Development of a microparticle-based on-site immunoassay for the detection of atrazine in soil and water samples
Min
Li†
*a,
Robert S.
Wu
a,
Shiow-Fen
Tsai
a,
Steven M.
Rosen
a,
Joseph
DiCesare
b,
Jane S. C.
Tsai
a and
Salvatore J.
Salamone
a
aRoche Diagnostics Corporation, 9115 Hague Road, Indianapolis, IN 46250, USA
bThe Perkin-Elmer Corporation, 50 Danbury Road, Wilton, CT 06897-0208, USA
First published on 26th November 2002
Abstract
Atrazine is widely used as a herbicide in agriculture and has been identified as a major groundwater contaminant in the US. Because of the possible hazard associated with its usage, there is a need for an efficient and economic screening method for on-site field testing of atrazine and other s-triazine herbicides in soil and water. We have developed a rapid, on-site test for the detection of atrazine based on the principle of microparticle agglutination inhibition immunoassay. The test detects 50 µg kg−1 (0.050 ppm) atrazine in soil samples with direct extraction and 1.0 µg L−1 atrazine in water samples when coupled with solid phase extraction.
Introduction
Atrazine is the most widely used herbicide in corn and sorghum production for control of broadleaf and grassy weeds in the United States. It was first marketed to US farmers in 1959 and in 1991, 51 million pounds of active ingredient of atrazine were applied to 40 million corn acres (62% of the US crop).1 Despite the fact that its usage has been restricted since 1993, the widespread use and moderate persistence of this compound have led to the problem of soil and ground water contamination. Because of possible long term health risks such as cancer and cardiovascular damage,2 an efficient and economic screening method is needed to monitor atrazine and other s-triazine herbicides in soil and water. Immunoassays meet these requirements and over the past three decades they have been increasingly utilized in the field of environmental pollutant monitoring. The early formats of immunoassays are based on enzyme-linked immunosorbent assay (ELISA)3,4 or radioimmunoassay (RIA).5 These heterogeneous immunoassays require multiple-step sample preparation as well as instruments for result reading. Novel formats which are much easier and quicker to perform have been developed and marketed more recently. For example, several microparticle-based, homogeneous immunoassay platforms have been successfully applied in the on-site screening of drugs of abuse.6–8 These on-site immunoassays can be performed without extra sample preparation procedures and the results can be interpreted visually without the use of a reading instrument.We have developed a rapid on-site test for the detection of atrazine based on the ‘Abuscreen OnTrak’ format, one of the Roche on-site immunoassay formats for drugs of abuse testing.6–9 The ‘Abuscreen OnTrak Immunoassay’ is a ‘Microparticle Agglutination Inhibition Immunoassay’ (MAI-IA) that does not require the use of an instrument for result reading/interpretation. The MAI-IA format is based on the competition between analyte derivatized-latex microparticle conjugates and the free analytes in the specimen for binding to a limited amount of free antibody in solution. The analyte analog molecules are conjugated to a macromolecular carrier and then ‘labeled’ onto uniform latex microparticles through covalent coupling. The test is performed by pipetting a sample aliquot into a well on a test slide followed by addition of reagents (1 drop each) from 3 reagent dropper bottles. The resulting mixture is allowed to run through winding capillary tracks on the testing slide, during which time agglutination of the labeled microparticles would occur if the target analyte is not present or is below the preset cutoff value (negative result). On the other hand, a smooth milky appearance is indicative of a positive result. Like other Abuscreen OnTrak immunoassays for drug testing, this atrazine test is visualized by the occurrence of agglutination of atrazine-derivatized microparticles, effected by the addition of the anti-atrazine antibody. When free atrazine is present, agglutination is inhibited and the degree of inhibition correlates to the amount of free atrazine present in the sample tested, which competes for the same binding sites of the antibody as the atrazine analog on derivatized microparticles does. The test takes less than 5 min and has been configured to have a cutoff detection limit of 50 μg kg−1 atrazine in soil samples with direct extraction. When coupled with solid phase extraction with reversed phase cartridges, the cutoff detection limit is readily set at 1.0 μg L−1 atrazine in water samples. In this report, we describe the design of immunogen, production of antibody, synthesis of atrazine–protein conjugates, and performance of the test.
