Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension
Joanne T. Brindle*a, Jeremy K. Nicholsona, Peter M. Schofieldb, David J. Graingerc and Elaine Holmesa
aBiological Chemistry, Biomedical Sciences Division, Sir Alexander Fleming Building, Faculty of Medicine, Imperial College of Science Technology and Medicine, Exhibition Road, South Kensington, London, UK SW7 2AZ. E-mail: j.ashfield@ic.ac.uk; Fax: 020 75943226; Tel: 020 75943145
bDepartment of Cardiology, Papworth Hospital NHS Trust, Cambridge, UK CB3 8RE
cDepartment of Medicine, University of Cambridge, Box 157, Addenbrooke’s Hospital, Cambridge, UK CB2 2QQ
First published on 17th December 2002
Abstract
The application of chemometric methods to 1H NMR spectroscopic data has been documented for pathophysiological processes. In this study we show the application of 1H NMR-based metabonomics to investigate a relationship between serum metabolic profiles and hypertension. Although hypertension can be defined using blood pressure measurements, the underlying aetiology and metabolic effects are not so readily identified. Serum profiles for patients with low/normal systolic blood pressure (SBP ≤ 130 mm Hg; n = 28), borderline SBP (131–149 mm Hg; n = 19) and high SBP (≥ 150 mm Hg; n = 17) were acquired using 1H NMR spectroscopy. Orthogonal signal correction followed by principal components analysis were applied to these NMR data in order to facilitate interpretation, and the resulting chemometric models were validated using Soft Independent Modelling of Class Analogy. Using 1H NMR-based metabonomics, it was possible to distinguish low/normal SBP serum samples from borderline and high SBP samples. Borderline and high SBP samples, however, were indiscriminate from each other. Our preliminary results showed that there was a relationship between serum metabolic profiles and blood pressure which, in part, was due to lipoprotein particle composition differences between the samples. Furthermore, our results indicated that serum pathology associated with blood pressure is apparent at SBP values > 130 mm Hg, which the WHO and ISH currently define as the limit between normal and high-normal.
Introduction
Hypertension, or high blood pressure is one of the most prevalent clinically significant abnormalities in the Western world, often occurring with no physical symptoms. It is, however, a major health problem and can lead to an increased risk of atherosclerosis, as well as diabetes mellitus, haemorrhagic stroke and kidney failure.1–4 In a minority of cases, a single well-defined cause of hypertension can be identified, for example Conn’s syndrome, which is characterised by excessive production of aldosterone. In the majority of cases, however, the root cause of hypertension remains unknown and the disorder is classified as ‘essential hypertension’; the symptoms can be treated but not the underlying cause.Routinely blood pressure is measured using a sphyngomanometer, a relatively inexpensive and non-invasive procedure, to identify hypertension in individuals. Several epidemiological and pharmacological studies have reported definitions for high blood pressure and the data indicate that, for all stages of hypertension, the treatment should aim to reduce systolic blood pressure to below 150 mm Hg and the diastolic blood pressure to below 90 mm Hg.5 New guidelines for the management of hypertension have been published in 1999 by the World Health Organisation (WHO) and the International Society of Hypertension (ISH). These are based on the definition and classification of hypertension provided by the JNC VI (1997),6 and are detailed in Table 1. The new classification defines a blood pressure of 120/80 mm Hg as optimal and 130/85 mm Hg as the limit between normal and high-normal.
| Pressure | Systolic blood pressure/mm Hg | Diastolic blood pressure/mm Hg |
|---|---|---|
| Optimal | 120 | 80 |
| Normal | <130 | <85 |
| High-normal | 130–139 | 85–89 |
| Stage 1 hypertension | 140–159 | 90–99 |
| Stage 2 hypertension | 160–179 | 100–109 |
| Stage 3 hypertension | >180 | >110 |
Although hypertension can be easily defined using blood pressure measurements, the underlying aetiology and/or correlated metabolic effects are not so readily identified from a single blood pressure measurement. For example, it has been reported that there is an interrelation between serum lipids and blood pressure;7 Zavaroni et al. have reported significantly raised systolic and diastolic pressures in subjects who had increased levels of triglycerides and decreased levels of HDL-cholesterol.8 The aim of our study was to extend this concept to determine whether there is a relationship between the metabolic profile of serum and blood pressure which is independent of the extent of coronary heart disease. One method that can be used to obtain metabolic profiles of information-rich biofluids, without requiring pre-selection of measurable analytes, is 1H NMR spectroscopy.9 In order to reduce the complexity of biofluid NMR data and facilitate analysis, automatic data-reduction followed by chemometric methods, for example, principal components analysis (PCA) and partial least squares-discriminant analysis (PLS-DA), can be applied. An efficient NMR-based metabonomic approach to understanding pathophysiological processes has been developed and is well documented in the literature.10–12 To further optimise the metabonomic approach, data filtering can be applied prior to chemometric analysis. Orthogonal signal correction (OSC) is one such filtering method that serves to remove variation within the NMR data that is not correlated to the focus of the study.13 Data-filtering is particularly pertinent to human metabonomic studies as there is immense variability in human populations compared to laboratory-controlled animal studies.
