10−4 M, 2.48 × 10−4 M, 4.96 × 10−4 M, 1.04 × 10−3 M). Q band region: 4.92 × 10−5 M ZnTPP; pyridine concentrations in the range 0 M to 0.049 M (0 M, 4.96 × 10−5 M, 9.92 × 10−5 M, 1.49 × 10−4 M, 1.98 × 10−4 M, 2.48 × 10−4 M, 4.96 × 10−4 M, 9.92 × 10−4 M, 1.98 × 10−3 M, 3.97 × 10−3 M, 5.95 × 10−3 M, 9.92 × 10−3 M, 0.015 M, 0.020 M, 0.030 M, 0.049 M). | ||
An alternative method of analysing the absorption data is the Scatchard plot19 of (A − A0)/[Py] against (A − A0), where A0 is the absorbance with no pyridine present and A is the absorbance after addition of a given amount of pyridine, which should have a slope of −k. An example is shown in Fig. 3.
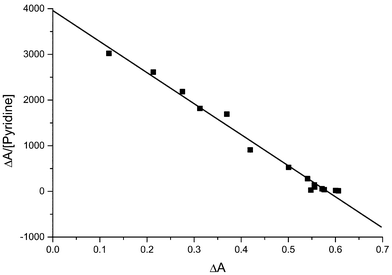 | ||
| Fig. 3 Scatchard plot for a titration of 4.92 × 10−5 M ZnTPP with pyridine. The absorbance was measured at 561.5 nm. The slope is −6796 ± 233, giving a log k value of 3.832 ± 0.015. | ||
Analysis of our absorption spectroscopic data by both methods gave log k values in the range 3.5–3.9 and an average log k value of 3.69 ± 0.18, in agreement with literature, Table 1.
Resonance Raman spectroscopy
The RR spectrum of ZnTPP in dichloromethane is shown in Fig. 4(A). The spectrum is dominated by the five main resonance enhanced bands,20ν2 (1550 cm−1), ν4 (1353 cm−1), ν1 (1236 cm−1), ν6 (1005 cm−1) and ν8 (387 cm−1). This is typical of metallo-tetraphenylporphyrin spectra recorded using Soret excitation.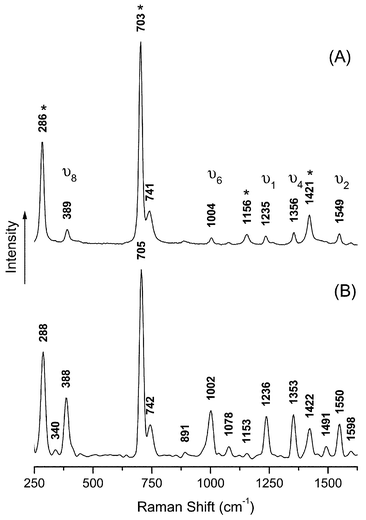 | ||
| Fig. 4 Resonance Raman spectra using 441.6nm excitation of ZnTPP in dichloromethane solution. Solvent bands are marked with an asterisk, *. (A) 4.92 × 10−5 M ZnTPP; (B) 4.92 × 10−5 M ZnTPP + 0.049M pyridine. All ZnTPP converted to PyZnTPP. | ||
Fig. 4(B) shows the RR spectrum of a ZnTPP solution at the end of a titration when a large excess of pyridine is present. Superficially the spectrum appears unchanged. However, it is clear that the main bands have significantly gained in intensity compared to the solvent bands (700 and 280 cm−1). This is to be expected because the Soret band is shifted towards the excitation wavelength (441.6 nm) and so the degree of resonance enhancement will be greater.
Thus, it should be possible to monitor the reaction between ZnTPP and pyridine using the intensity of a ZnTPP band with the help of a solvent band as a calibrant. The solvent signal is not resonance enhanced and hence is assumed to remain unchanged, whilst the porphyrin signal will be intensified as more pyridine binds and the degree of enhancement increases.
There are very few methods for extracting thermodynamic information from resonance Raman data and the method used here is based on that published by Michaelian et al.21 Its basis is that the signal at a given wavelength may be broken down into components from each of the species present and:
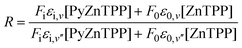 | (1) |
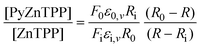 | (2) |
Substituting x into eqn. (2) gives:
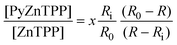 | (3) |
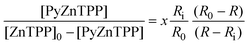 | (4) |
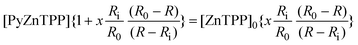 | (5) |
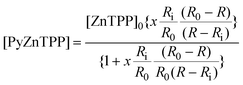 | (6) |
We investigated the change in the ratio of the intensities of the resonance enhanced porphyrin bands and the solvent band at 700 cm−1. In the experiments where the ZnTPP concentration was not fixed the ratio was divided by the ZnTPP concentration because the dilution by the pyridine solution naturally causes the porphyrin signal to decrease relative to the solvent signal. An example of a Scatchard plot from such resonance Raman data is shown in Fig. 5. The experiments using fixed ZnTPP concentration gave similar results, as shown in Fig. 6.