Materials and methods
General
All solvents were obtained from Fisher Scientific (Pittsburgh, PA). Bovine serum albumin (BSA) and keyhole limpet hemocyanin (KLH) were acquired from Sigma-Aldrich (St. Louis, MO) and Calbiochem (San Diego, CA), respectively. Trinitrobenzenesulfonic acid (TNBS) was obtained from Pierce Chemical Co. (Rockford, IL). Carboxyl modified microparticles were product of Seradyn (Indianapolis, IN). Coomassie protein assay reagent was purchased from Bio-Rad (Hercules, CA). Bovine alkaline phosphatase conjugated, affinity purified goat anti-rabbit IgG and alkaline phosphatase conjugated anti-sheep IgG were obtained from Zymed Laboratories, Inc. (San Francisco, CA). Weathered soil samples consisting of 65% clay and 35% sand were purchased from Environmental Resource Associates (Arvada, CO). All other chemicals and/or reagents were from Sigma-Aldrich (St. Louis, MO). ELISA data were recorded with a SLT multiple well reader. A RaPID Assays® unit and reagents were purchased from Ohmicron Environmental Diagnostics, (now Strategic Diagnostics Inc., Newark, Delaware).Chemical synthesis
Preparation of atrazine–KLH immunogen
Dimethylformamide (DMF, 5 mL) was added to a mixture of compound 1 (Fig. 1, 285 mg, 1.00 mmoL), NHS (N-hydroxysuccinimide, 127 mg, 1.10 mmoL), and DCC (Dicyclohexylcarbodiimide, 206 mg, 1.00 mmoL) and the resulting mixture was stirred under argon at room temperature for 4 h. After the reaction mixture was frozen at −10 °C overnight, the supernatant (1.25 mL) was added dropwise into a cold KLH solution (25 mL, 10 mg mL−1) in 50 mM, pH 7.5 phosphate buffer. The resulting solution was stirred at room temperature overnight. The reaction solution was transferred to a dialysis tubing with molecular weight cutoff of 2,000 and dialyzed extensively against pH 7.5, 50 mM phosphate buffer. The dialyzed solution was filtered through a sterile 0.2 μm filter unit.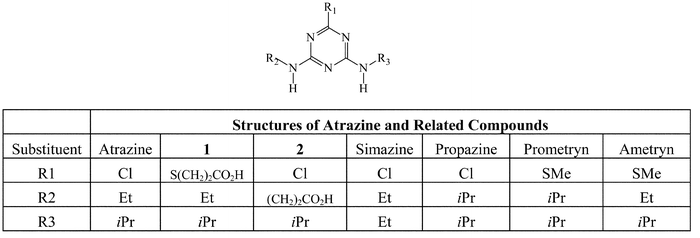 | ||
| Fig. 1 Structures of atrazine and related compounds. | ||
Preparation of atrazine–BSA conjugate I
The conjugate was made using the procedure similar to the preparation of atrazine–KLH immunogen, except that only two molar equivalents of the NHS ester of compound 1 were allowed to react with BSA in a mixture of pH 7.5, 50 mM phosphate buffer/DMF (2.5/1, v/v).Preparation of atrazine–BSA conjugate II
The conjugate was made using the procedure identical to the preparation of atrazine–BSA conjugate I, except that the NHS ester of compound 2 (Fig. 1) was used in the conjugation with BSA.Characterization of the immunogen and conjugates
The protein concentrations were determined by the Coomassie protein assay11 and BSA was used as the reference. For the immunogen, the degree of atrazine hapten substitution on carrier proteins was calculated based on the absorbance difference at 420 nm between the TNBS derivatized immunogen and TNBS derivatized native carrier protein.12,13 On the other hand, the substitution ratios of atrazine–BSA conjugates, which were used for further conjugation with microparticles, were estimated by using ELISA.Animal immunization
Four sheep and four rabbits were placed on an immunization program using a method adapted from the procedure of Erlanger.14 Each animal received multiple site injections across the back using 1 mg of the atrazine–KLH immunogen emulsified with Complete Freund′s Adjuvant. At the second week, the animals received booster immunizations containing 1 mg of the immunogen emulsified in Incomplete Freund′s Adjuvant. The boost injections were repeated twice in the following 2 weeks, followed by a monthly injection of 0.5 mg of the immunogen in Incomplete Freund′s adjuvant for a period of 6 months. The sheep and rabbits were then bled and antisera were separated from the clot by centrifugation. Screening of the antisera was done using ELISA.Enzyme-linked immunosorbant assay (ELISA) for evaluation of antiserum affinity to atrazine–BSA conjugates
