Fourier-transform Raman characterization of brazilwood trees and substitutes
Howell G. M.
Edwards
*a,
Luiz F. C.
de Oliveira
ab and
Mark
Nesbitt
c
aDepartment of Chemical and Forensic Sciences, University of Bradford, Bradford, UK BD7 1DP. E-mail: h.g.m.edwards@bradford.ac.uk
bNúcleo de Espectroscopia e Estrutura Molecular, Departamento de Química, Instituto de Ciências Exatas, Universidade Federal de Juiz de Fora, Juiz de Fora, MG, 36036-330, Brazil
cCentre for Economic Botany, Royal Botanic Gardens, Kew, Richmond, Surrey, UK TW9 3AE
First published on 25th November 2002
Abstract
In this work we have applied Fourier-transform Raman spectroscopy to the analysis of several archival samples of brazilwoods from different geographical origins and of different ages. Samples of Caesalpinia echinata Lam. (from Brazil, South America), Caesalpinia sappan L. (East Indies, Asia), Haematoxylum brasiletto Karsten (Central America) and Haematoxylum campechianum L. (North America) were analysed in order to isolate key Raman biomarker bands which could provide the basis for an identification protocol. Previously recorded Raman spectra of brazilin and brazilein pigments extracted from genuine brazilwood of Brazilian origin provided a foundation for the nondestructive spectral discrimination between a sample of false ‘brazilwood’, which consisted of an inferior wood substratum stained with genuine pigment, and the true specimens. The provision of well-documented specimens of determinable age from the archival collection facilitated the evaluation of the effects of temporal degradation on the observed spectra, which could be used to further test the experimental protocols for nondestructive verification of samples in the archive with questionable assignment or provenance.
Introduction
In mediaeval times a thriving trade was developed between Europe and Asia in a wood derived from the Caesalpinia sappan L. tree, from which bright reddish–purple colours were extracted for textile dyeing.1 Under the name brasilium or brasyll, this wood later became to be known as brazilwood, although its origin was clearly the East Indies. The dye extracted from brazilwood during the Middle Ages was exotic and very expensive, and the transport from Asia to Europe was also very difficult, increasing the price of the pigment used as a colouring agent for cotton, woollen cloth, and as red ink.2 In 1500 Pedro Alvares Cabral discovered the land now known as Brazil, firstly named by Cabral as Santa Cruz, but afterwards taking its name from the characteristic red wood from trees flourishing along the coastline. However, it was not then realized that the ‘brazilwood’ from Brazil was a different species of tree from its Asian counterpart, the South American version being Caesalpinia echinata Lam. The situation was further complicated by the discovery of other red woods from Central American sources, which properly belong to the Haematoxylum genus. In Table 1 there is a description of the accepted names and synonyms of the main species investigated in this work, following the International Legume Database and Information Service World Database of Legumes (http://www.ildis.org/).| Accepted name | Synonym | Common name used in 19th century trade | Distribution |
|---|---|---|---|
| Caesalpinia echinata Lam. | Guilandina echinata (Lam.) Spreng. | Brazilwood, Pernambuco | Wild in coastal Brazil; also cultivated in southern Brazil |
| Caesalpinia sappan L. | Biancaea sappan (L.) Tod. | Sappan wood | Native to Malaysia; cultivated throughout south and southeast Asia |
| Haematoxylum brazilleto Karsten | Haematoxylum boreale S. Watson | Nicaragua wood, Lima wood, Peach wood, Wood of St. Martha | Wild in Nicaragua and Venezuela |
| Haematoxylum campechianum L. | Logwood, Palo del Brasil, Jamaica wood | Wild in Yucatan, Mexico and Belize; cultivated in the Caribbean, especially Jamaica. Some very limited cultivation in South Asia. |
The new source of red wood from South America, ‘arbor brasilia’, was subjected to particularly vigorous deforestation from as early as 1501 and by 1652 the Brazilian forests were ravaged of brazilwood for the European market.3 This was gradually replaced with Haematoxylum brasiletto Karsten from Central America, which unfortunately also became known as brazilwood, adding to the confusion for botanists and historians.