In this report, we have used 1H NMR spectroscopy to obtain a metabolic profile of serum taken from human subjects with low/normal systolic blood pressure (SBP ≤ 130 mm Hg), borderline SBP (131–149 mm Hg) and high SBP (≥150 mm Hg). SBP was chosen over diastolic blood pressure (DBP) as the prime determinant of the hypertensive state.5 OSC was applied to the NMR data to focus the study on the metabolic effects relating only to blood pressure. Principal components analysis (PCA) was then performed on these NMR data in order to investigate whether there was a relationship between blood pressure and serum metabolic profiles, and to determine whether the relationship concurred with the current WHO/ISH definition of hypertension. The chemometric models constructed for the three SBP groups were validated using soft independent modelling of class analogy (SIMCA).
Methods
Sample collection
Blood was drawn from consecutive patients as they attended Papworth Hospital (Cambridgeshire, UK). Samples were allowed to clot in plastic tubes for 2 h at room temperature, and the serum was collected by centrifugation. Aliquots of serum were stored at −80 °C until assayed. Single blood pressure values were determined for each subject using a manual sphyngomanometer and were classified as low/normal SBP (≤ 130 mm Hg; n = 12 female, n = 16 male); borderline SBP (131–149 mm Hg; n = 5 female, n = 14 male); and high SBP (≥ 150 mm Hg; n = 7 female, n = 10 male).1H NMR Spectroscopy
Prior to NMR analysis, serum samples (150 μl) were diluted (10% 2H2O v/v, 0.9% NaCl w/v (350 μl)) and placed in 5 mm high quality NMR tubes (Goss Scientific Instruments Ltd.). Conventional 1H NMR spectra of the serum samples were measured at 600.22 MHz on a Bruker Avance-600 spectrometer, using the following pulse sequence: RD–90°–t1–90°–tm–90°–acquire free induction decay (FID). RD represents a relaxation delay of 1.5 s during which the water resonance was selectively irradiated, and t1 corresponds to a fixed interval of 4 μs. The water resonance was irradiated for a second time during the mixing time (tm, 150 ms). For each sample, 64 FIDs were collected into 32K data points using a spectral width of 8389.3 Hz, and an acquisition time of 1.95 s. The FIDs were multiplied by an exponential weighting function corresponding to a line broadening of 0.3 Hz prior to Fourier transformation. The acquired NMR spectra were corrected for phase and baseline distortions using XWINNMR (version 2.1, Bruker Gmbh, Germany), and referenced to lactate (CH3 δ 1.33).Data-reduction of NMR data
The 1H NMR spectra (δ 10–0.2) were automatically data-reduced to 245 integral segments of equal length (δ 0.04) using AMIX (Analysis of MIXtures software package, version 2.5, Bruker, Germany). Each segment consisted of the integral of the NMR region to which it was associated. In order to remove the effects of variation in the suppression of the water resonance, and also the effects of variation in the urea signal caused by partial cross solvent saturation via solvent exchanging protons, the region δ 6.0 to 4.5 was set to zero integral. The data were normalised to total spectral area in Excel (Microscoft, USA) and then exported to the SIMCA-P software package (version 8.0 Umetrics, Sweden). All subsequent analysis was performed after centring the variables to zero.Principal components analysis (PCA)
Principal components analysis (PCA) is a bilinear decomposition method used for overviewing ‘clusters’ within multivariate data. The NMR data (X) were represented in K-dimensional space (where K is equal to the number of chemical shift regions) and reduced to a few principal components (or latent variables) which described the maximum variation within the data, independent of any knowledge of class membership (i.e. ‘unsupervised’). The principal components were displayed as a set of ‘scores’ (t) that highlighted clustering or presence of outliers, and a set of ‘loadings’ (p) that described the influence of input variables on t.Orthogonal signal correction (OSC)