Polystyrene microplates were coated with a conjugate (I or II) solution in phosphate-buffered saline (PBS)/0.01% azide at a concentration of 5.0 µg mL−1. The PBS/azide buffer was made by adding 250 mg of KH2PO4, 1.38 g of Na2HPO4, 250 mg of KCl, 9.0 g of NaCl, and 0.01% NaN3 into 1 L of water. The coating was conducted at either room temperature for 2 h or 4 °C overnight. The plates were then washed three times with PBS/0.1% Tween 20, followed by addition of 50 µL aliquots of a 1% BSA solution and 50 µL diluted solutions of an anti-atrazine antiserum (rabbit antiserum or sheep antiserum in PBS containing 1% BSA and 0.01% azide), respectively. The resulting plates were incubated at 37 °C for 2 h. After five washes with PBS/Tween 20, 50 µL aliquots of a diluted solution of corresponding bovine alkaline phosphatase–conjugated anti-rabbit IgG or anti-sheep IgG were added to the wells. The plates were incubated at 37 °C for 2 h, washed three times with PBS/Tween 20, and then 50 µL aliquots of a 4-nitrophenyl phosphate solution in pH 9.8 diethanolamine buffer were added. After incubation at 37 °C for about 30 min, the enzymatic reactions were stopped by the addition of 50 µL aliquots of a 3.0 M NaOH solution and the plates were read immediately at 405 nm.The ELISA assays were also performed in the presence of free atrazine as well as a number of structurely related s-triazines for evaluating their ability to inhibit antiserum binding to atrazine–BSA conjugates. In these cases, a 50 µL aliquot of a free atrazine (or one of the related s-triazines) solution in 1% BSA was employed in lieu of the 50 µL 1% BSA aliquot used in the previous section.
Direct extraction of soil samples
Part of the soil samples were spiked with atrazine at a concentration of 5.05 μg g−1 and the value was certified with GC-MS by Environmental Resource Associates (Arvada, CO). Soil samples with 25, 50, 100, 200 and 500 μg kg−1 of atrazine were prepared through dilution of the certified samples with unspiked soil. All soil samples (20 g sample size), including unspiked ones for use as controls, were extracted respectively with 20 mL of methanol–ethylene glycol (60/40, v/v, containing 4% NaCl and 2% PVP). Aliquots (∼11 μL) of the extraction solutions were directly applied onto the OnTrak plates respectively for assay; the recovery of the extraction was assessed with a RaPID Assays® system for atrazine manufactured by Ohmicron Environmental Diagnostics.Solid phase extraction of water samples
De-ionized water samples were spiked with known levels of atrazine with eight preparations at each level. A defined volume was withdrawn with a plastic syringe from each of the spiked samples and then pushed through a pre-wet C18 cartridge, respectively. The cartridge was immediately eluted with 1 mL of methanol–ethylene glycol (60/40, v/v, containing 4% NaCl and 2% PVP) and an aliquot of the eluant was measured by the Ohmicron RaPID Assays® system and the on-site OnTrak immunoassay, respectively.Development of the MAI-IA based on-site atrazine immunoassay
Latex microparticles (0.8 micron), prepared according to a previously published procedure,6 were covalently coated with a set of atrazine–BSA conjugate (I or II) and BSA mixtures,15 respectively, where the ratios of atrazine–BSA conjugate vs. BSA ranged from 1∶1, 0.5∶1, 0.25∶1, and 0.125∶1. Each batch of the microparticles coated at a specific atrazine–BSA conjugate/BSA ratio was then mixed with various dilutions (titers) of the anti-atrazine antibody and a reaction buffer (50 mM HEPES, pH 7.2) to effect the agglutination reaction. To perform the test, 11 µL of testing sample is dispensed into a mixing well of a capillary agglutinography slide followed by one drop (50 µL) each of antibody buffer, reaction buffer and sensitized microparticles solution. The added liquids are stirred for approximately 3 s and the solution is moved to allow contact with the