Chemically, the major compound which is isolated from Caesalpinia is brazilin (structure 1A), which forms the deep red brazilein (structure 1B) on exposure to air and light, during which process the secondary hydroxyl group is oxidised to a quinonoid moiety. The resonance of this group with the conjugated system gives rise to the deep red colour of the brazilein in brazilwood. The haematoxylin (structure 1C) and haematein (structure 1D) extracted from Haematoxylum (false brazilwood) are chemically very closely related to brazilin and brazilein, respectively, through a similar oxidative process; the haematoxylin and haematein each have an additional phenolic hydroxyl group in the 8-position over the structures of brazilin and brazilein.4 Most of the chemical and colourant properties of these compounds can be found in textbooks about natural colourants and dyestuffs.5,6
There is much interest currently in identifying the source of artefacts related to these woods, which were used in antiquity for the manufacture of medicines, musical instruments and decorative objects for housewares; the sourcing of wood samples in herbaria, museums and archaeological sites is of critical importance for the establishment of trade routes with ancient cultures and this is now receiving attention. Since only small quantities of archival wood in herbaria are normally available, the destruction of valuable specimens using wet chemical extraction is not favoured. Hence, the purpose of this work was the assessment of FT-Raman spectroscopy for the nondestructive characterization of brazilwood and related specimens; here, the minimisation of fluorescence from the natural material without chemical or mechanical pretreatment is a prime objective for which the near-infrared laser excitation at 1064 nm is excellent. In a preliminary study, we have been able to characterize chemically pure brazilin and brazilein which had been extracted from some documented samples of native Brazilian Caesalpinia echinata Lam.; this has been of fundamental importance in the present work for the unambiguous identification of these chemical compounds in wood samples.7 Although it was not possible similarly to obtain specimens of haematoxylin and haematein extracted from Haematoxylum trees, a preliminary target of the current work is the identification of key biomarkers for these chemical compounds which differentiate them from their brazilin and brazilein analogues.
Further complication in accessing suitable specimens from herbarial and arboreal archives is the proliferation of common names for genuine brazilwood and its congeners. Examples are shown in Table 2 and include Nicaragua wood, Pernambuco wood, logwoods, Kayu spang redwood and sappan wood. In the current work, we have been fortunate to access some twenty samples of ‘brazilwoods’ from the Economic Botany Collection, Royal Botanic Gardens, Kew, which were collected between 1849 and 1993. These provide the basis for the well-documented specimens used in the derivation of the Raman spectroscopic protocol for identification of genuine brazilwood and for the testing of this protocol against examples of questionable authenticity and nomenclature.
| Specimen | RBG catalogue number | Date | Country of origin | Number of specimens | Common name | Provenance details |
|---|---|---|---|---|---|---|
| 1. Haematoxylum brasiletto | 21521 21522 7293 | 1900 | Mexico | 3 | Brazilwood | Exposition Universelle, Paris |
| 2. Haematoxylum brasiletto | 71256 | 1933 | ? | 1 | Nicaragua wood | Trade: Bowes Loddiges |
| 3. Haematoxylum brasiletto | 75899 | 1993 | Nicaragua | 1 | Nicaragua wood | Collected from living specimen in Nicaragua |
| 4. Haematoxylum campechianum | 24771 | ? | West Indies | 1 | Bastard logwood | Unknown |
| 5. Haematoxylum campechianum | 7296 | 1895 | USA | 1 | Bastard logwood | Trade: A. and Br. Lascalles, New York |
| 6. Haematoxylum campechianum | 72917 | 1992 | USA | 1 | Bastard logwood | Collected from living specimen in Fairchild Tropical Garden, Florida |
| 7. Haematoxylum campechianum | 58064 | ? | ? | 1 | Jamaica logwood | Trade: Royal Pharmaceutical Society |
| 8. Haematoxylum campechianum | 59728 | ? | ? | 1 | Logwood | Trade: A. Hill and Son |
| 9. Caesalpinia echinata | 58129 | 1878 | ? | 1 | — | Trade: Royal Pharmaceutical Society |
| 10. Caesalpinia echinata | 67796 | 1849 | ? | 1 | — | Trade |
| 11. Caesalpinia echinata | 6968 | 1879 | Brazil | 1 | — | Natural History Museum, London |
| 12. Caesalpinia echinata | 6969 | 1920 | Brazil | 1 | Pernambuco wood | Trade: W.E. Hill and Sons |