PCA was performed on 1H NMR data of human serum after application of orthogonal signal correction (OSC). The class identity (systolic blood pressure) was used as a response vector, Y, to describe the variation between the sample classes (different blood pressure groups). The OSC method then located the longest vector describing the variation between the samples which was not correlated with the Y vector, and removed it from the data matrix. The resultant dataset had thus been filtered to allow pattern recognition focused on the variation correlated to features of interest within the sample population, rather than non-correlated, orthogonal variation.Validation using soft independent modelling of class analogy (SIMCA)
To ensure that the NMR data were not over-fitted as a result of OSC, the data were validated using Soft Independent Modelling of Class Analogy (SIMCA). Separate PCA models were built for the low/normal blood pressure samples, the borderline blood pressure samples and the high blood pressure samples. SIMCA was then applied to the low/normal SBP model and the classification performance was assessed by predicting class membership, in terms of distance from the model, of the borderline and high SBP samples. The procedure was repeated for the borderline SBP model and the high SBP model in turn, to classify samples not represented in each of these models. The critical distance from the model used corresponded to a 0.05 level, and defined a 95% tolerance interval.Results and discussion
The 600 MHz 1H NMR spectra of human sera from patients with low/normal SBP (≤130 mm Hg), borderline SBP (131–149 mm Hg) and high SBP (≥150 mm Hg) were visually compared (Fig. 1a–c). The profiles of the NMR spectra had many similar characteristic peaks reflecting the tight homeostatic control of serum in general. On close visual inspection, however, differences were apparent between the NMR spectra from different blood pressure groups, notably around δ 0.8, 1.6, 2.1, 2.2, 2.4 and 3.2 (Fig. 1a–c). Overall, there were more similarities between the serum spectral profiles of the borderline SBP samples and the profiles of the high SBP samples. The chemical components contributing to the major peaks shown in Fig. 1 were assigned to the spectra on the basis of previously published data.14–16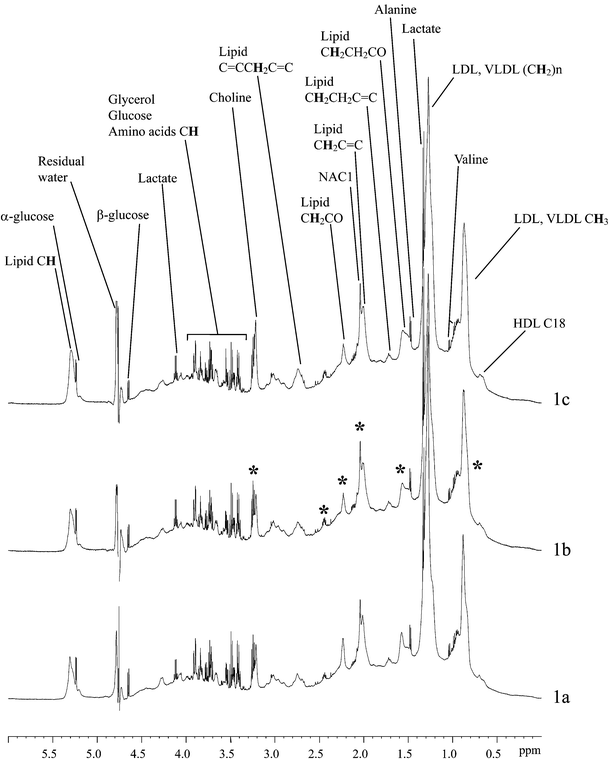 | ||
| Fig. 1 600 MHz 1H NMR spectra of serum samples from typical subjects with low/normal SBP (a), borderline SBP (b) and high SBP (c). * regions of variation between the spectra. | ||
To determine whether it was possible to distinguish between samples of different systolic blood pressure values based on their NMR spectra, and hence define a relationship between serum profile and blood pressure, PCA was performed on OSC-filtered NMR data (Fig. 2a–c). PCA indicated that the serum profiles of samples obtained from patients with low/normal SBP were clearly different from the profiles obtained from patients with borderline or high SBP, as demonstrated by the clustering observed in the PCA scores, t[1] (Fig. 2a and b). In contrast, serum profiles from borderline or high SBP samples were very similar; no separation based on blood pressure was observed in the PCA scores (Fig. 2c). These results supported the observations made via visual inspection of the NMR data; however, the application of OSC and PCA led to a reduction in data complexity and, therefore, facilitated interpretation. To ensure the NMR data were not over-fitted, the OSC-filtered PCA models were validated using SIMCA, the results are shown in Table 2. Overall, the results confirm and validate those obtained from PCA. Both borderline and high SBP models were able to classify low/normal SBP samples as being non-borderline and non-high SBP, respectively. Borderline and high SBP models, however, were not able to discriminate each other, demonstrating the similarity between the borderline and high SBP serum metabolic profiles.