capillary. The liquid moves through the capillary, fills the viewing area at the end of the capillary, and then appears as fine flocculates (agglutinated) or milky suspension. Each test result was visually inspected when the reaction is completed (within 5 min after all the reagents were mixed). The degree of agglutination is visually scored according to a set of ‘agglutination rating standard’ that correlates the various degrees of agglutination to a score range of 0 to 4 points in 0.5 increments. A score of 4 represents the highest degree of agglutination whereas a score of 0 represents complete inhibition of the agglutination reaction (smooth milky appearance with no visible sign of agglutination). The atrazine–BSA conjugate/BSA ratio and antibody titer were optimized so that the assay would score 3.5 or 4.0 points in the absence of free atrazine (negative result), and would score in the range of 0 to 1.5 points once the free atrazine concentration reached 50 μg L−1 or greater in the sample tested (positive result). With the assay parameters thus determined, a set of standard solutions corresponding to atrazine concentrations between 0 and 500 μg L−1 produced agglutination at various degrees that yielded scores between 4 and 0 points (Fig. 2).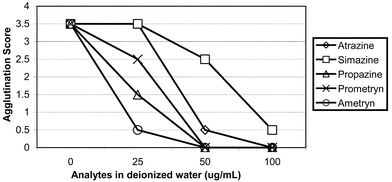 | ||
| Fig. 2 Evaluation of anti-atrazine antibody reaction to atrazine and structure-related herbicides by OnTrak Immunoassay. The optimized OnTrak Immunoassay was performed using rabbit anti-atrazine antibody and conjugate II-coated microparticles. | ||
Results and discussion
Compound 1 (Fig. 1) was chosen as the hapten for conjugation to the carrier protein KLH. The compound was converted to NHS ester by using DCC as the coupling reagent; the active ester was directly utilized in the conjugation with KLH to give the KLH–immunogen. The substitution ratio of the hapten on available Lys residues of KLH was determined to be 75%. After extensive dialysis and the subsequent filtration, the immunogen filtrate retained a KLH concentration of 3.90 mg mL−1 and the overall protein recovery was 70%.The immunogen thus prepared was given to four sheep and four rabbits for immunization. After six months, the animals were bled and the antisera were screened using ELISA. It was found that the antisera of rabbit displayed the highest affinity (binding) towards atrazine–BSA conjugates, and yet 50% of the affinity could be inhibited by 7 ng of free atrazine. The latter property is important for achieving desirable sensitivity in the final assay format. The selected rabbit antisera also showed reasonable cross-reactivity towards several related s-triazine herbicides, among which simazine was found to be less cross-reactive than others (Table 1). The cross-reactivity among these s-triazines was later confirmed in the OnTrak immunoassay using conjugate II-coated microparticles and the rabbit antisera (Fig. 2). The optimized OnTrak Atrazine assay (cutoff 50 µg L−1) can also be used for detecting propazine (showing a cutoff between 25 and 50 µg L−1), ametryn (cutoff 25 µg L−1), prometryn (cutoff between 25 and 50 µg L−1), and simazine (cutoff 100 µg L−1). The assay performance observed between the two immunoassay formats for detecting the atrazine structurally related compounds is generally compatible even though ELISA is a heterogeneous immunoassay and allows longer incubation time for the assay to reach equilibrium. The cross-reactivity of the anti-atrazine antisera to other s-atrazines appears to be a quite general phenomenon that has been seen with both monoclonal and polyclonal antibodies.16–20 On the other hand, the antisera of sheep were found to have poorer cross-reactivity to propazine in the ELISA assay (Table 1). Since a reasonable cross-reactivity towards herbicides of similar structures is essential for an on-site screening method, it was decided to use the rabbit antisera in the assay as the antibody reagent.