| 13. Caesalpinia echinata | 71043 | 1933 | Brazil | 1 | Ibira Pitanga wood | Trade: Bowes Loddiges |
| 14. Caesalpinia sappan | 21451 | ? | India | 1 | — | J. S. Gamble, Indian Forest Service |
| 15. Caesalpinia sappan | 25458 | ? | India | 1 | Sappan wood | Trade: Thomas Glover |
| 16. Caesalpinia sappan | 57893 | 1880 | India | 1 | Sappan wood | Trade: Royal Pharmaceutical Society |
| 17. Caesalpinia sappan | 59394 | 1910 | Sarawak | 1 | Kayu spang redwood | Imperial Institute, London, specimen 33754 |
| 18. Caesalpinia sappan | 59400 | ? | India | 1 | Sappan wood | Indian Museum, London |
Experimental
Specimens
Twenty specimens of wood (Table 2) from genuine brazilwood specimens and substitutes were provided from the Royal Botanic Gardens, Kew. These were of the Leguminosae–Caesalpinioidae family and comprised Haematoxylum brasiletto Karsten (5 specimens), Haematoxylum campechianum L. (5 specimens), Caesalpinia echinata Lam. (5 specimens) and Caesalpinia sappan L. (5 specimens). The specimens had been collected at various times between 1849 and 1993 from locations in the Caribbean, Central America, North America, Brazil, India and the Far East, in some cases by importers, and in others directly from the countries concerned. All wood specimens were characterized by a deep red colour but only those of the Caesalpinia echinata Lam. specimens from Brazil can truly be described as brazilwood, other common names deriving from logwood and sappan wood or variants of these. In some cases, the country of origin of the specimens cannot be specified and there is some doubt about their taxonomic classification.Instrumental
The Raman spectra were obtained from a Bruker IFS66 with FRA106 Raman module, with 1064 nm excitation from a Nd3+/YAG laser, equipped with germanium detector cooled with liquid nitrogen; spectra were recorded with up to 4000 spectral accumulations at 4 cm−1 resolution over the wavenumber range between 100 and 3500 cm−1. The laser power was kept at a minimum value (less than 20 mW at the source) to avoid degradation problems. All the samples were studied without modification or further treatment, and the Raman spectrum of each sample was obtained at least twice to assure reproducibility.Results and discussions
The vibrational spectra of pure brazilin and brazilein have been discussed in a recent paper;7 the compounds were extracted from a genuine Brazilian specimen of Caesalpinia echinata Lam. In that work, we assigned some Raman and infrared vibrational bands as biomarkers in the 1500–1700 cm−1 region, where the vibrational modes ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups can be observed.7 Also we gave for comparison purposes the Raman spectrum of an archival specimen of Caesalpinia echinata Lam., from the Royal Botanic Gardens, dating from about an eighty years ago.7 At first sight, the comparison shows that the spectra are very close, and the biomarker bands could be employed to certify the assignment of the wood as a typical brazilwood.
C groups can be observed.7 Also we gave for comparison purposes the Raman spectrum of an archival specimen of Caesalpinia echinata Lam., from the Royal Botanic Gardens, dating from about an eighty years ago.7 At first sight, the comparison shows that the spectra are very close, and the biomarker bands could be employed to certify the assignment of the wood as a typical brazilwood.
In this study there are several questions which need to be addressed and to which the Raman spectroscopic method may provide answers:
(1) Is it possible to identify nondestructively a spectral feature characteristic of brazilein or
brazilin
, the red pigments present in genuine, documented examples of archival brazilwood material?
(2) Do woods of similar appearance to genuine brazilwood, or alternative botanical species, have any features which facilitate their discrimination from the genuine species?
(3) Despite the close chemical similarity between brazilein and
haematein
, is it possible to identify the
Haematoxylum
and
Caesalpinia
genera unequivocally using Raman spectroscopy?
(4) Can Raman spectroscopy discriminate between the visually similar South America and East Indies sources of brazilwoods, namely
Caesalpinia echinata
Lam. and
Caesalpinia sappan
L.?
(5) Does the influence of ageing or degradation of material in storage obscure the classification of genuine or imitation brazilwoods?
(6) Is it possible to use
Raman spectroscopy
to identify ‘fake’ brazilwood, which can consist of a common base wood treated with genuine
brazilein
pigment
dye
, or at least with any kind of red dye?