![Scatchard plot for the titration of ZnTPP with pyridine monitored using RR spectroscopy. The data was produced for the ratio between the intensity of the porphyrin band at 1005 cm−1 and the intensity of the solvent band at 700 cm−1. The ratio was divided by the total ZnTPP concentration, [ZnTPP]t, to correct for dilution by the pyridine solution. The slope is −5465 ± 366, giving a log k value of 3.738 ± 0.030.](/image/article/2003/AN/b209526b/b209526b-f5.gif) | ||
| Fig. 5 Scatchard plot for the titration of ZnTPP with pyridine monitored using RR spectroscopy. The data was produced for the ratio between the intensity of the porphyrin band at 1005 cm−1 and the intensity of the solvent band at 700 cm−1. The ratio was divided by the total ZnTPP concentration, [ZnTPP]t, to correct for dilution by the pyridine solution. The slope is −5465 ± 366, giving a log k value of 3.738 ± 0.030. | ||
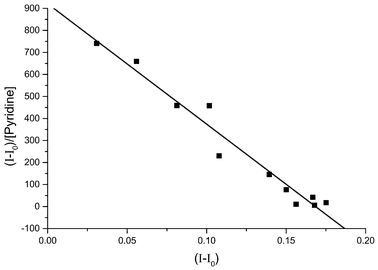 | ||
| Fig. 6 Scatchard plot for the titration of ZnTPP with pyridine monitored using RR spectroscopy. The data was produced for the ratio between the intensity of the porphyrin band at 1550 cm−1 and the intensity of the solvent band at 700 cm−1. The slope is −6659 ± 399, giving a log k value of 3.823 ± 0.027. | ||
Analysis of our RR data by both methods gave log k values in the range 3.2–4.3 and an average log k value of 3.65 ± 0.32. This agrees with our absorption data and also with the published literature values (see Table 1).
Picoline binding to ZnTPP
In order to verify that our RR method was indeed capable of providing information about the equilibrium binding constant, we also investigated the binding of three related compounds to the same template molecule, ZnTPP. 2-, 3- and 4-picoline are closely related to pyridine, with the crucial addition of a methyl group in the 2, 3 or 4 position respectively. Steric hindrance means that the binding constant for 2-picoline is significantly lower than that for the other compounds.Using the method detailed above, we obtained the results given in Table 2. Once again the RR data gave values that corresponded well with literature values.
Discussion
The results of our RR investigation into the binding of pyridine to ZnTPP have elucidated values for the equilibrium binding constant, k, which match those of our own absorption experiments and previously published values. Whilst this is not an unexpected outcome in its own right, the method of calculation is noteworthy.It should be emphasised that the RR spectrum of ZnTPP has been used to quantify the amount of pyridine present in a solution. Moreover, there was no need to record the spectrum of pyridine at any point in the procedure. It is only the ZnTPP template which is monitored at all times.
This indirect quantification is made possible because the absorption spectra of the two ZnTPP species, the bound and unbound states, are significantly different. When the pyridine binds in the axial position, the entire spectrum (Soret and Q bands) is red-shifted. In particular, the Soret band maximum moves from 418 nm to 427 nm—towards the wavelength being use to excite the Raman scattering (441.6 nm). The direct effect of this is that the scattering from PyZnTPP will be significantly stronger than that from unbound ZnTPP.
Thus, as increasing amounts of pyridine are added, the PyZnTPP signal will begin to dominate the recorded spectrum. However, the formation of PyZnTPP cannot be monitored by the increasing intensity of any new bands as the main features of the Raman spectrum lie in the same positions. Moreover, direct monitoring of the increase in intensity of a particular band is not possible because the Raman signal naturally tends to fluctuate due to changes in the laser power output and small variations in focus. However, this may be overcome by using the solvent signal as an internal standard to normalise the data—any fluctuation in the porphyrin signal caused by instrument response will be mirrored in the solvent signal.
Simply dividing the intensity of a porphyrin band by the intensity of a solvent band proved an effective basis for calculating the equilibrium constant for the axial ligand binding process. The intensity could be used to solve eqn. (6) and hence to determine the equilibrium constant. The intensity of each of the five resonance enhanced porphyrin bands could be used in this way and yielded similar results.
Investigation of the three substituted pyridines by RR spectroscopy also gave log k values that matched our own uv-vis studies and the previously reported values, Table 2. This confirms that our method is not specific to the ZnTPP–pyridine system and may be applied to other ligands and substrates so long as the ligand binding induces a change in the absorption spectrum and hence to the degree of RR enhancement.