| Amount (ng) of the herbicide needed to inhibit 50% of Bmaxa | |||||
|---|---|---|---|---|---|
| Reagents | Atrazine | Simazine | Propazine | Prometryn | Ametryn |
| a Bmax is the maximum antiserum binding to atrazine–BSA conjugates. | |||||
| Atrazine derivative I and rabbit anti-atrazine | 30 | 185 | 15 | 24 | 15 |
| Atrazine derivative II and rabbit anti-atrazine | 7 | 62.5 | 15 | 15 | 15 |
| Atrazine derivative II and sheep anti-atrazine | 15 | 7 | 500 | 15 | 15 |
Another key reagent of the on-site atrazine immunoassay is the atrazine-derivatized microparticles. In order to prepare this reagent, the atrazine label had to be attached to a macromolecular spacer15,21 such as IgG or BSA with a desirable molar ratio of atrazine to the spacer at approximately 1∶1. The atrazine–spacer conjugate was then coupled to the carboxylated microparticles. In this case, we chose BSA as the spacer and the two atrazine–BSA conjugates (I and II) were evaluated in the preparation of the microparticle reagent. Atrazine–BSA conjugates I and II were prepared, respectively, from the conjugation of the corresponding NHS esters with BSA in a 2 to 1 molar ratio. It was estimated by ELISA that each mole of BSA contains ∼1.2 moles of atrazine label in the conjugates thus formed. As opposed to the case of the immunogen where high atrazine substitution ratio was achieved, the indirect TNBS assay is not accurate enough to measure the very low atrazine substitution ratio of the atrazine–BSA conjugates. Both conjugates were then coupled to microparticles to produce two reagents containing different linkers. Conjugate II has a somewhat different hapten structure (compound 2, Fig. 1) and it was hoped that the derivatized microparticles made from this conjugate might be more easily displaced from the antibody binding site by the free atrazine molecules. The antibody used in this assay was raised against the antigen made from the immunogen that has the same hapten as in conjugate I and would therefore be expected to bind less well to conjugate II. Having such an ability to tune the degree of displacement of the derivatized microparticles is important for performance adjustment of the assay, since the degree of the displacement is directly related to the sensitivity of the assay. In the process of the assay development, it was observed, as anticipated, that the microparticle reagent derivatized with atrazine–BSA conjugate II demonstrated greater sensitivity towards free atrazine than did the reagent made from conjugate I. Thus, the former microparticle reagent (derivatized with conjugate II) was adopted in the OnTrak assay.
With both the antibody and microparticle reagents available, a test program with 45 soil samples spiked with varying amounts of atrazine was started along with 9 blank control samples. The recovery of each extraction was quantitated with Ohmicron’s RaPID Assays® for atrazine, a commercial instrument-based atrazine screening apparatus based on magnetic beads-based immunoassay, and the averaged recovery at all levels (25 through 500 µg kg−1, Table 2) was found to be slightly above 100%. Aliquots of the extraction were then applied onto OnTrak assay plates and corresponding scores were assigned according to the degree of individual agglutination on each plate. The blank control and 25 µg kg−1 samples, which were below the pre-set cutoff level of 50 µg kg−1, showed negative results with OnTrak assay (3.5 points, Table 2), while all 36 spiked samples with 50 µg kg−1 or higher atrazine displayed positive results (0.5 point or lower).