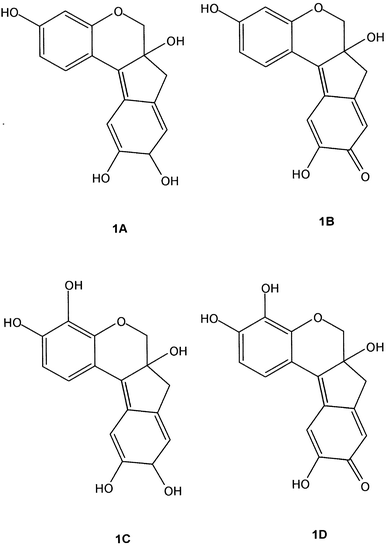
Although the sharpest and strongest intensity Raman band in the pigment brazilein characteristic of Caesalpinia echinata Lam., genuine brazilwood from Brazil, occurs at 1607 cm−1 (Fig. 1), this also has a close accidental counterpart in wood lignin at 1603 cm−1;8–12 hence, the Raman spectra of genuine brazilwood shows a broadened feature in this region. The identification of brazilein in the natural woods therefore relies on the presence of the broad band at ca. 1570 cm−1 and the weaker features at 728 and 764 cm−1, whose tentative assignments to aromatic ring quadrant in-phase deformations can be observed in Table 1. Also, a sharp band at 1167 cm−1 is present in all documented brazilwoods and in the brazilein spectrum, as a medium intensity feature, but in brazilin is present only as a very weak feature and, hence, must arise after the oxidisation of the pigment from its exposure to air. It is worth noting that the vibrational assignments made in Table 3 are only tentative and that most of the modes are certainly coupled and also must have some contribution from the wood cellulose. In this sense, the multiplet observed in the region between 1100 and 1150 cm−1 in the Raman spectra of the investigated samples can be assigned to the signature of softwood.11
 | ||
| Fig. 1 FT-Raman spectra of Caesalpinia echinata Lam. specimens. Details of each specimen are given in Table 2. | ||
| Specimen 9 | Specimen 10 | Specimen 11 | Specimen 12 | Specimen 13 | Assignment |
|---|---|---|---|---|---|
| a sh shoulder, w weak, s strong, vw very weak, m medium. | |||||
| 1660 sh,w | 1660 sh,w | 1660 sh,w | 1660 sh,w | 1660 sh,w | ν(C=O) lignin conjugated |
| 1610 sh,w | 1607 sh,w |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) + ν(C C) + ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) dye O) dye |
|||
| 1600 s | 1603 s | 1600 s | 1600 s | 1600 s | ν(C–C) lignin aromatic ring |
| 1570 s | 1570 s | 1564 s | 1574 s | 1565 s |
ν(C–C) + ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) dye O) dye |
| 1550 mw | 1541 w | 1550 w | 1548 mw | ||
| 1502 | 1498 m | 1502 m | |||
| 1452 vw | 1443 w | ||||
| 1426 | 1430 br | 1439 w | |||
| 1367 | 1367 | 1375 | 1374 | 1377 | |
| 1325 ms | 1325 ms | 1321 m | 1325 m | 1321 ms | ν(C–C) ν(C=O) composed of syringyl and guaiacylligin modes |
| 1249 m | 1246 w | 1249 w | 1250 w | ||
| 1223 w | 1223 w | 1226 w | 1223 w | δ(C–C) | |
| 1203 m | 1203 w | 1203 w | δ(C–C) | ||
| 1164 s | 1164 m | 1167 w | 1164 m | 1161 w | δ(C–H) + δ(C–C) |
| 1145 sh,w | δ(C–H)i.p. | ||||
| 1111 w | 1115 w | 1118 w | 1125 w | δ(C–H)i.p. wood cellulose | |
| 1095 w | 1095 w | 1095 w | 1092 w | δ(C–H)i.p. wood cellulose | |
| 1030 w | 1030 w | 1033 w | 1036 w | 1039 w | δ(C–H)i.p. |
| 931 vw | 931 vw | 928 vw | |||
| 905 vw | 905 vw | 895 vw | 902 vw | 895 vw | δ(C–H)o.o.p. |
| 764 w | 764 w | 764 w | 764 w | 764 w | δ(C–H)i.p. |
| 725 mw | 728 mw | 728 w | 725 w | 728 w | δ(C–H)i.p. |
| 669 w | 669 vw | ||||
| 548 m | 548 mw | 548 w | 548 w | holocellulose | |
| 446 ms | 446 m | 443 w | 446 mw | 439 w | |
| 374 w | 377 w | holocellulose | |||
| 348 w | 348 w | ||||
| 315 m | 315 w | 308 w | 315 w | 308 w | |
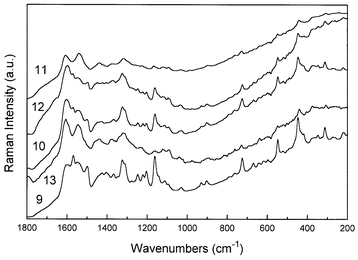
Significantly, although specimens of Caesalpinia echinata Lam. have these characteristic bands, those of Caesalpinia sappan L. do not (Fig. 2). A problem arises here in the authenticity of the archival specimens, since several could be of questionable attribution. Potentially, therefore, Raman spectroscopy could provide a nondestructive diagnostic method for discrimination between Caesalpinia echinata Lam. and Caesalpinia sappan L. woods; in itself this could not only provide a better classification of archival arboreal specimens of questionable provenance, but also generates novel information about the trade routes from which these specimens were obtained—since C. echinata is of South America origin and C. sappan comes from the East Indies.