Porphyrin concentrations used here are ca. 5 × 10−5 M and the detection limits for pyridine, 3-, and 4-picoline were estimated to be ca. 4 × 10−5 M, and that for 2-picoline was ca. 1 × 10−3 M. The method described could reasonably be applied to any system in which ligand binding induces a change in the absorption spectrum. In the specific case of ZnTPP, the method is particularly suited for monitoring binding by nitrogenous Lewis bases, such as pyridine and substituted pyridines as well as amines and other related compounds.22
On the basis of these results, we propose to use a metalloporphyrin, such as ZnTPP, as a RR template to detect analyte molecules. The changes to the absorption spectrum apply equally to the solid state as to the solution state and so the binding of an axial ligand should still alter the degree of resonance enhancement.
Conclusion
In this paper we report the first use of the RR spectra of a template molecule (ZnTPP) to quantify an analyte molecule (pyridine). The thermodynamic information extracted from the RR data agreed with our own and previously published values elucidated by absorption spectroscopy. These results therefore provide the ‘proof of principle’ for the development of a miniature sensor for small analytes based on a metalloporphyrin RR template.Acknowledgements
We are grateful to the EPSRC for funding this work and for a studentship to APH.References
- C. Rodger, W. E. Smith, G. Dent and M. Edmondson, J. Chem. Soc., Dalton Trans., 1996, 5, 791 RSC.
- K. Kneipp, H. Kneipp, R. Manoharan, E. B. Hanlon, I. Itzkan, R. R. Dasari and M. S. Feld, Appl. Spectrosc., 1998, 52, 1493 Search PubMed.
- C. J. L. Constantino, T. Lemma, P. A. Antunes and R. Aroca, Anal. Chem., 2001, 73, 3674 CrossRef CAS.
- C. J. L. Constantino, T. Lemma, P. A. Antunes and R. Aroca, Spectrochim. Acta, Part A, 2002, 58, 403 Search PubMed.
- J. R. Miller and G. D. Dorough, J. Am. Chem. Soc., 1952, 74, 3977 CrossRef CAS.
- D. M. Collins and J. L. Hoard, J. Am. Chem. Soc., 1970, 92, 3761 CrossRef CAS.
- S. J. Cole, G. C. Curthoys, E. A. Magnusson and J. N. Phillips, Inorg. Chem., 1972, 11, 1024 CrossRef CAS.
- M.-S. Liao and S. Scheiner, J. Chem. Phys., 2002, 116, 3635 CrossRef CAS.
- K. M. Kadish and D. Schaeper, Chem. Commun., 1980, 1273 RSC.
- S. J. Cole, G. C. Curthoys and E. A. Magnusson, J. Am. Chem. Soc., 1970, 92, 2991 CrossRef CAS.
- G. C. Vogel and J. R. Stahlbush, Inorg. Chem., 1977, 16, 950 CrossRef CAS.
- M. Nappa and J. S. Valentine, J. Am. Chem. Soc., 1978, 100, 5075 CrossRef CAS.
- O. W. Kolling, Inorg. Chem., 1979, 18, 1175 CrossRef CAS.
- C. H. Kirksey, P. Hambright and C. B. Storm, Inorg. Chem., 1969, 8, 2141 CrossRef CAS.
- T. G. Spiro and P. Stein, Ann. Rev. Phys. Chem., 1977, 28, 501 Search PubMed.
- D. P. Strommen and K. Nakamoto, J. Chem. Educ., 1977, 54, 474 Search PubMed.
- R. J. H. Clark and T. J. Dines, Angew. Chem. Intl. Ed. Engl., 1986, 25, 131 CrossRef.
- L. M. Proniewicz, J. Golus, K. Nakamoto and J. R. Kincaid, J. Raman Spectrosc., 1995, 26, 27 CAS.
- K. A. Connors, Binding Constants, John Wiley and Sons, 1987 Search PubMed.
- T. G. Spiro, R. S. Czernuszewicz and X.-Y. Li, Coord. Chem. Rev., 1990, 100, 541 CrossRef CAS.
- K. H. Michaelian, K. E. Rieckhoff and E.-M. Voigt, J. Phys. Chem., 1977, 81, 1489 CrossRef CAS.
- R. M. Izatt, J. S. Bradshaw, K. Pawlak, R. L. Brening and B. J. Tarbet, Chem. Rev., 1992, 92, 1261 CrossRef CAS.
- L. M. Kadish, L. R. Shiue, R. K. Rhodes and L. A. Bottomley, Inorg. Chem., 1981, 20, 1274 CrossRef.
| This journal is © The Royal Society of Chemistry 2003 |