| Atrazine level/µg kg−1 | Dilution factor | Average recovery/µg L−1 | SD/±µg L−1 | % Recovery | OnTrak result (score) |
|---|---|---|---|---|---|
| a A 20 g soil sample spiked with the amount specified in the table was extracted with 20 mL methanol/ethylene glycol (60/40, v/v, containing 4% NaCl and 2% PVP) in each case. The extract was filtered and an aliquot was then diluted with water according to the corresponding dilution factor. The diluted solution was quantitated by the Ohmicron’s atrazine assay kit. Aliquots (11 µL) of the original, undiluted extract were visually estimated by the OnTrak atrazine immunoassay. A score was assigned based on the degree of the agglutination observed. | |||||
| 0 | 10 | 1.61 | 0.3 | — | Negative (3.5) |
| 25.0 | 10 | 25.8 | 1.32 | 103 | Negative (3.5) |
| 50.0 | 20 | 51.8 | 2.96 | 104 | Positive (0.5) |
| 100 | 40 | 101.9 | 3.62 | 102 | Positive (0) |
| 200 | 100 | 219.6 | 10.8 | 110 | Positive (0) |
| 500 | 200 | 631.6 | 57.3 | 113 | Positive (0) |
To test the on-site atrazine immunoassay’s ability to detect atrazine in water samples at concentrations around EPA’s maximum allowed level (3 µg L−1), 40 water samples were spiked with a certified concentrated atrazine solution at level 0.25, 1.00, 2.50, and 5.00 µg L−1. A defined volume (50 mL) of each spiked solution was pushed through a pre-conditioned C-18 cartridge via a plastic syringe. The cartridge was immediately eluted with a mixture of methanol/ethylene glycol and aliquots of the eluant were then assayed with the RaPid Assay® and the OnTrak microparticle atrazine immunoassay, respectively. The recovery of the extraction ranged from 91 to 99% (Table 3). At or above the pre-determined cutoff level of 1.00 µg L−1 atrazine in water, the on-site assay gave positive results for all 24 spiked samples. On the other hand, the 8 samples that were spiked with 0.25 µg L−1 atrazine showed negative results. Further studies indicated that the detection limit in water sample could be readily lowered to 0.1 µg L−1, provided that 10 times more volume of water be run through a single C-18 cartridge.
| Atrazine level /µg L−1 | Atrazine (ng) in 50 mL water | Average recoverya/µg L−1 | SDa/±µg L−1 | % Recovery | OnTrak result (score) |
|---|---|---|---|---|---|
| a Average of eight replicate runs at each level. Atrazine was spiked into de-ionized water samples. Fifty milliliters of water was withdrawn with a plastic syringe from each of the spiked samples and then pushed through a pre-wet C18 cartridge, respectively. The cartridge was immediately eluted with 1 mL of methanol/ethylene glycol (60/40, v/v, containing 4% NaCl and 2% PVP) and an aliquot of the eluant was measured by the Ohmicron RaPID Assays® system and the microparticle OnTrak atrazine immunoassay, respectively. | |||||
| 0.25 | 12.5 | 12.4 | 0.4 | 99 | Negative (3.5) |
| 1.00 | 50.0 | 45.5 | 2.0 | 91 | Positive (0) |
| 2.50 | 125 | 121 | 8.1 | 97 | Positive (0) |
| 5.00 | 250 | 230 | 8.9 | 92 | Positive (0) |
Matrix effects on the solid phase extraction and assay procedure were also studied for some commonly occurring salts (Table 4). At the cutoff of 1.00 µg L−1 level, very high levels of Na2SO4 and NaCl appeared to have some negative impact on the extraction recoveries (77% and 78%, respectively). Nevertheless, the assay still displayed positive results for all 16 samples at these levels. At 250 µg L−1 level, all salts under examination had virtually no effect on extraction as well as final OnTrak atrazine immunoassay results. Finally, the specificity of the OnTrak atrazine immunoassay was established by the negative results obtained with soil and water samples that were spiked respectively with pentachlorophenol, benzene, toluene, or xylenes at the cutoff levels.