 | ||
| Fig. 2 FT-Raman spectra of Caesalpinia sappan L. specimens. Details of each specimen are given in Table 2. | ||
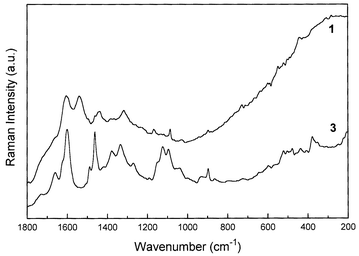
Comparison of the molecular structures of the red pigment formed by aerial oxidation in the cut woods of Caesalpinia and Haematoxylum trees reveals that we would expect to observe very similar Raman spectra from brazilein and haematein, respectively. In a previous study,7 we reported the first Raman spectra of the reduced and oxidised pigments, brazilin and brazilein, which had been extracted from genuine specimens of brazilwood from Caesalpinia echinata Lam. We have not been able to obtain extracts of haematoxylin and haematein to verify the band wavenumbers characteristic of these materials in their Raman spectra, but we would suggest that since the only difference between the brazilein and haematein (and also the reduced counterparts) is a simple phenolic –OH group, then their spectra would be very similar to each other. Fig. 3 shows the typical Raman spectra of Caesalpinia samples, together with the spectra of pure brazilin and brazilein compounds. Based on the features present in the spectra of both brazilwoods, mainly concerning the Raman band at ca. 1570 cm−1, it is possible to attribute that in Caesalpinia echinata Lam. there is a predominance of the brazilein structure as a major component, while in Caesalpinia sappan L. the major constituent spectroscopically is probably brazilin.
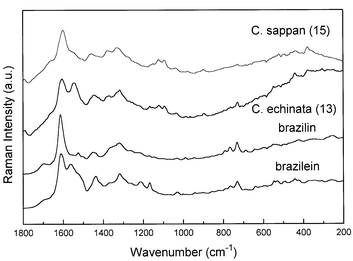 | ||
| Fig. 3 Comparison between the FT-Raman spectra of Caesalpinia echinata Lam. (specimen 15, Table 2), Caesalpinia sappan L. (specimen 13) and the pure compounds brazilin and brazilein, extracted from Caesalpinia echinata Lam.7 | ||
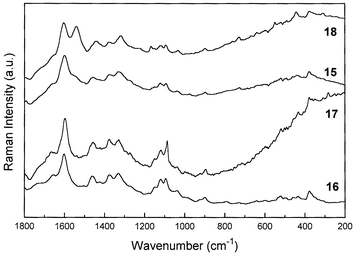
In Fig. 4 and 5 are depicted the Raman spectra of Haematoxylum campechianum L. and Haematoxylum brasiletto Karsten specimens, respectively. The Raman spectra of H. campechianum specimens show two different patterns, in a similar way observed to C. sappan species. Only three specimens have their origin displayed: specimens 5 and 6, from the USA, and specimen 4 from the West Indies; the other specimens do not have their origin or age defined. In this sense, the comparison of the Raman spectra from the known specimens indicates that there is a similarity between H. campechianum specimens from USA (specimen 5) and West Indies (specimen 4), since the isolated band at ca. 1600 cm−1, which is present in all spectra, seems to be characteristic of the haematoxylin compound. On the other hand, specimen 6, with a provenance from the USA, depicts a strong band at 1086 cm−1 in the vibrational region characteristic of softwood,11 despite the presence of the doublet at 1550–1600 cm−1 which is characteristic of haematoxylin. In this situation, as this specimen is one of the well documented collections, it is likely that the Raman spectrum is showing the contributions of both the wood and the dye, probably due to a poor content of the pigment at the sample; there is a weak doublet due to wood cellulose near 1095 and 1120 cm−1 in these samples. Specimen 8 does not have a defined origin and age, however, the label on this sample has the legend ‘Jamaica logwood’, suggestive of a Central American location for this sample. However, the Raman spectrum of specimen 8 is very similar to that of C. echinata specimens, suggesting some misinterpretation about the origin of this particular specimen. In Fig. 5, the difference between the Raman spectra of the two specimens of H. brasiletto (1 and 3, Table 1) is very clear; it seems that specimen 3 shows a Raman spectrum that is very similar to softwoods,8 whilst specimen 1 resembles the characteristic Raman spectra of C. echinata, mainly due to the presence of the doublet at 1550–1600 cm−1. As specimen 3 is a recent and secure identification from the wild, the observed Raman spectrum could be perhaps due to a very poor content of dye present at the sample, as