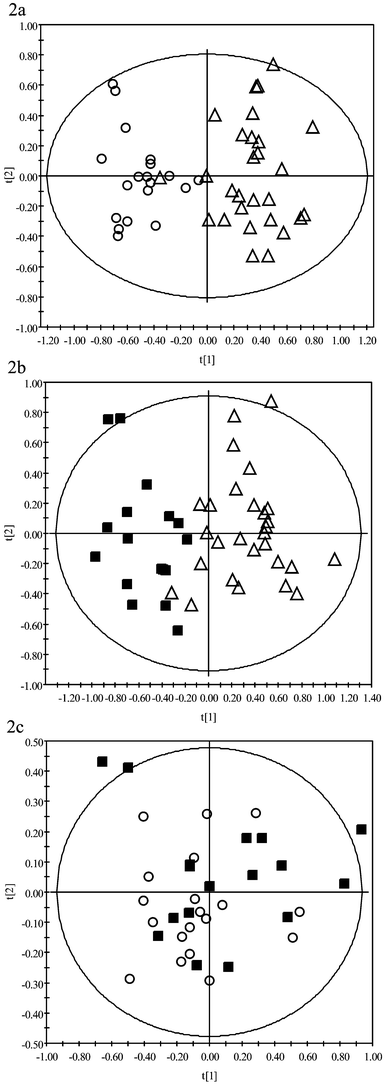 | ||
| Fig. 2 PCA scores comparing 1H NMR spectroscopic data of serum taken from subjects with low SBP (open triangles), borderline SBP (open circles) and high SBP (black squares). OSC had been applied to the NMR data prior to PCA in order to remove non-correlated variance components. | ||
| Blood pressure PCA model | Percentage of correct classifications | ||
|---|---|---|---|
| Low SBP samples | Borderline SBP samples | High SBP samples | |
| Low SBP model | (≥86%) | 47% | 53% |
| Borderline SBP model | 86% | (≥89%) | 35% |
| High SBP model | 82% | 16% | (≥88%) |
In addition to blood pressure measurements, the coronary artery status of the subjects in this study had been determined by coronary angiography as previously described.17 Although hypertension is a known risk factor for coronary heart disease (CAD), there was no association between high blood pressure and angiographic CAD in our cohort. Of the individuals above the 50th percentile for CAD severity, 23% had high SBP, 43% had borderline SBP with the remainder having low/normal SBP. This was compared with 31% and 44% in the individuals below the 50th percentile for CAD severity (p = 0.6177, Chi squared test). We can, therefore, conclude that potential covariation between SBP and CAD severity cannot account for the classification of the individuals on the basis of blood pressure reported here.
Initial investigation of the PCA loadings that corresponded to the scores for the three SBP groups suggested that lipid moieties, listed in Table 3, were, in part, responsible for causing the observed separation of low/normal SBP samples from borderline and high SBP samples (Table 3). Whilst there may be a relationship between serum lipids and blood pressure, as indicated by the PCA loadings, we suggest that it is lipoprotein particle composition exposed in the NMR profile, for example degree of fatty acid side chain unsaturation and lipoprotein-protein molecular interactions, that is important in discriminating between different SBP groups, rather than absolute lipid concentrations. Lipid parameters (HDL-cholesterol, LDL-cholesterol and triglycerides) were measured for each sample in our cohort using traditional clinical chemistry methods. There was found to be no significant differences in these parameters between each SBP group (ANOVA single factor analysis, 99% confidence interval).
| Bucket region (δ) | Assignment | Chemical shift (δ) and multiplicity |
|---|---|---|
| 0.86 | Lipid: | |
| LDL CH3(CH2)n | 0.84 (t) | |
VLDL CH3CH2CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) | 0.87 (t) | |
| 0.90 | Cholesterol C21 | 0.91 |
| 1.22 | Lipid CH3CH2CH2 | 1.22 (m) |
| 1.26 | Lipid, mainly LDL | 1.26 (m) |
| CH3CH2(CH2)n | 1.25 (m) | |
| 1.30 | Lipid, mainly VLDL | 1.29 (m) |
| (CH2)n | ||
| 1.34 | Lipid CH2CH2CH2CO | 1.32 (m) |
| 3.22 | Choline N(CH3)3+ | 3.21 (s) |
Our results have shown the existence of a relationship between systolic blood pressure and the serum metabolic profile of an individual. In view of the current definition and guidelines for the management of hypertension, our results suggest that a target of 150 mm Hg for maximum systolic blood pressure, at all stages of hypertension, is high. We have illustrated that the serum profile at a SBP > 130 mm Hg (defined as the limit between normal and high-normal by WHO/ISH) is indiscriminate from the serum profile at a SBP ≥ 150 mm Hg, characterised by NMR spectroscopic-based metabonomic analysis. Our findings suggest pathological changes in serum, that are related to blood pressure, are evident in the NMR serum profile before the SBP reaches values currently defined as hypertensive. A full analysis of our data is the subject of ongoing work, but these preliminary findings are in agreement with published data that reports an interrelationship between serum lipids and blood pressure, for example the Tromso Study and the Framingham Study.18,19 Furthermore, previous studies have also reported lipid abnormalities may be present in early and borderline hypertension20,21 as we too have demonstrated.
Acknowledgements
This work was supported by Metabometrix Ltd.References
- E. Escobar, J. Hum. Hypertens., 2002, 16, S61 CrossRef.
- F. Contreras, M. Rivera, J. Vasquez, M. A. De la Parte and M. Velasco, J. Hum. Hypertens., 2000, 14, S26 CrossRef.
- S. Tuhrim, Curr. Cardiol. Rep., 2002, 4, 158 Search PubMed.
- M. S. Parmar, Br. Med. J., 2002, 325, 85 Search PubMed.
- W. C. Cushman, Am. J. Hypertens., 2001, 14, 226S CrossRef CAS.
- The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The Sixth Report National High Blood Pressure Education Programme, National, Lung, and Blood Institute, National Institutes of Health. Arch. Intern. Med., 1997, 157, 2413.
- G. K. Goode, J. P. Miller and A. M. Heagerty, The Lancet, 1995, 11, 362 Search PubMed.
- I. Zavaroni, E. Bonora, M. Pagliara, E. Dallaglio, L. Luchetti, G. Buonanno, P. A. Bonati, M. Bergonzani, L. Gnudi, M. Passeri and G. Reaven, New Engl. J. Med., 1989, 320, 702 Search PubMed.
- J. K. Nicholson and I. D. Wilson, Prog. Nucl. Magn. Reson. Spectrosc., 1989, 21, 449 CrossRef CAS.
- J. K. Nicholson, J. C. Lindon and E. Holmes, Xenobiotica, 1999, 29, 1181 CrossRef CAS.
- J. C. Lindon, E. Holmes and J. K. Nicholson, Prog. Nucl. Magn. Reson. Spectrosc., 2001, 39, 1 CrossRef CAS.
- J. K. Nicholson, J. C. Connelly and E. Holmes, Nature Reviews, 2002, 1, 154 Search PubMed.
- B. M. Beckwith-Hall, J. T. Brindle, R. H. Barton, M. Coen, E. Holmes, J. K. Nicholson and H. Antti, Analyst, 2002, 127, 1283 RSC.
- J. K. Nicholson, P. J. D. Foxall, M. Spraul, D. R. Farrant and J. C. Lindon, Anal. Chem., 1995, 67, 793 CrossRef CAS.
- M. Ala-Korpela, Prog. Nucl. Magn. Reson. Spectrosc., 1995, 27, 475 CrossRef CAS.
- M. Lui, J. K. Nicholson, J. A. Parkinson and J. C. Lindon, Anal. Chem., 1997, 69, 1504 CrossRef.
- D. J. Grainger, P. R. Kemp, J. C. Metcalfe, A. C. Liu, R. M. Lawn, N. R. Williams, A. A. Grace, P. M. Schofield and A. Chauhan, Nature Medicine, 1995, 1, 74 Search PubMed.
- K. H. Bonnaa and D. S. Thelle, Circulation, 1991, 83, 1305 Search PubMed.
- W. P. Castelli and K. Anderson, Am. J. Med., 1986, 80, 23 CrossRef CAS.
- C. Lemne, A. Hamsten, F. Karpe, P. Nilssonehle and U. Defaire, Hypertension, 1994, 24, 605 Search PubMed.
- B. M. Pannier, M. S. Cambillau, V. Vellaud, V. Atger, N. Moatti and M. E. Safar, Clin. Invest. Med., 1994, 17, 42 Search PubMed.
| This journal is © The Royal Society of Chemistry 2003 |