| Matrix (level/µg mL−1) | Atrazine (ng) in 50 mL water | Average recoveryb/µg L−1 | SDb/±µg L−1 | % Recovery | OnTrak result (score) |
|---|---|---|---|---|---|
| a Spiked water level was fixed at 1.00 µg L−1 for all the samples in this study. b Average of eight replicate runs obtained with Ohmicron RaPid Assay®. c Positive results are those with scores of 1.5 or below. | |||||
| Water | 50.0 | 45.5 | 2.0 | 91 | Positivec |
| Na2SO4 (1.0 × 104) | 50.0 | 38.7 | 2.0 | 77 | Positive |
| NaNO3 (250) | 50.0 | 48.3 | 2.1 | 97 | Positive |
| NaCl (5.9 × 104) | 50.0 | 39.2 | 1.4 | 78 | Positive |
| CuSO4 (250) | 50.0 | 46.5 | 2.2 | 93 | Positive |
| CaCl2 (250) | 50.0 | 44.9 | 2.5 | 90 | Positive |
| FeCl3 (250) | 50.0 | 58.0 | 4.3 | 116 | Positive |
| MgSO4 (250) | 50.0 | 43.3 | 3.6 | 87 | Positive |
Conclusion
In summary, we have prepared an immunogen which produced antibody with satisfactory affinity toward atrazine and atrazine–BSA conjugate II and applied the antibody and conjugate in the development of a microparticle-based, on-site immunoassay for atrazine. The microparticle reagent was made from the coupling of atrazine–BSA conjugate II to carboxylated uniform latex microparticles. Agglutination of the derivatized microparticles was effected by the addition of the antibody reagent; inhibition of this process occurred when free atrazine molecules were present in a sample being tested. The degree of the microparticle agglutination inhibition was proportional to the amount of free atrazine and the whole event is easily visualized in less than 5 min, giving rise to a fast and economic on-site atrazine test for soil and water samples.References
- US Environmental Protection Agency Nonpoint Source News—Notes, November–December 1992, Issue No. 25.
- J. T. Stevens and D. D. Sumner, in Handbook of Pesticide Toxicology, ed. W. J. Hayes, Jr and E. R. Laws, Jr, Academic Press, New York, NY, 1991 Search PubMed.
- C. Wittmann and B. Hock, Vom Wasser, 1990, 75, 115–126 Search PubMed.
- M. G. Weller, L. Weil and R. Niessner, Mikrochim. Acta, 1992, 108, 29–40 CAS.
- M. Vanderlaan, B. E. Watkins and L. Stanker, Environ. Sci. Technol., 1988, 22, 247–254 CAS.
- S. J. Salamone and S. Vitone, US Pat. 5,618,926, 1997.
- D. J. Crouch, M. L. Cheever, D. M. Andrenyak, D. J. Kuntz and D. L. Loughmiller, J. Forensic Sci., 1998, 43, 35–40 Search PubMed.
- D. J. Crouch, J. F. Frank, L. J. Farrell, H. M. Karsch and J. E. Klaunig, J. Anal. Toxicol., 1998, 22, 493–502 CAS.
- J. Towt, S.-C. J. Tsai, M. R. Hernandez, A. D. Klimov, C. V. Kravec, S. L. Rouse, H. S. Subuhi, B. Twarowska and S. J. Salamone, J. Anal. Toxicol., 1995, 19, 504–510 CAS.
- M. H. Goodrow, R. O. Harrison and B. D. Hammock, J. Agric. Food Chem., 1990, 38, 990 CrossRef CAS.
- M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- A. R. Goldfarb, Biochemistry, 1966, 5, 2570–2574 CrossRef CAS.
- S. L. Snyder and P. Z. Sobocinski, Anal. Biochem., 1975, 64, 284–288 CrossRef CAS.
- B. F. Erlanger, Methods Enzymol., 1980, 70, 85–104 Search PubMed.
- R. S. Wu, A. J. McNally, I. A. Pilcher and S. J. Salamone, Bioconjugate Chem., 1997, 8, 385–390 CrossRef CAS.
- C. Wittmann and B. Hock, Food Agric. Immunol., 1989, 1, 211–224.
- S. Wust and B. Hock, Anal. Lett., 1992, 25, 1025–1037.
- B. Dunbar, B. Riggle and G. Niswender, J. Agric. Food Chem., 1990, 38, 433–437 CrossRef CAS.
- T. Giersch, J. Agric. Food Chem., 1993, 41, 1006–1011 CrossRef CAS.
- J. M. Schlaeppi, W. Fory and K. Ramsteiner, J. Agric. Food Chem., 1989, 37, 1532–1538 CrossRef CAS.
- M. Li, S.-F. Tsai, S. M. Rosen, R. S. Wu, K. B. Reddy, J. DiCesare and S. J. Salamone, J. Agric. Food Chem., 2001, 49, 1287–1292 CrossRef CAS.
Footnote |
| † Current address: 88 Becks Blvd., Ringoes, NJ 08551, USA. |
| This journal is © The Royal Society of Chemistry 2003 |