a result of a recent collection or even a part of the bark with a very low concentration of dye, as tentatively explained above in a similar case. As a result of the Raman spectra obtained for the H. brasiletto specimens, probably it seems unlikely that all are misidentified, however the results strongly suggest this situation. Interestingly, there is a hint in an old study13 that there are two forms of H. campechianum, named Bastard logwood, very poor in dye, and True logwood, very rich in dye content. Perhaps variation in dye content could account for some of the results shown in our investigation.
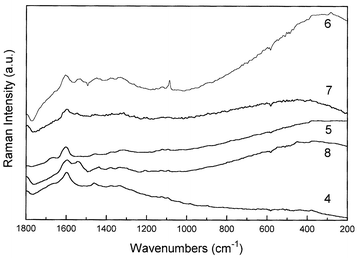 | ||
| Fig. 4 FT-Raman spectra of Haematoxylum campechianum L. species. Details of each specimen are given in Table 2. | ||
 | ||
| Fig. 5 FT-Raman spectra of Haematoxylum brasiletto Karsten species. Details of each specimen are given in Table 2. | ||
In Fig. 6 we compare the Raman spectra of selected specimens of each one of the brazilwoods studied here, from which we can differentiate between the two botanical species using their characteristic Raman bands. The comparison shows that the bands of genuine brazilwood at 728, 764 and 1167 cm−1 are absent from the H. brasiletto specimens, which have features at 1660, 1463, 1270, 1150, 1123, 897 and 377 cm−1 that are not present in brazilwood. A second botanical species, Haematoxylum campechianum, originating from the West Indies and the Southern USA was dissimilar to the H. brasiletto in that these prominent features were absent; the specimens alleged to be H. campechianum, in fact, have Raman spectra which are very closely similar to C. echinata. Also, specimen 17 (Table 1), which is alleged to be a specimen of C. sappan from Sarawak, has a Raman spectrum which is dissimilar from the other specimens of C. sappan; the vibrational spectrum (Fig. 3) is very close to that of H. brasiletto with all the prominent characteristic features of the latter at 1660, 1463, 1270, 1150, 1123, 900 and 377 cm−1 being present. However, all the similarities suggested here must be taken with care, since all the specimens present the same kind of pigment and perhaps should present some analogies on their Raman spectra. Also, it seems very reaSonable that some kind of degradation could affect the archival materials, leading to the results shown here.
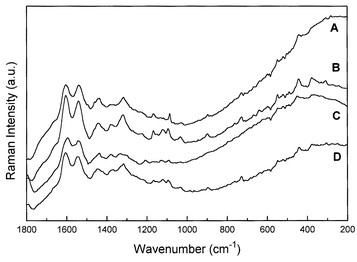 | ||
| Fig. 6 FT-Raman spectra of H. brasiletto (specimen 1, A), C. sappan (specimen 18, B), H. campechianum (specimen 8, C) and C. echinata Lam., (specimen 13, D). | ||
A difficult, but very important, discrimination for Raman spectroscopy is provided for samples of C. echinata (from Brazil) and C. sappan (from the East Indies). The most significant Raman spectral difference between the well-documented specimens 13 and 16 (Table 1) is the presence of the doublet in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching region, 1550–1620 cm−1 of the former and a singlet for the latter, with a medium intensity triplet in the 1000–1200 cm−1 region for the C. sappan, occurring only with significantly weaker intensity for C. echinata. In this protocol, it seems that Raman spectroscopy has the potential to provide a nondestructive analytical discrimination between these two Caesalpinia botanical species. However, there appears to be a discrepancy in that one specimen which has been assigned to C. sappan (specimen 18, Table 1) with a questionable country of origin (India), in fact has a spectrum remarkably similar to that of C. echinata (specimen 13)! It is therefore strongly suggestive from the Raman spectroscopic analysis of specimen 18 that it has been incorrectly attributed to a C. sappan botanical source from an unknown country of origin and that it is probably C. echinata. However, it is worth mentioning that these species contain the same pigment, and relative variations of it on the sample surface should be affecting the obtained Raman signal, and we must be careful on this analysis.
C stretching region, 1550–1620 cm−1 of the former and a singlet for the latter, with a medium intensity triplet in the 1000–1200 cm−1 region for the C. sappan, occurring only with significantly weaker intensity for C. echinata. In this protocol, it seems that Raman spectroscopy has the potential to provide a nondestructive analytical discrimination between these two Caesalpinia botanical species. However, there appears to be a discrepancy in that one specimen which has been assigned to C. sappan (specimen 18, Table 1) with a questionable country of origin (India), in fact has a spectrum remarkably similar to that of C. echinata (specimen 13)! It is therefore strongly suggestive from the Raman spectroscopic analysis of specimen 18 that it has been incorrectly attributed to a C. sappan botanical source from an unknown country of origin and that it is probably C. echinata. However, it is worth mentioning that these species contain the same pigment, and relative variations of it on the sample surface should be affecting the obtained Raman signal, and we must be careful on this analysis.
The effect of ageing of resins and pigments on their Raman spectra through environmental degradation, in which atmospheric oxidation, radiative fission and bacterial scission of biomolecular structures are possibly expected to occur, has been noted in the application of Raman spectroscopy to the characterization of biomaterials from archaeological excavations. Resin degradation in paleo-Indian grave artifacts, linens from sarcophagi and human tissue such as skin, hair and nail from cold and hot desert burials have been studied using Raman spectroscopy and the following have been noted: (1) A broadening of observed band widths and a ‘smearing out’ of structural information in the spectra through bond scission and ring-opening reactions.14–16 (2) A diminution of spectral intensity in certain functional groups such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–S bonds.17–22 (3) Creation of new bands arising from incorporation of material from the burial or storage environment.23–25
O and S–S bonds.17–22 (3) Creation of new bands arising from incorporation of material from the burial or storage environment.23–25
In the present work, we notice the effect of specimen age on the Raman spectra using the documented archival specimens from expeditions in which materials from the same botanical species have been collected over approximately 150 years. An interesting comparison is made of C. echinata specimens in Fig. 1: specimens 11, 12 and 13 were collected in 1879, 1920 and 1933 from Brazil. Clearly, specimens 11 and 13 are very similar indeed to each other, but 9, 10 and 12 are different in that the characteristic doublet in the 1550–1620 cm−1 region has disappeared and new bands have appeared in the lower wavenumber region, 300–800 cm−1. With some differences in relative intensity, specimens 9, 10 and 12 are also assignable to the same species set—and come from the period 1849–1920. The situation is complicated in that only one of these specimens, 12, is known to have a clearly defined country of origin (Brazil)—the others are from unknown geographical locations. We cannot be certain of the discrepancy between the Raman spectral data for the two sets of C. echinata specimens (viz. 11, 13 and 9, 10, 12) but this could perhaps reflect environmental differences in storage before deposition was made in the Economic Botany Collection Kew.
Conclusions
Nondestructive Raman spectroscopic analysis of archival well-documented specimens of ‘brazilwoods’ from different botanical species and from different geographical locations has provided a possible protocol for the discrimination between visually closely similar materials. All the results shown above strongly suggest that it is possible to use Raman spectroscopy for the analytical identification of the main features of some brazilwood trees, mainly between the Caesalpinia species. However, several important details must be taken into account, as for example the geographical origin of each one of the samples, age of each species, and probably the dye content, since the Raman spectrum is very sensitive to the presence of the pigment. It has also been shown some of the Raman bands that could be used as biomarkers in the identification of brazilwoods, but this identification is based only in the comparison with the previous work on the vibrational spectra of brazilin and brazilein,7 the main dyes of brazilwood from Brazil. This fact impllies there is a good possibility for separation of C. echinata and C. sappan species. There are several problems in separating Haematoxylum from Caesalpinia. The key evidence for this are specimens 3 and 6 (Table 2), collected recently from living material and of certain identification, but the Raman protocol seems poor to identify. Further work is needed using extracts from recently harvested wood of Haematoxylum species.Acknowledgements
LFCO wishes to thank CAPES (Brazil) for the grant of a research fellowship during the time of which this work was carried out.References
- R. A. Defilipps, Arch. Nat. Hist., 1998, 25, 103 Search PubMed.
- W. P. Armstrong, Herbalgram, 1994, 32, 30 Search PubMed.
- P. F. Souza, in Plants and Plant Sciences in Latin America, ed. F. Verdoorn, Chronica Botanica Company, Waltham, MA, USA, 1945, pp. 111–119 Search PubMed.
- H. Puchtler, S. N. Meloan and F. S. Waldrop, Histochemistry, 1985, 85, 353.
- A. G. Perkin and A. E. Everest, The Natural Organic Colouring Matters, Monographs on Industrial Chemistry, ed. E. Thorpe, Longmans, Green and Co., London, 1918, ch. IX, pp. 345–383 Search PubMed.
- C. L. Green, Natural Colorants and Dyestuffs—A Review of Production, Markets and Development Potential, Food and Agriculture Organization of the United Nations, Rome, 1995, pp. 47–50 Search PubMed.
- L. F. C. de Oliveira, H. G. M. Edwards, E. S. Velozo and M. Nesbitt, Vib. Spectrosc., 2002, 28, 243 CrossRef CAS.
- T. P. Schultz, M. C. Templeton and G. D. McGinnis, Anal. Chem., 1985, 57, 2867 CrossRef CAS.
- P. A. Evans, Spectrochim. Acta, Part A, 1991, 47, 1441 CrossRef.
- R. C. Kenton and R. L. Rubinovitz, Appl. Spectrosc., 1990, 44, 1377 Search PubMed.
- I. R. Lewis, N. W. Daniel, Jr., N. C. Chaffin and P. R. Griffiths, Spectrochim. Acta, Part A, 1994, 50, 1943 CrossRef.
- M. Takayama, T. Johjima, T. Yamanaka, H. Wariishi and H. Tanaka, Spectrochim. Acta, Part A, 1997, 53, 1621 CrossRef.
- J. H. Holland, Bull. Misc. Inf., R. Bot. Gard., 1916, 9, 211 Search PubMed.
- M. T. Kirchner, H. G. M. Edwards, D. Lucy and A. M. Pollard, J. Raman Spectrosc., 1997, 28, 171 CrossRef CAS.
- H. G. M. Edwards and M. J. Falk, J. Raman Spectrosc., 1997, 28, 211 CrossRef CAS.
- H. G. M. Edwards, L. F. C. de Oliveira and A. Quye, Spectrochim. Acta, Part A, 2001, 57, 2831 Search PubMed.
- H. G. M. Edwards, D. W. Farwell and A. Quye, J. Raman Spectrosc., 1997, 28, 243 CrossRef CAS.
- W. Akhtar, H. G. M. Edwards, D. W. Farwell and M. Nutbrown, Spectrochim. Acta, Part A, 1997, 53, 1021 CrossRef.
- H. G. M. Edwards and M. G. Sibley, Spectrochim. Acta, Part A, 1997, 53, 2373 CrossRef.
- M. Gniadecka, H. G. M. Edwards, J. P. H. Hansen, O. F. Nielsen, D. H. Christensen, S. E. Guillen and H. C. Wulf, J. Raman Spectrosc., 1999, 30, 147 CrossRef CAS.
- H. G. M. Edwards, D. W. Farwell and D. D. Wynn-Williams, Spectrochim. Acta, Part A, 1999, 55, 2691 Search PubMed.
- H. G. M. Edwards, L. F. C. de Oliveira and A. Quye, Spectrochim. Acta, Part A, 2001, 57, 2831 Search PubMed.
- H. G. M. Edwards and M. J. Falk, Appl. Spectrosc., 1997, 51, 1134 Search PubMed.
- H. G. M. Edwards, D. W. Farwell, D. L. A. de Faria, A. M. F. Monteiro, M. C. Alfonso, P. De Blasis and S. Eggers, J. Raman Spectrosc., 2001, 32, 17 CrossRef CAS.
- A. S. Wilson, H. G. M. Edwards, D. W. Farwell and R. C. Janaway, J. Raman Spectrosc., 1999, 30, 378 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |